Abstract
PolyI:C is a nucleotide pattern molecule that induces cross-presentation of foreign Ag in myeloid dendritic cells (DC) and MHC Class I-dependent proliferation of cytotoxic T lymphocytes (CTL). DC (BM or spleen CD8α+) have sensors for dsRNA including polyI:C to signal facilitating cross-presentation. Endosomal TLR3 and cytoplasmic RIG-I/MDA5 are reportedly responsible for polyI:C sensing and presumed to deliver signal for cross-presentation via TICAM-1 (TRIF) and IPS-1 (MAVS, Cardif, VISA) adaptors, respectively. In fact, when tumor-associated Ag (TAA) was simultaneously taken up with polyI:C in DC, the DC cross-primed CTL specific to the TAA in a syngenic mouse model. Here we tested which of the TICAM-1 or IPS-1 pathway participate in cross-presentation of tumor-associated soluble Ag and retardation of tumor growth in the setting with a syngeneic tumor implant system, EG7/C57BL6, and exogenously challenged soluble Ag (EG7 lysate) and polyI:C. When EG7 lysate and polyI:C were subcutaneously injected in tumor-bearing mice, EG7 tumor growth retardation was observed in wild-type and to a lesser extent IPS-1−/− mice, but not TICAM-1−/− mice. IRF-3/7 were essential but IPS-1 and type I IFN were minimally involved in the polyI:C-mediated CTL proliferation. Although both TICAM-1 and IPS-1 contributed to CD86/CD40 upregulation in CD8α+ DC, H2Kb-SL8 tetramer and OT-1 proliferation assays indicated that OVA-recognizing CD8 T cells predominantly proliferated in vivo through TICAM-1 and CD8α+ DC is crucial in ex vivo analysis. Ultimately, tumor regresses > 8 d post polyI:C administration. The results infer that soluble tumor Ag induces tumor growth retardation, i.e., therapeutic potential, if the TICAM-1 signal coincidentally occurs in CD8α+ DC around the tumor.
Keywords: cross-presentation, dendritic cell, TLR3, TICAM-1 (TRIF), tumoricidal CTL
Introduction
Cytotoxic T lymphocytes (CTL) and natural killer (NK) cells are two major effectors for antitumor cellular immunity. These effectors are driven through activation of dendritic cells (DC) and/or macrophages (Mf), which is mediated by pattern-recognition receptors (PRRs) for the recognition of microbial patterns.1,2 Antigen (Ag) presentation and upregulation of NK cell-activating ligands are major events induced in DC/Mf in response to PRRs, which link to evoking CTL- and NK-antitumor immunity, respectively. The immune-potentiating function of specific components of the classical adjuvants are largely attributable to the ligand activity of PRRs (CpG DNA/TLR9, polyI:C/TLR3, monophosphoryl lipid (MPL) A/TLR4, Pam2/TLR2, etc.).3 That is, the DC/Mf competent to drive effectors are generated through PRR signal in inflammatory nest where affected cells and recruited immune cells encounter exogenous or endogenous PRR ligands. Since studying the functional properties of PRRs in tumor immunity is on the way using a variety of possible ligands and cell biological analyses, immune responses reflecting the total adjuvant potential around Ag-presenting cells (APC) in local inflammatory nests are not always elucidated even in mice.
RNA-sensing PRR pathways, including TLR3-TICAM-1, TLR7-MyD88 and RIG-I/MDA5-IPS-1 participate in driving Type I IFN induction and cellular immunity in DC subsets.1,4,5 Type I IFN and the IFNAR pathway in DC and other cells reportedly evoke and amplify T cell immunity.5,6 TLR7 resides exclusively in plasmacytoid DC7 whereas TLR3 mainly exists in myeloid DC/Mf and epithelial cells.8 They are localized on the membrane of the endosome and deliver the signal via their adaptors, MyD88 and TICAM-1.7,8 RIG-I and MDA5 are ubiquitously distributed to a variety of mouse cells and signal the presence of cytoplasmic viral products through IPS-1.9 Thus, TLR3 and RIG-I/MDA5 are candidates associated with DC maturation to drive effector cells.10 Indeed, viral dsRNA analog, polyI:C, is a representative ligand for TLR3 and MDA5 and induces polyI:C-mediated DC-NK reciprocal activation.11,12 These are also true in human DC.13
The point of this study is by which pathway antitumor CTL are induced for tumor regression in a mouse tumor-implant model. It has been postulated that DC present exogenous tumor Ag to the MHC Class I-restricted Ag-presentation pathway and proliferate CD8 T cells specific to the extrinsic Ag. When tumor cells provide soluble and insoluble exogenous Ag, this Class I Ag presentation occurs mostly TAP/proteasome-dependent, suggesting the pathway partly sharing with that for endogenous Ag presentation. This DC’s ability to deliver exogenous Ag to the pathway for MHC Class I-restricted Ag presentation has been described as cross-presentation.14 DC cross-presentation leads to the cross-priming and proliferation of Ag-specific CD8 T cells in vivo and in vitro.14-18 A variety of PAMP15,16 and intrinsic DAMP17 as well as other factors including Type I IFN,5,18 CD4+ T cells19 and NKT cells20 augment cross-priming in tumor-bearing mice. However, by what molecular mechanism polyI:C enhances CTL induction in tumor-bearing mice remains largely unsettled.
Here, we made an EG7 tumor-implant mouse system and treated the mice with s.c.-injected ovalbumin (OVA)-containing cell lysates (Ag) and polyI:C. Spleen CD8α+ DC turn CTL-inducible when stimulated with Ag and polyI:C. In either case of s.c., i.p., or i.v. injection of polyI:C, the TLR3/TICAM-1 pathway predominantly participates in CD8α+ DC cross-priming and antitumor CTL induction. Earlier studies using non-tumor models, suggested that both TLR3 and MDA5 appeared to participate in polyI:C-dependent CTL induction.21 TLR3 is predominantly involved in primary Ag response and Th1 skewing,22 while MDA5 participates in secondary Ag response.23 Importance of TLR3 in induction of cross-priming was first suggested by Schulz et al., who used OVA/polyI:C-loaded or virus-infected xenogenic (Vero) cells and mouse DC.16 Here we demonstrate that the antitumor polyI:C activity is sustained by the TICAM-1 pathway in any route of injection in tumor-implant mice: antitumor CTL responses are mostly abrogated in TICAM-1−/− but not IPS-1−/− mice.
Results
Properties of EG7 tumor with high MHC in tumor-loading mice
The properties of the EG7 line we used are consistent with those reported previously.24,25 It expressed high MHC Class I (H2-Kb) and no Qa-1b or Rae-1 (Fig. S1). The expression levels of these proteins were barely changed before and after implantation of EG7 cells into mice. Cell viability was not affected by in vitro stimulation with polyI:C only (Fig. S1B). However, a batch-to-batch difference of cell viability may have affected the rate of tumor growth in each mouse tumor-implant experiment.
CD8+ T cells are responsible for tumor retardation by polyI:C
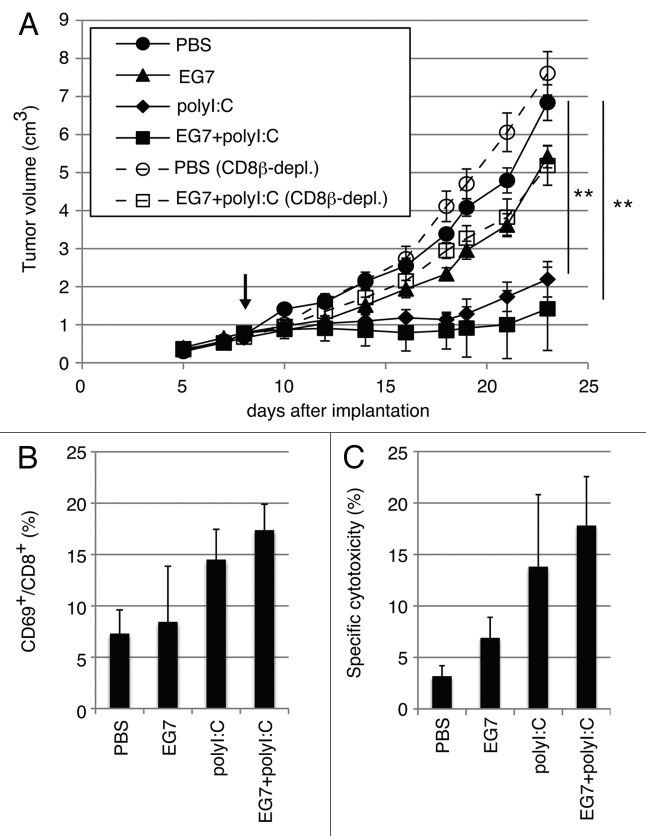
EG7 cells (2 × 106) were inoculated into the back of C57BL/6 (WT), and the indicated reagents were subcutaneously (s.c.) injected around the EG7 tumor (Fig. 1A). Growth retardation of tumor was observed by treatment with polyI:C or polyI:C plus EG7 lysate (Fig. 1A). EG7 lysate only had no effect on tumor regression. When CD8β+ T cells were depleted before EG7 lysate/polyI:C treatment, polyI:C-mediated tumor growth suppression was cancelled (Fig. 1A), suggesting the participation of CD8 T cells in tumor growth suppression. The therapeutic potential of polyI:C appeared to be more reproducible in the presence of EG7 lysate than in the absence, judged from the increases of activated CD8+ T cells (Fig. 1B) and cytotoxic activity (Fig. 1C) of LN T cells isolated from the mice sacrificed after the last therapy. Yet, the EG7 Ag could be more or less supplied from the implant tumor. NK1.1+ cells did not participate in this EG7 tumor regression in this setting (data not shown).
Figure 1. PolyI:C induces CTL-mediated tumor regression. (A) WT mice were challenged with EG7 cells and were treated with PBS (●), EG7 lysates (▲), polyI:C (◆) and EG7 lysates + polyI:C (■). The adjuvant therapy was started at the time indicated by the arrow and the indicated reagents injected twice per week. One of the two PBS groups (○) and one of the two EG7 lysates + polyI:C groups (◻) were treated with anti-CD8β ascites in order to deplete CD8+ T cells once a week. Each group had 3–5 mice. (B) Draining inguinal LNs were harvested 24 h after the last treatment and the proportion of CD69-expressing CD8+ cells were counted. (C) LN cells were co-cultured with MMC-treated EG7 cells for 3 d and subjected to 51Cr release assay to evaluate CTL activity. E/T = 50. All error bars used in this figure show ± SEM. Data are representative of two independent experiments. One-way analysis of variance (ANOVA) with Bonferroni’s test was performed to analyze statistical significance. **, p < 0.01.
Since EG7 lysate contains OVA, OVA-specific T cells in draining LN and spleen of the WT mice were counted by tetramer assay after the last therapy (Fig. S2A and B). The numbers of tetramer-positive cells were prominently increased in LN and spleen in mice with EG7 lysate and polyI:C. We confirmed the importance of simultaneous administration of Ag plus polyI:C for OVA-specific CTL induction as in Figure S2C, where pure Ag (OVA) was used instead of EG7 lysate for immunotherapy. The polyI:C adjuvant function appeared to be more efficient in the mixture of pure Ag than in polyI:C alone. Tumor regression (Fig. S2C) and OVA-specific CTL induction (Fig. S2D) were clearly observed in this additional experiment. To obtain reproducible data, we employed the EG7 lysate/polyI:C combination therapy as follows.
IFN-inducing pathways are involved in PolyI:C-derived EG7 growth retardation
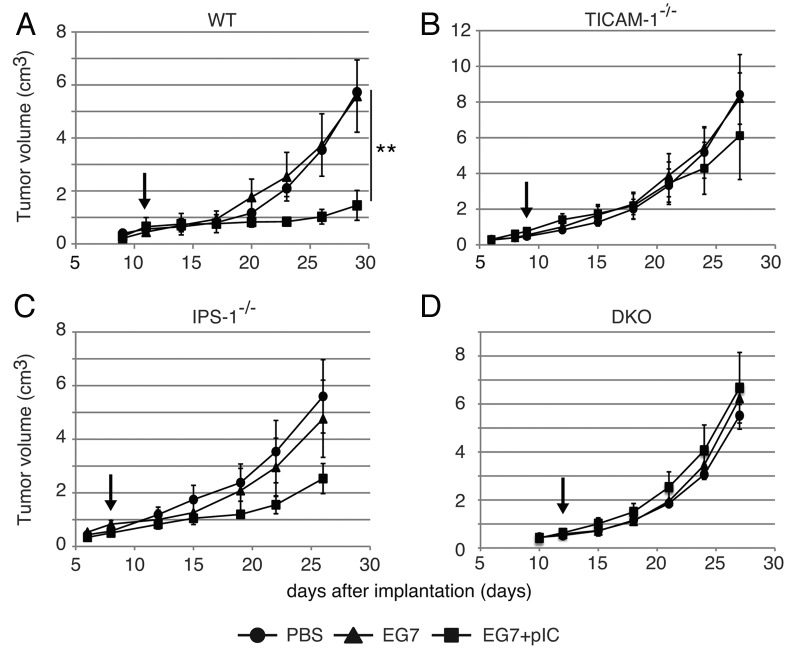
We next inoculated EG7 cells (2 × 106) into the back of C57BL/6 (WT), TICAM-1−/−, IPS-1−/−, or TICAM-1/IPS-1 double-deficient (DKO) mice (Fig. 2). We s.c. administered EG7 lysate with or without polyI:C around the tumor. The EG7 lysate was the soluble fraction of EG7 which removed insoluble debris by centrifugation. The EG7 lysate contained unprecipitated micro-debris and soluble Ag. No other emulsified reagent was added for immunization. Thus, the adjuvant function of polyI:C per se is reflected in the tumor growth, although polyI:C had to be injected into mice twice a week. Retardation of tumor growth was observed > 8 d after immunization with EG7 lysate + polyI:C in WT mice, though no growth retardation without polyI:C (Fig. 2A). The polyI:C-mediated tumor growth suppression was largely abrogated in TICAM-1−/− (Fig. 2B) and to a lesser extent in IPS-1−/− mice (Fig. 2C), and completely in TICAM-1/IPS-1 DKO mice (Fig. 2D). Hence, TICAM-1 plays an important role in inducing polyI:C-mediated tumor growth retardation in the s.c. setting we employed.
Figure 2. PolyI:C-induced tumor retardation is dependent on the TICAM-1 pathway. Antitumor effect of polyI:C on various KO mice were evaluated by using in vivo mouse tumor implant model. EG7 cells were inoculated to WT (A), TICAM-1−/− (B), IPS-1−/− (C) and DKO mice (D) on day 0. PBS (●), EG7 lysates (▲) or EG7 lysates + polyI:C (■) were s.c. administered around the tumor. The adjuvant therapies were started at the time indicated by the arrows and injected twice per week. Each group have 3–4 mice and error bar shows ± SEM. Data are representative of two independent experiments. **, p < 0.01
CD8 T cell activation induced by the TICAM-1 pathway
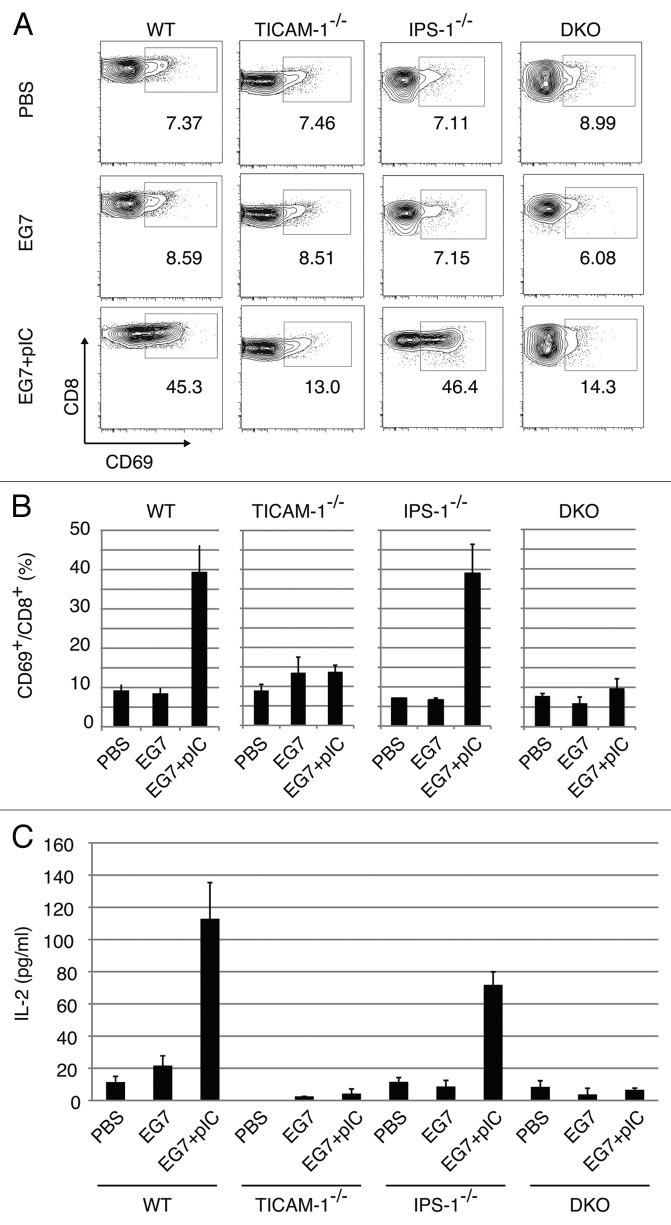
CD8 T cell activation in the inguinal LN was tested with polyI:C + EG7 lysate in EG7 tumor-bearing mice using CD69 as an activating marker. Twenty-four hours after the last polyI:C + EG7 sec.c. treatment, cells were harvested from the LN excised (Fig. 3A). FACS profiles of total cells from each mouse group are shown in Fig. S3. By combination therapy with EG7 lysate and polyI:C, T cells were activated in WT and IPS-1−/− mice, but the proportion of CD8 T cells was not affected by the therapy (Fig. S4A). Under the same conditions, T cells were barely activated in TICAM-1−/− mice in response to polyI:C (Fig. 3A). The proportion of CD69+ cells are indicated in Figure 3B. IL-2 (Fig. 3C) and IFNγ (Fig. S4B) were highly induced in the WT and IPS-1−/− LN cells, while they were not induced in TICAM-1−/− or DKO cells. IFNγ levels were upregulated only in polyI:C-treated tumor-bearing mice, although the WT > IPS-1−/− profile for IFNγ production was reproducibly observed (Fig. S4B).
Figure 3. CD8 T cells in the draining LNs are activated through the TICAM-1 pathway by polyI:C. Draining inguinal LNs were harvested from tumor-bearing mice 24 h after the last treatment. LN cells were stained with CD3ε, CD8α and CD69, and the cells gated on CD3ε+CD8α+ are shown (A). Spleen cells in each group of mice were stained separately, the CD8 levels in gated cells being variably distributed in FACS analyses. The average frequency of activated CD8 T cells defined by CD69 expression is shown (B). Alternatively, LN cells from the indicated mice were cultured for further 3 d in vitro and IL-2 production was measured by CBA assay (C).
In vivo proliferation of CD8 T cells judged by tetramer assay and IFNγ induction
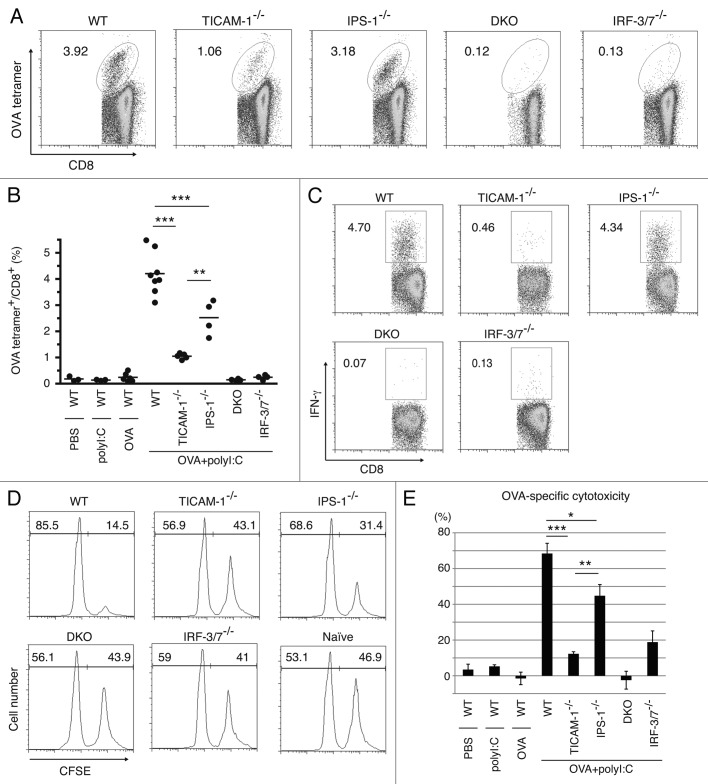
We next tested whether i.p. injection of polyI:C plus OVA induces CTL proliferation. PolyI:C and OVA were i.p. injected into mice and the polyI:C-dependent cross-priming of CD8 T cells were examined using the OVA tetramer assay. OVA-specific CD8 T cells were clonally proliferated in WT and IPS-1−/− mice, but not in TICAM-1/IPS-1 DKO and IRF-3/7−/− mice (Fig. 4A). Proliferation of OVA-specific CD8 T cells were severely suppressed in TICAM-1−/− mice (Fig. 4A), suggesting that polyI:C-mediated cross-priming of CD8 T cells largely depends on the TICAM-1 pathway followed by IRF-3/7 activation in the i.p. route. The results were reproduced in additional experiments using more mice (Fig. 4B) and TLR3−/− mice (Fig. S5A and B). The polyI:C cytokine response, where IFNα is IPS-1-dependent while IL-12p40 is TICAM-1-dependent, was also confirmed in serum level by polyI:C i.p. injection (Fig. S5E). Specific induction of IFNγ (Fig. 4C) was also observed in parallel with the results of Figure 4A.
Figure 4. TICAM-1 and IRF-3/7 are essential for polyI:C-induced antigen-specific CTL expansion. WT, TICAM-1−/−, IPS-1−/−, TICAM-1/IPS-1 DKO and IRF-3/7−/− mice were i.p. administered with the combination of OVA and polyI:C. After 7days, splenocytes were harvested and stained with CD8α and OVA tetramer (A). The average percentages of OVA-specific CTL are shown (B). Alternatively, splenocytes were cultured in vitro in the presence of SL8 for 8 h and IFNγ production was measured by intracellular cytokine staining (C). To assess the killing activity, in vivo CTL assay was performed. The combinations of OVA and polyI:C were administered i.v. to each group of mice and 5 d later, cytotoxicity was measured (D). The data shown are collaborate or representative of at least three independent experiments. One-way analysis of variance (ANOVA) with Bonferroni’s test was performed to analyze statistical significance. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Whether or not i.v. injection of polyI:C plus OVA induces Ag-specific CTL and cytotoxicity was next checked. OVA-specific OT-1 proliferation and cytotoxicity (Fig. 4D and E) were observed in in vivo analyses of WT and IPS-1−/− CD8 T cells but not of TICAM-1−/−, TICAM-1/IPS-1 DKO, and IRF-3/7−/− mice in the i.v. setting.
Since TICAM-1 is the adaptor for TLR3 as well as cytoplasmic helicases,24 we confirmed the level of cross-priming being decreased in TLR3−/− mice and an expected result was obtained (Fig. S5A and B). Furthermore, in IFNAR−/− mice, OVA-specific CTL induction was slightly reduced compared with that in WT mice, but higher than in TICAM-1−/− mice (Fig. S5C and D). Hence, in vivo cross-presentation induced by polyI:C mostly depends on the TLR3-TICAM-1 pathway followed by transcriptional regulation by IRF-3/7 in any administration route, and is further promoted by Type I IFN presumably produced by the stromal cells through the IPS-1 pathway.26
IPS-1 induces DC maturation but not cross-priming in vivo
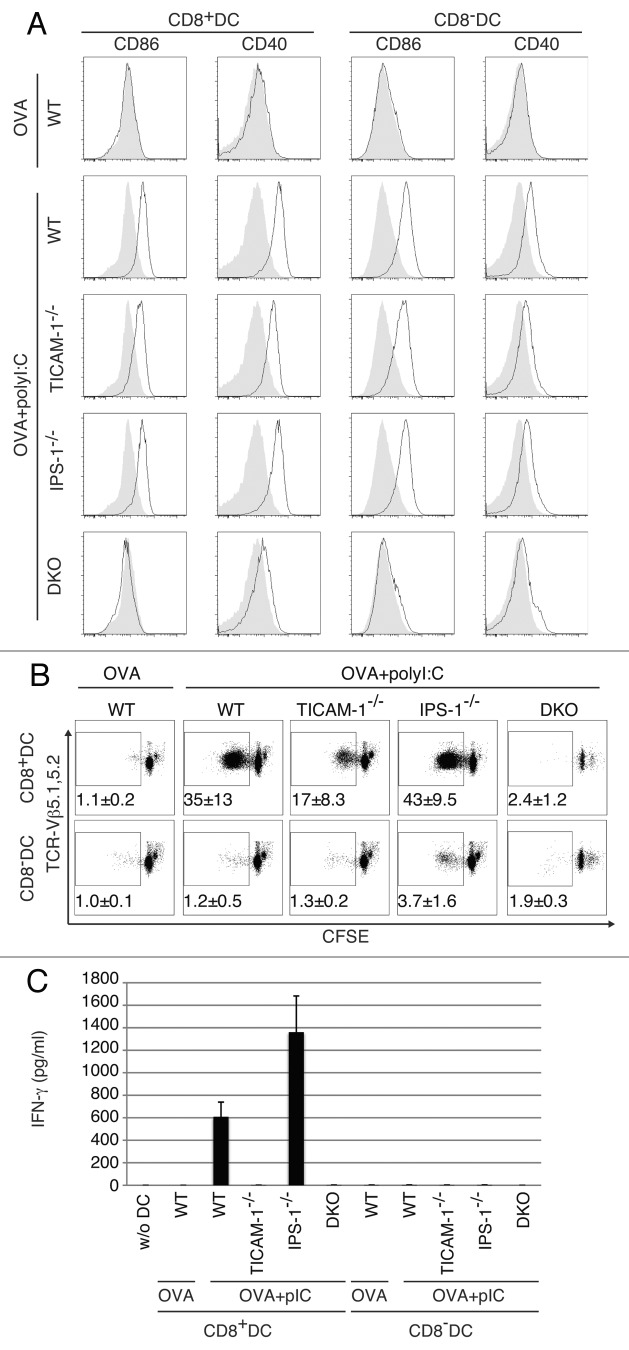
Spleen DC maturation by i.v.-injected polyI:C was tested ex vivo using CD8α+ DC and CD8α- DC isolated from WT or KO mice with no tumor as indicated in Figure 5A. The maturation markers CD86 and CD40 were upregulated on both CD8α+ and CD8α- DC from WT mice when they were stimulated with OVA and polyI:C. Treatment of DC with OVA only did not induce upregulation of CD86 and CD40. Although the expression levels of CD86 and CD40 were a little less in CD8α+ and CD8α- DC from TICAM-1−/− or IPS-1−/− mice than those from WT mice, both CD86 and CD40 were sufficiently upregulated even in the abrogation of either one pathway in polyI:C-injected mice. The CD86 and CD40 shifts were completely abolished in DKO mice (Fig. 5A). Thus, the TICAM-1 pathway participates in both potent co-stimulation and cross-priming, while the IPS-1 pathway mainly participates only in integral co-stimulation in myeloid DC.
Figure 5. TICAM-1 in CD8α+ DC is more important than IPS-1 in polyI:C-induced cross-priming. OVA and polyI:C were administered i.v. and 4 h later, CD8α+ and CD8α- DC were isolated from the spleen. CD86 and CD40 expressions were determined by FACS (A). Filled gray and black line show isotype control and target expression, respectively. Alternatively, CD8α+ and CD8α- DC were co-cultured with CFSE-labeled RAG2−/−/OT-1 T cells for 3 d. The cross-priming activity of each DC subset was determined with sequential dilution of CFSE (B) and IFNγ production (C). IFNγ was measured by CBA assay. The data shown are representative of two independent experiments. Err bar shows SD.
We next assessed in vitro proliferation of OT-1 cells. CD8α+ and CD8α- DC were prepared from PBS, polyI:C, OVA and OVA/polyI:C-treated mice, and mixed in vitro with CFSE-labeled OT-1 cells. WT, TICAM-1−/− and IPS-1−/− mice were used for this study. OT-1 proliferation was observed with CD8α+ DC but not CD8α- DC when OVA + polyI:C was injected (Fig. 5B). Furthermore, the OT-1 proliferation barely occurred in the mixture containing TICAM-1−/− CD8α+ DC. Thus, OT-1 proliferation is triggered by the TICAM-1 pathway in CD8α+ DC. Again, IPS-1 had almost no effect on OT-1 proliferation with CD8α+ DC in this setting. In the mixture, IFNγ was produced in the supernatants of WT and IPS-1−/− CD8α+ DC but not TICAM-1−/− DC by stimulation with OVA + polyI:C (Fig. 5C). No IFNγ was produced in the supernatants of CD8α- DC even from WT mice, which results are in parallel with those of OT-1 proliferation. In any case irrespective of tumor-bearing or not, Ag, polyI:C and the TICAM-1 pathway are mandatory for CD8α+ DC to cross-prime and proliferate OVA-specific CD8 T cells.
We checked the TICAM-1- or IPS-1-specific gene expressions related to Type I IFN and MHC Class I presentation using genechip and qPCR (Fig. S6). PolyI:C-mediated upregulation of Tap1, Tap2 and Tapbp messages diminished in TICAM-1−/− BMDC (Fig. S6A). The levels of these genes were hardly affected in IPS-1−/− BMDC (data not shown). PolyI:C-mediated upregulation was observed with MDA5 (Ifih1) in CD8α- and CD8α+ DCs (Fig. S6B). Surprisingly, other factors including TLR3, TICAM-1 and MAVS messages were all downregulated in response to polyI:C in CD8α+ DC (Fig. S6B), for the reason as yet unknown.
Effect of TLR3-mediated IFN-inducing pathway on antitumor CTL induction
PolyI:C is a dsRNA analog capable of incorporating into the endosome and cytoplasm by exogenous administration in vitro.27,28 However, no evidence has been proposed that polyI:C is internalized into the endosome of CD8α+ DC where TLR3 is expressed in vivo. Peritoneal (PEC) Mf and bone marrow-derived DC22 usually phagocytoze polyI:C and deliver them into the endosome. In mouse CD8α+ DC direct internalization of polyI:C has remain unproven. Using labeled polyI:C and anti-mouse TLR3 mAb, 11F8,22 we checked whether the exogenously-added polyI:C encountered with TLR3 in CD8α+ DC in vitro. TLR3 (green) was merged with TexasRed-polyI:C 30–120 min after polyI:C stimulation in the culture (Fig. 6A). The quantities of CD8α+ and CD8α- DC where FITC-polyI:C was incorporated were determined by FACS analysis (Fig. 6B). Thus, the process by which polyI:C injected reaches the endosomal TLR3 is delineated in the CD8α+ DC.
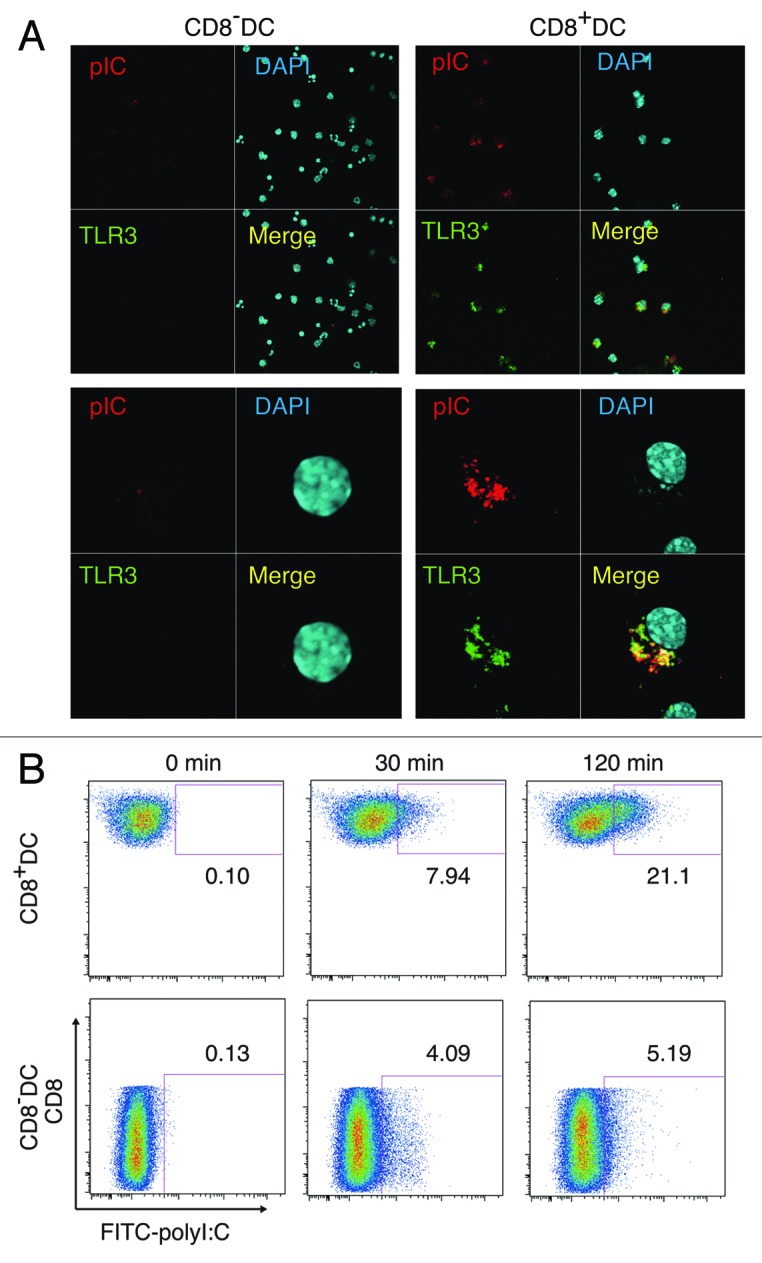
Figure 6. PolyI:C encounters TLR3 in CD8α+ DC. CD8α+ and CD8α- DC were isolated by FACSAriaII and stimulated with 20 µg/ml TexasRed-polyI:C for 2 h. Then cells were stained with Alexa647-antiTLR3 and subjected to confocal microscopic analysis (A). Alternatively, splenic DC isolated by MACS were incubated with FITC-polyI:C for the time shown in figure and analyzed the degrees of polyI:C uptake by FACS (B). Data shown are the representative of three independent experiments.
Discussion
PolyI:C is an analog of virus dsRNA, and acts as a ligand for TLR3 and RIG-I/MDA5. PolyI:C has been utilized as an adjuvant for enhancement of antitumor immunity for a long time.29 However, the mechanistic background of the therapeutic potentials of polyI:C against cancer has been poorly illustrated. It induces antitumor NK activation through DC-NK cell-to-cell interaction when CD8α+ DC TLR3 is stimulated in the spleen.11 Besides myeloid cells, however, some tumor cell lines express TLR3 and dsRNA targeting tumor cells may affect the growth rate of tumors,30 where the receptor-interacting protein (RIP) pathway is involved downstream of TICAM-1.31 Here we showed evidence that polyI:C injection facilitates maturation of TLR3-positive CD8α+ DC (i.e., APC) to trigger CTL induction against exogenous soluble Ags including EG7 lysate or OVA. The TICAM-1 adaptor for TLR3 and IRF-3/7 are involved in the cross-presentation signal in CD8α+ DC, but the molecule/mechanism downstream of TICAM-1 that governs cross-presentation remains elusive. Since most of the tumor-associated Ags (TAA) are predicted to be liberated from tumor cells as soluble Ags, the TICAM-1 pathway in CD8α+ DC would be crucial for driving of tumor-specific CTL around the tumor microenvironment. In any route of polyI:C injection, this is true as shown first in this study. Although TICAM-1 is an adaptor of other cytoplasmic sensors, DDX1, DDX21 and DHX36,32 the antitumor CTL responses are merely relied on TLR3 of CD8α+DC in this system. Taken together with previous reports,11,12 TICAM-1 signaling triggers not only NK activation but also CTL induction.
TLR3 and MDA5 are main sensors for dsRNA and differentially distributed in myeloid cells.33,34 TLR3 is limitedly expressed in myeloid, epithelial and neuronal cells,33 whereas MDA5 is ubiquitously expressed including non-myeloid stromal cells.33 Several reports suggested that i.v. injection of polyI:C predominantly stimulate the stromal cells which express IFNAR,26 thereby robust type I IFN are liberated from these cells to be a systemic response including cytokinemia and endotoxin-like shock.35,36 Both TLR3 and MDA5 link to the IRF-3/7-activating kinases leading to the production of IFNα/β.37,38 Once IFNα/β are released, IFNAR senses it to amplify the Type I IFN production,39 and reportedly this amplification pathway involves cross-priming of CD8 T cells in viral infection.18 Tumor progression or metastasis can be suppressed through the IFNAR pathway.40 These scenarios may be right depending on the conditions employed. Our message is related to what signal pathway is fundamentally required for induction of antitumor CTL in DC. The CTL response is almost completely abrogated in TICAM-1−/− and IRF-3/7−/− mice, but largely remains in IPS-1−/− and IFNAR−/− mice when Ag and polyI:C are extrinsically administered. The results are reproducible in some other tumor-implant models (data not shown), and even in IFNAR−/− mice, TICAM-1-specific genes are upregulated to confer tumor cytotoxicity (Fig. S6, Azuma et al., unpublished data). In addition, the upregulation of these genes is independent of IPS-1 knockout in DC. Our results infer that the primary sensing of dsRNA in CD8α+ DC is competent to induce cross-presentation, which minimally involves the IPS-1 or IFNAR amplification pathway, at least at a low dose of polyI:C. Yet, subsequent induction of Type I IFN via the IFNAR may further amplify the cross-priming.18,41 Further studies are needed as to which of the TICAM-1-inducible genes link to the cross-presentation in CD8α+ DC.
The main focus of this study was to identify the pathway for transversion of immature DC to the CTL-driving phenotype by co-administration of polyI:C with soluble Ag. The IPS-1 pathway, although barely participates in antitumor CTL driving, can upregulate CD40/CD86 co-stimulators on the membranes of splenic CD8α+ and CD8α- DC in response to polyI:C, suggesting that MDA5 does function in the cytoplasm of splenic CD8α+ and CD8α- DC to sense polyI:C. However, effective CTL induction happens only in CD8α+ DC when stimulated with polyI:C. CD8α+ DC express TLR3 but CD8α- DC do not, and CD8α+ DC with no TLR3 fail to induce CTL, suggesting that integral co-stimulation by MDA5/IPS-1 is insufficient for DC to induce cross-priming of CD8 T cells: antitumor CTL are not induced until the TICAM-1 signal is provided in DC. At least, sole effect of the IPS-1 pathway and upregulation of co-stimulators on CD8α+ DC is limited for cross-priming and induction of antitumor CTL, which result partly reflects those in a previous report where IPS-1 and TICAM-1 harbor a similar potential for CD8 T cell proliferation when polyI:C (Alum-containing) is employed as an adjuvant for CD8α+ DC to test proliferation of anti-OVA CTL.21
A question is why TICAM-1 is dominant to IPS-1 for response to exogenously-added polyI:C in CD8α+ DC. The answer is rooted in the difference of functional behavior between BMDC and CD8α+ DC. TLR3 levels are variable depending upon subsets of DC,22 which affects DC subset-specific induction of cellular immune response. The high TLR3 expression (partly surface-expressed) is situated in CD8α+ DC before polyI:C stimulation, which is distinct from the properties of F4/80+ Mf and presumably BMDC of low TLR3 expression. The polyI:C-uptake machinery15 appears to efficiently work in concert with the TLR3/TICAM-1 pathway in CD8α+ DC and this tendency is diminished when CD8α+ DC are pretreated with Alum + polyI:C.21 Furthermore, there are functional discrepancies between CD8α+ splenic DC and GM-CSF-induced BMDC, which appears to reflect the difference of their TLR3 levels.22 These results on CD8α+ DC encourage us to develop dsRNA adjuvant immunotherapy supporting TAA soluble vaccines for cancer applicable to humans, which possess the counterpart of CD8α+ DC.
There are two modes of dsRNA-mediated DC maturation, intrinsic and extrinsic modes that are governed by the IPS-1 and TICAM-1 pathways, respectively.9,34 It is important to elucidate the in vivo qualitative difference in the two pathways in tumor-loading mice. TLR3+ DC/Mf are responsible for CTL driving via an extrinsic route in viral infection.34 Previous data suggested that dsRNA in infectious cell debris, rather than viral dsRNA produced in the cytoplasm of Ag-presenting cells or autophagosome formation, contribute to fine tuning of DC maturation through extrinsic dsRNA recognition.16 It is reported that dsRNA-containing debris are generated secondary to infection-mediated cell death,41 and DC phagocytose by-stander dead cells. Likewise, soluble tumor Ags released from tumor cells usually are extrinsically taken up by APC in patients with cancer.42 If CTL are successfully induced in therapeutic biotherapy targeted against cancer cells, this extrinsic TICAM-1 pathway must be involved in the therapeutic process.
Cross-presentation occurs in a TAP-dependent43 and -independent fashions.44,45 The peptides are transported by TAP into the endoplasmic reticulum (ER) and loaded onto MHC Class I for presentation at the cell surface. ER and phagosome might fuse each other for accelerating cross-presentation.46 Another possibility is that cross-presentation occurs in early endosomes where TLR3 resides. This early endosome cross-presentation does not always depend on TAP42-44 but requires TLR stimulation.34 TLR4/MyD88 pathway is involved in the TAP-dependent early endosome model,43 where recruitment of TAP to the early endosomes is an essential step for the cross-presentation of soluble Ag. These models together with our genechip analysis of polyI:C-stimulated BMDC suggested that some ER-associated proteins are upregulated in BMDC by polyI:C-TICAM-1 pathway. The results infer that the TLR3/TICAM-1 rather than the TLR4/MyD88 pathway more crucially participates in cross-presentation in response to dsRNA or viral stimuli and facilitates raising CTL antitumor immunity in APC.
Although multiple RNA sensors couple with TICAM-1 and signal to activate the Type I IFN-inducing pathway,25 at least TLR3 in the CD8α+ DC are critical in CTL driving. CD8α+ DC are a high TLR3 expresser, while BMDC express TLR3 with only low levels.22 CD8α- DC do not express it.22 The Ag presentation and TLR3 levels in CD8α+ DC appear reciprocally correlated with the phagocytosing ability of DC. Although the TLR3 mRNA level is downregulated secondary to polyI:C response after maturation, this may not be related to the CD8α+ DC functions. Yet, polyI:C might interact with other cytoplasmic sensors for DC maturation,32,47
The route of administration and delivery methods may be important for culminate the polyI:C adjuvant function. The toxic problem has not overcome in the adjuvant therapy using polyI:C35,36 and this is a critical matter for clinical introduction of dsRNA reagents to immunotherapy. The most problematic is the life-threatening shock induced by polyI:C. Recent advance of polyI:C study suggests that PEI-jet helps efficient uptake of polyI:C into peritoneal macrophages.48 LC (poly-l-lysine and methylcellulose) has been used as a preservative to reduce the toxic effect of polyI:C.49 Nanotechnological delivery of polyI:C results in efficient tumor regression.50 There are many subsets of DC that can be defined by surface markers, and selecting an appropriate administration route can target a specific DC subset. The route for s.c. administration usually mature dermal/epidermal DC or Langerhans cells.51,52 Some DC subsets with unique properties specialized to CTL induction would work in association with the route of polyI:C administration. Attempting to develop more harmless and efficient dsRNA derivatives will benefit for establishing human adjuvant immunotherapy for cancer.
Materials and Methods
Mice
TICAM-1−/− and IPS-1−/− mice were made in our laboratory and backcrossed more than eight times to adapt C57BL/6 background.12 IRF-3/7−/− and IFNAR−/− mice were kindly provided by T. Taniguchi (University of Tokyo, Tokyo, Japan). TLR3−/− mice were kindly provided by S. Akira (Osaka University, Osaka, Japan). Rag2−/− and OT-1 mice were kindly provided from Drs N. Ishii (Tohoku University, Sendai, Japan). Rag2−/−/OT-1 mice were bred in our laboratory. All mice were maintained under specific pathogen-free conditions in the animal facility of the Hokkaido University Graduate School of Medicine. Animal experiments were performed according to the guidelines set by the animal safety center, Hokkaido University, Japan.
Cells
EG7 and C1498 cells were purchased from ATCC and cultured in RPMI1640/10% FCS/55 µM 2-ME/1 mM sodium pyruvate and RPMI1640/10% FCS/25 ng/ml 2-ME, respectively. Mouse splenocytes, OT-1 T cell, CD8α+ DC and CD8α- DC were harvested from the spleen and cultured in RPMI1640/10% FCS/55 µM 2-ME/10 mM HEPES.41 B16D8 cells were cultured in RPMI/10% FCS as described previously.12
Reagents and antibodies
Ovalbumin (OVA) and polyI:C (polyI:C) were purchased from SIGMA and Amersham Biosciences, respectively. OVA257–264 peptide (SIINFEKL: SL8) and OVA (H2Kb-SL8) Tetramer were from MBL. Following Abs were purchased: anti-CD3ε (145-2C11), anti-CD8β (53-6.7), anti-CD11c (N418), anti-CD16/32 (93), anti-CD69 (H1.2F3) and anti-IFNγ(XMG1.2) Abs from BioLegend, anti-B220 (RA3-6B2), anti-CD4 (L3T4), anti-CD40 (1C10), anti-CD86 (GL1), and anti-MHC I-SL8 (25-D1.16) Abs from eBiosciences, anti-TCR-Vβ5.1/5.2 Ab and ViaProbe from BD Biosciences. The Rat anti-mouse TLR3 mAb (11F8) was kindly provided by David M. Segal (National Institute of Health, Bethesda, MD). To rule out LPS contamination, we treated OVA or other reagents with 200 µg/ml of Polymixin B for 30 min at 37°C before use. Texas Red- or FITC-labeled poly(I:C) was prepared using the 5′ EndTag™ Nucleic Acid Labeling System (Vector Laboratories) according to the manufacturers instructions.
Tumor challenge and poly I:C therapy
Mice were shaved at the back and s.c. injected with 200 µl of 2 × 106 syngenic EG7 cells in PBS. Tumor volumes were measured at regular intervals by using a caliper. Tumor volume was calculated by using the formula: Tumor volume (cm3) = (long diameter) × (short diameter)2 × 0.4. A volume of 50 µl of a mixture consisting of the lysate of 2 × 105 EG7 cells with or without 50 µg of poly I:C (polyI:C) was s.c. injected around the tumor. We added no other emulsified reagent for immunization since we want to role out the conditional effect of the Ag/polyI:C. The treatments were started when the average of tumor volumes reached at 0.4–0.8 cm3 and performed twice per week. EG7 lysate were prepared by three times freeze/thaw cycles (-140°C/37°C) in PBS, with removal of cell debris by centrifugation at 6,000 g for 10 min.53 To deplete CD8 T cells, mice were i.p. injected with hybridoma ascites of anti-CD8β mAb. The dose of antibody and the treatment regimens were determined in preliminary studies by using the same lots of antibody used for the experiments. Depletion of the desired cell populations by this treatment was confirmed by FACS for the entire duration of the study.
Evaluation of T cell activity in tumor-bearing mice
Draining inguinal LN cells were harvested from tumor-bearing mice after 24 h from the last polyI:C treatment. The activity of T cells was evaluated by CD69 expression and IL-2/IFNγ production. These cells were stained with FITC-CD8α, PE-CD69, PerCP/Cy5.5-7AAD and APC-CD3ε. To check cytokine production, LN cells were cultured for 3 d in vitro in the presence or absence of EG7 lysates and IL-2 and IFNγ productions were determined by Cytokine Beads Array (CBA) assay (BD). To assess the cytotoxic activity of CTL, standard 51Cr release assay was performed. For CTL expansion, 2.5 × 106 LN cells were co-cultured with 1.25 × 105 mitomycin C-treated EG7 cells in the presence of 10 U/ml IL-2 for 5 d. Then, LN cells were incubated with 51Cr-labeled EG7 or C1498 cells for 4 h and determined cytotoxic activity. The cell-specific cytotoxicity was calculated with subtracting the cytotoxity for C1498 from for EG7 cells.
Antigen-specific T cell expansion in vivo
Mice were i.p. immunized with 1 mg of OVA and 150 µg of poly I:C. After 7 d, spleens were homogenized and stained with FITC-CD8α and PE-OVA Tetramer for detecting OVA-specific CD8 T cell populations. For intracellular cytokine detection, splenocytes were cultured with or without 100 nM OVA peptide (SIINFEKL; SL8) for 8 h and 10 µg/ml of Brefeldin A (Sigma-Aldrich) was added to the culture in the last 4 h. Then cells were stained with PE-anti-CD8α and fixed/permeabilized with Cytofix/Cytoperm (BD Biosciences) according to manufacturer’s instruction. Then, fixed/permeabilized cells were further stained with APC-anti-IFNγ. Stained cells were analyzed with FACSCalibur (BD Biosciences) and FlowJo software (Tree Star).
In vivo CTL assay
The in vivo CTL assay was performed as described.54 In brief, WT, TICAM-1−/−, MAVS−/− and IRF-3/7−/− mice were i.v. administered with PBS, 10 µg of OVA or OVA with 50 µg of polyI:C. After 5 d, 2 × 107 target cells (see below) were i.v. injected to other irrelevant mice and 8 h later, the OVA-specific cytotoxicity was measured by FACSCalibur. Target cells were 1:1 mixture of 2 µM SL8-pulsed, 5 µM CFSE-labeled splenocytes and SL8-unpulesed, 0.5 µM CFSE-labeled splenocytes. OVA-specific cytotoxicity was calculated with a formula: {1-(Primed [CFSEhigh(%)/CFSElow(%)]/Unprimed [CFSEhigh(%)/CFSElow(%)]} × 100.
DC preparation
DCs were prepared from spleens of mice, as described previously.55 In brief, collagenase-digested spleen cells were treated with ACK buffer and then washed with PBS twice. Then splenocytes were positively isolated with anti-CD11c MicroBeads. CD11c+ cells were acquired routinely about ≥ 80% purity. Further, to highly purify CD8α+ and CD8α- DCs, spleen DC were stained with FITC-CD8α, PE-B220, PE/Cy7-CD11c and PerCP5.5-7AAD. CD8α+ or CD8α- CD11c+B220- DCs were purified on FACSAriaII (BD). The purity of the cells was ≥ 98%.
OT-1 proliferation assay
Ten micrograms of OVA with or without 50 µg of polyI:C were i.v. injected to WT, TICAM-1−/−, IPS-1−/− and DKO mice. After 4 h, CD8α+ or CD8α- DC were purified from the spleen. 2.5 × 104 CD8α+ or CD8α- DC were co-cultured with 5 × 104 1 µM CFSE-labeled Rag2−/−/OT-1 T cells for 3 d in 96-well round bottom plate. These cells were stained with PE-anti-TCR-Vβ5.1,5.2 and APC-anti-CD3ε and T cell proliferation was analyzed by CFSE dilution using FACSCalibur. Additionally, IFNγ in the culture supernatant was measured by CBA assay.
Statistical analysis
P-values were calculated with one-way analysis of variance (ANOVA) with Bonferroni’s test. Error bars represent the SD or SEM between samples.
Supplementary Material
Disclosure of Potenial Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We are grateful to Drs. T. Taniguchi (University Tokyo, Tokyo), N. Ishii (Tohoku University, Sendai) and D.M. Segel (NCI, Bethesda) for providing us with IRF-3/7−/− mice, OT-1 mice and anti-monse TLR3 mAb, respectively. Invaluable discussions about the peptide vaccine therapy with Dr. N. Satoh (Sapporo Medical University, Sapporo) are gratefully acknowledged. We thank Drs H. Takaki, J. Kasamatsu, H.H. Aly, and H. Shime in our lab for their critical comments on this study.
This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, and Culture (Specified Project for Advanced Research, MEXT) and the Ministry of Health, Labor, and Welfare of Japan, and by the Takeda and the Waxmann Foundations. Financial supports by a MEXT Grant-in-Project “The Carcinogenic Spiral” is gratefully acknowledged.
Glossary
Abbreviations:
- APC
antigen-presenting cells
- CTL
cytotoxic T lymphocytes
- DAMP
damage-associated molecular pattern
- DC
dendritic cells
- IFN
interferon
- IPS-1
IFNβ promoter stimulator-1
- MDA5
melanoma differentiation associated gene 5
- Mf
macrophages
- NK
natural killer
- OVA
ovalbumin
- PAMP
pathogen-associated molecular pattern
- PRR
pattern-recognition receptors
- PV
poliovirus
- RIG-I
retinoic acid inducible gene-1
- SL8
an OVA tetramer
- TICAM-1
Toll-IL-1 receptor homology domain-containing molecule-1
- TLR
Toll-like receptor
- WT
wild-type
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19893
References
- 1.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seya T, Shime H, Ebihara T, Oshiumi H, Matsumoto M. Pattern recognition receptors of innate immunity and their application to tumor immunotherapy. Cancer Sci. 2010;101:313–20. doi: 10.1111/j.1349-7006.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–8. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–37. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longman RS, Braun D, Pellegrini S, Rice CM, Darnell RB, Albert ML. Dendritic-cell maturation alters intracellular signaling networks, enabling differential effects of IFN-alpha/beta on antigen cross-presentation. Blood. 2007;109:1113–22. doi: 10.1182/blood-2006-05-023465. [DOI] [PubMed] [Google Scholar]
- 6.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diebold SS. Recognition of viral single-stranded RNA by Toll-like receptors. Adv Drug Deliv Rev. 2008;60:813–23. doi: 10.1016/j.addr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Oshiumi H, Seya T. Antiviral responses induced by the TLR3 pathway. Rev Med Virol. 2011 doi: 10.1002/rmv.680. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Yoneyama M, Fujita T. RIG-I family RNA helicases: cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007;18:545–51. doi: 10.1016/j.cytogfr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Seya T, Matsumoto M. The extrinsic RNA-sensing pathway for adjuvant immunotherapy of cancer. Cancer Immunol Immunother. 2009;58:1175–84. doi: 10.1007/s00262-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akazawa T, Ebihara T, Okuno M, Okuda Y, Shingai M, Tsujimura K, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci U S A. 2007;104:252–7. doi: 10.1073/pnas.0605978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, et al. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med. 2010;207:2675–87. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrot I, Deauvieau F, Massacrier C, Hughes N, Garrone P, Durand I, et al. TLR3 and Rig-like receptor on myeloid dendritic cells and Rig-like receptor on human NK cells are both mandatory for production of IFN-gamma in response to double-stranded RNA. J Immunol. 2010;185:2080–8. doi: 10.4049/jimmunol.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–8. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, et al. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J Immunol. 2003;170:4102–10. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 16.Schulz O, Diebold SS, Chen M, Näslund TI, Nolte MA, Alexopoulou L, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–92. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 17.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–15. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 19.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med. 2007;204:2641–53. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol. 2008;180:683–7. doi: 10.4049/jimmunol.180.2.683. [DOI] [PubMed] [Google Scholar]
- 22.Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, Goldsmith PK, et al. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol. 2011;186:2422–9. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Cella M, Gilfillan S, Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J Immunol. 2010;184:2751–5. doi: 10.4049/jimmunol.0903201. [DOI] [PubMed] [Google Scholar]
- 24.Carbone FR, Bevan MJ. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989;169:603–12. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asano J, Tada H, Onai N, Sato T, Horie Y, Fujimoto Y, et al. Nucleotide oligomerization binding domain-like receptor signaling enhances dendritic cell-mediated cross-priming in vivo. J Immunol. 2010;184:736–45. doi: 10.4049/jimmunol.0900726. [DOI] [PubMed] [Google Scholar]
- 26.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, et al. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206:2967–76. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe A, Tatematsu M, Saeki K, Shibata S, Shime H, Yoshimura A, et al. Raftlin is involved in the nucleocapture complex to induce poly(I:C)-mediated TLR3 activation. J Biol Chem. 2011;286:10702–11. doi: 10.1074/jbc.M110.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh K, Watanabe A, Funami K, Seya T, Matsumoto M. The clathrin-mediated endocytic pathway participates in dsRNA-induced IFN-beta production. J Immunol. 2008;181:5522–9. doi: 10.4049/jimmunol.181.8.5522. [DOI] [PubMed] [Google Scholar]
- 29.Talmadge JE, Adams J, Phillips H, Collins M, Lenz B, Schneider M, et al. Immunomodulatory effects in mice of polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose. Cancer Res. 1985;45:1058–65. [PubMed] [Google Scholar]
- 30.Conforti R, Ma Y, Morel Y, Paturel C, Terme M, Viaud S, et al. Opposing effects of toll-like receptor (TLR3) signaling in tumors can be therapeutically uncoupled to optimize the anticancer efficacy of TLR3 ligands. Cancer Res. 2010;70:490–500. doi: 10.1158/0008-5472.CAN-09-1890. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–52. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, et al. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34:866–78. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–64. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–12. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Absher M, Stinebring WR. Toxic properties of a synthetic double-stranded RNA. Endotoxin-like properties of poly I. poly C, an interferon stimulator. Nature. 1969;223:715–7. doi: 10.1038/223715a0. [DOI] [PubMed] [Google Scholar]
- 36.Berry LJ, Smythe DS, Colwell LS, Schoengold RJ, Actor P. Comparison of the effects of a synthetic polyribonucleotide with the effects of endotoxin on selected host responses. Infect Immun. 1971;3:444–8. doi: 10.1128/iai.3.3.444-448.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasai M, Shingai M, Funami K, Yoneyama M, Fujita T, Matsumoto M, et al. NAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J Immunol. 2006;177:8676–83. doi: 10.4049/jimmunol.177.12.8676. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2:378–86. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- 40.Ogasawara S, Yano H, Momosaki S, Akiba J, Nishida N, Kojiro S, et al. Growth inhibitory effects of IFN-beta on human liver cancer cells in vitro and in vivo. J Interferon Cytokine Res. 2007;27:507–16. doi: 10.1089/jir.2007.0183. [DOI] [PubMed] [Google Scholar]
- 41.Ebihara T, Shingai M, Matsumoto M, Wakita T, Seya T. Hepatitis C virus-infected hepatocytes extrinsically modulate dendritic cell maturation to activate T cells and natural killer cells. Hepatology. 2008;48:48–58. doi: 10.1002/hep.22337. [DOI] [PubMed] [Google Scholar]
- 42.Chaput N, Conforti R, Viaud S, Spatz A, Zitvogel L. The Janus face of dendritic cells in cancer. Oncogene. 2008;27:5920–31. doi: 10.1038/onc.2008.270. [DOI] [PubMed] [Google Scholar]
- 43.Burgdorf S, Schölz C, Kautz A, Tampé R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–66. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 44.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–65. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Kurotaki T, Tamura Y, Ueda G, Oura J, Kutomi G, Hirohashi Y, et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007;179:1803–13. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 46.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–31. doi: 10.1016/S0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 47.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu CY, Yang HY, Monie A, Ma B, Tsai HH, Wu TC, et al. Intraperitoneal administration of poly(I:C) with polyethylenimine leads to significant antitumor immunity against murine ovarian tumors. Cancer Immunol Immunother. 2011;60:1085–96. doi: 10.1007/s00262-011-1013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitano S, Kageyama S, Nagata Y, Miyahara Y, Hiasa A, Naota H, et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res. 2006;12:7397–405. doi: 10.1158/1078-0432.CCR-06-1546. [DOI] [PubMed] [Google Scholar]
- 51.Kushwah R, Hu J. Complexity of dendritic cell subsets and their function in the host immune system. Immunology. 2011;133:409–19. doi: 10.1111/j.1365-2567.2011.03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Galea-Lauri J, Wells JW, Darling D, Harrison P, Farzaneh F. Strategies for antigen choice and priming of dendritic cells influence the polarization and efficacy of antitumor T-cell responses in dendritic cell-based cancer vaccination. Cancer Immunol Immunother. 2004;53:963–77. doi: 10.1007/s00262-004-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durand V, Wong SY, Tough DF, Le Bon A. Shaping of adaptive immune responses to soluble proteins by TLR agonists: a role for IFN-α/β. Immunol Cell Biol. 2004;82:596–602. doi: 10.1111/j.0818-9641.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- 55.Yamazaki S, Okada K, Maruyama A, Matsumoto M, Yagita H, Seya T. TLR2-dependent induction of IL-10 and Foxp3+ CD25+ CD4+ regulatory T cells prevents effective anti-tumor immunity induced by Pam2 lipopeptides in vivo. PLoS One. 2011;6:e18833. doi: 10.1371/journal.pone.0018833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.