Abstract
Autophagy is an important mechanism in cancer cell survival and tumor growth and plays both pro- and anti-oncogenic roles. However, the biochemical basis for these diverse functions is not well understood. Our work provides new evidence for the existence of two separate autophagic programs regulated in an opposite manner by the von Hippel-Lindau tumor suppressor (VHL). These programs, marked by differential requirements for LC3B vs. LC3C, play tumor-promoting and tumor-suppressing roles in renal cancer.
Keywords: autophagy, HIF, LC3B/LC3C, renal cancer, VHL
Sufficient access to nutrients is a fundamental requirement of cancer cells for survival and proliferation. Thus, tumor angiogenesis, which provides nutrients from the blood, is considered to be a hallmark of cancer and is a useful target for anticancer therapies. It is, however, increasingly clear that, in addition to extracellular sources of nutrients, cancer cells also efficiently utilize intracellular nutrients through the process of macroautophagy to drive oncogenic growth and survival.
Autophagic recycling of organelles, protein aggregates, and/or cytoplasm yields simple metabolites for use in cell growth or bioenergetics, and can destroy damaged structures to prevent toxicity. However, autophagy can be a double-edged sword: on the one hand, cancer cells exploit autophagy to enhance the nutrient supply, while on the other hand, too much autophagy leads to self-depletion and triggers cell death. At the same time, there is genetic evidence from mouse model systems that knockdown of certain canonical autophagic regulators, such as BECN1, ATG5, or ATG7, promotes tumor development. Opposing roles for autophagy in cancer—tumor promoting vs. tumor suppressing—may result from differing interactions with cooperating oncogenes. Alternatively, the impact of autophagy in oncogenesis may be determined by differences in the formation of autophagic vacuoles carrying distinct cargos in a tissue- or starvation-specific manner.
There are multiple steps in autophagic pathways in which the specificity of the autophagic process is determined. Autophagy begins with the formation of a phagophore that can originate by de novo formation or from diverse sources, such as the plasma membrane, Golgi, endoplasmic reticulum, or outer mitochondrial membrane. A ubiquitin-like conjugation system generates an ATG12–ATG5 conjugate that, with ATG16L1, carries out a final E3-like conjugation of phosphatidylethanolamine (PE) to a family of proteins known as the LC3/GABARAP paralogs. The lipidated forms are then inserted into the autophagosome membrane where they recruit cargo by binding to adaptor proteins, such as SQSTM1/p62, NBR1, and CALCOCO2/NDP52, that seem to carry some level of specificity to certain cargos. Human cells have at least seven different LC3 paralogs (LC3A/B/B2/C and GABARAP/L1/L2), which indicates a biochemical richness that could lead to both specificity and redundancy.
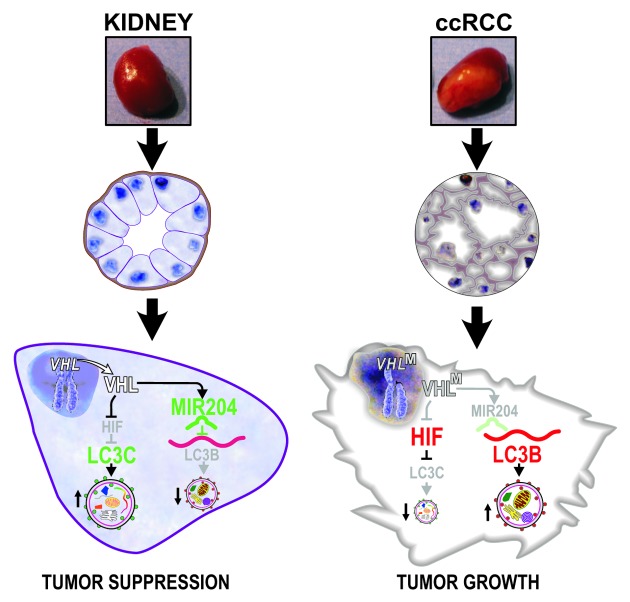
Our recently published results show that differential engagement of LC3 paralogs identifies separate autophagic programs that exert either pro- or anti-tumorigenic activities in renal cell carcinoma. Both autophagic programs are regulated by VHL. The LC3B autophagic program supports tumor formation by VHL- renal cancer cells in orthotopic xenografts. LC3B expression is significantly increased in high-grade human ccRCC tumors as compared with lower grade tumors. The second program, mediated by a lesser-known LC3 paralog, LC3C, has tumor-suppressing activity. Knockdown of LC3C in VHL-expressing cells induces the formation of tumors that would otherwise be suppressed by VHL tumor suppressor function. In addition, LC3C expression is significantly higher in normal human kidney tissue as compared with ccRCC tumors.
We found that VHL inhibits LC3B-mediated autophagy through induction of MIR204, which directly targets LC3B. In parallel, VHL, by inhibiting HIF, induces expression of LC3C, which carries tumor-suppressing autophagic activity (Fig. 1). At this point, the specific cargo by which LC3s mediate differential oncogenic effects is not yet understood, but one can speculate that specific oncogenic cargo can be targeted for lysosomal degradation. It is also becoming increasingly evident that there are noncanonical pathways regulating autophagy in a manner independent of the hierarchical recruitment of upstream regulators. These emerging regulatory mechanisms, along with the variety of LC3 activities that can be employed in autophagy, indicate that there is a wide biochemical and functional spectrum of autophagic pathways. Integrating our data with the established role of VHL in regulating angiogenesis produces a more global picture of VHL as a master tumor suppressor that regulates nutrient access for renal cancer cells from both extracellular and intracellular sources.
Figure 1. VHL inhibits LC3B and stimulates LC3C autophagic pathways. VHLM, mutant (inactive, VHL-) VHL; ccRCC, clear cell renal cell carcinoma. From Cancer Cell 2012; 21:532–46, 2012. Reproduced by permission of the publisher.
Clearly, autophagy represents an attractive target for the development of therapeutic approaches, particularly for the treatment of therapy-resistant cancers such as metastatic renal cancer. Moreover, targeting autophagy could be particularly beneficial in combination with, or as an alternative to, anti-angiogenic therapies or inhibition of the MTOR pathway, as both such approaches induce autophagy. For instance, the small molecule STF-62247 has been identified as a stimulator of autophagy leading to cell death and inhibition of RCC growth in xenograft models. In addition, inhibition of autophagy by chloroquine appears to cause robust tumor regression in the case of pancreatic cancer. Indeed, there is currently one phase I clinical trial looking at the treatment of metastatic ccRCC with the autophagy inhibitor hydroxychloroquine. However, the emerging complexity in autophagic pathways implies that the application of nonspecific autophagy inhibitors may not be as effective as originally hoped, as both tumor-promoting and tumor-suppressing pathways will be affected. Our data also showed that, while VHL- cancer cells rely heavily on the activity of LC3B-dependent autophagy during starvation, cells with VHL can activate both LC3B- and LC3C-dependent programs. Thus, selective targeting of LC3B-dependent autophagy may specifically kill VHL- cells while sparing nontransformed kidney cells. Looking ahead, it will be imperative to understand the role of individual autophagic pathways in different cancers and to develop small-molecule inhibitors capable of targeting them selectively.
Acknowledgment
This work was supported in part by the following grants to MCK:NCI CA122346, BLR&D VA Merit Award.
Glossary
Abbreviations:
- HIF
hypoxia inducible factor
- VHL
von Hippel-Lindau tumor suppressor, ccRCC, clear cell renal cell carcinoma
- VEGF
vascular endothelial growth factor
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20650