Abstract
Targeting dendritic cells (DC) through the release of suppressive factors is an effective means for tumors to escape immune control. We assessed the involvement of downstream signaling through the JAK2/STAT3 and p38 MAPK pathways in tumor-induced suppression of human DC development. Whereas the JAK2/STAT3 pathway has been pinpointed in mouse studies as a key regulator of myeloid suppression, in human DC this is less well established. We studied the effects of STAT3 inhibition on the suppression of monocyte-derived DC differentiation mediated by a short-list of four predominant suppressive factors and found that pharmacological STAT3 inhibition could only counteract the effects of IL-6. Accordingly, in testing a panel of supernatants derived from 11 cell lines representing various types of solid tumors, STAT3 inhibition only modestly affected the suppressive effects of a minority of supernatants. Importantly, combined interference in the STAT3 and p38 pathways completely prevented inhibition of DC differentiation by all tested supernatants and effected superior DC function, evidenced by increased allogeneic T cell reactivity with elevated IL-12p70/IL-10 ratios and Th1 skewing. Combined STAT3 and p38 inhibition also afforded superior protection against the suppressive effects of primary glioma and melanoma supernatants and induced a shift from CD14+ cells to CD1a+ cells in metastatic melanoma single-cell suspensions, indicating a potential for improved DC differentiation in the tumor microenvironment. We conclude that combined interference in the STAT3 and p38 MAPK signaling pathways is a promising approach to overcome tumor-induced inhibitory signaling in DC precursors and will likely support clinical immunotherapeutic strategies.
Keywords: dendritic cell, differentiation, IL-6, microenvironment, p38 MAPK, STAT3, suppression, tumor
Introduction
Dendritic cells (DC) are the most powerful antigen-presenting cells of the immune system, and play a crucial role in the induction and maintenance of immune responses.1 Unfortunately, under tumor conditions DC differentiation and activation are inhibited by high levels of tumor-derived suppressive factors, like IL-10, IL-6, PGE-2 and TGF-β. Disturbed DC differentiation is often accompanied by accumulation of immature myeloid cells and the development of alternatively activated (M2) macrophages and myeloid derived suppressor cells (MDSC), with detrimental effects on T cell immunity.2,3 Such a disturbed balance in myeloid differentiation in the microenvironment of tumors may thus seriously hamper the efficacy of anti-tumor T cell responses. Improving the function of DC in cancer patients is therefore a key issue in improving the outcome of immunotherapeutic treatments, because restored DC function will result in more efficient T-cell priming and may simultaneously alleviate the accumulation of suppressive myeloid cells and regulatory T cells (Tregs). In order to overcome tumor-induced suppression of DC, deeper understanding of the underlying pathways involved in DC suppression and differentiation is essential. Signal transducer and activator of transcription-3 (STAT3) is constitutively activated in many solid tumors4–6 as well as infiltrating immune effector cells (reviewed in refs. 7–8) and has emerged over the past few years as a very important negative regulator of inflammatory responses. DC from tumor-bearing mice have increased levels of phosphorylated (i.e., activated) STAT3 compared with DC from naïve mice.9 Tumour-induced STAT3 activity negatively regulates DC maturation by lowering the expression of MHC class II, CD80, CD86 and decreasing the production of IL-12.10 Inhibition of STAT3 expression in DC through the use of small molecule inhibitors increased T cell responses in tumor bearing mice.9 Moreover, in vivo STAT3 inhibition counteracted abnormal expansion and activation of MDSC.11 A study by Hussain et al. demonstrated that blocking STAT3 with a small molecule inhibitor could revert immune suppression in monocytes and macrophages from glioblastoma patients by inducing the expression of costimulatory molecules and inducing the proliferation of effector T cells.12 Since STAT3 is activated by many different suppressive cytokines that can be released in various combinations by different tumor types (reviewed in refs. 3 and 13), blocking STAT3 activity in DC and their precursors might be a promising generally applicable approach to overcome tumor-induced immune suppression and could result in increased DC functionality and anti-tumor immunity.
Another interesting suppression-related pathway is the p38 mitogen-activated protein kinase (MAPK) pathway. In a study by Xie et al. it was demonstrated that the differentiation of monocytes into immature DC was accompanied by activation of the Raf/MEK/ERK and PI3K/AKT signaling pathways, whereas p38 MAPK was identified as a negative regulator of DC differentiation.14 Accordingly, in a mouse model of Multiple Myeloma inhibition of p38 MAPK was shown to restore DC development and functionality that were disturbed by tumor-derived IL-10, IL-6 and TGFβ.15 The same group also demonstrated that p38 MAPK plays an important role in suppression of human DC function by Multiple Myeloma-derived factors. Wang et al. reported that monocyte-derived DC (MoDC) from Multiple Myeloma patients were impaired in phenotype and T-cell stimulatory capacity due to elevated production of IL-6 and that these suppressive effects could be partially overcome by inhibiting p38 MAPK activity.16 Zhao et al. more recently showed that ret transgenic mice with spontaneous skin melanoma had high serum levels of IL-6 and VEGF and that the observed in vivo suppression of DC could be overcome by systemic inhibition of p38.17
We investigated the involvement of STAT3 and p38 MAPK signaling in the inhibition of human DC differentiation by solid tumors. From a panel of suppressive factors (IL-10, IL-6, PGE2, TGFβ) STAT3 inhibition appeared to counteract the suppressive effects of IL-6 only, while for consistent and full protection against the suppressive effects of a range of solid tumors combined inhibition of the STAT3 and p38 MAPK pathways was required. Importantly, a dominant stimulatory effect on DC differentiation in metastatic melanoma suspensions was observed for p38 inhibition, indicative of its therapeutic potential for conditioning of the tumor microenvironment in support of immunotherapy.
Results
IL-10, IL-6, PGE2 and TGFβ hamper MoDC differentiation; STAT3 inhibition only blocks IL-6 effects
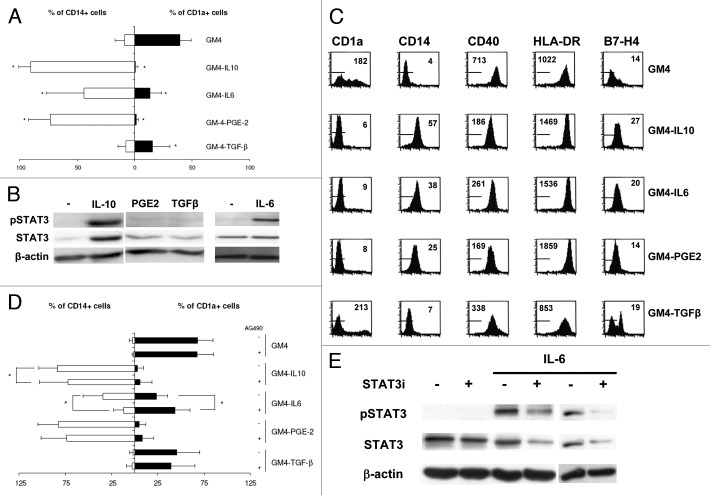
To investigate the effect of tumor derived factors on MoDC differentiation, IL-10, IL-6, PGE-2 or TGFβ were added at the start of differentiation at optimally suppressive and non-toxic concentrations.18 At day 6, the phenotype of the MoDC was determined using FACS analysis. As shown in Figure 1A and B, normal DC differentiation with GM-CSF and IL-4 (GM4) resulted in well differentiated DC expressing high levels of CD1a, whereas the monocytic marker CD14 was absent. Addition of any of the tumor-associated suppressive factors hampered DC differentiation, as demonstrated by decreased CD1a levels and maintained expression of CD14 (Figure 1A). Of note, TGFβ did not induce sustained expression of CD14. DC differentiated in the presence of any of the suppressive factors, except for TGFβ, also had reduced expression levels of the co-stimulatory molecule CD40, whereas the levels of HLA-DR were increased (Figure 1B). We also determined the expression levels of the costimulatory molecules CD80 and CD86, but no differences in levels were observed in DC differentiated in the presence of tumor-derived factors compared with the control DC (data not shown). The expression levels of the inhibitory molecule B7-H4 on DC were increased when DC were differentiated in the presence of IL-10 or IL-6, whereas addition of PGE2 during differentiation did not have an effect on B7-H4 expression. Addition of TGFβ however, induced a subpopulation of DC expressing high levels of B7-H4 (Figure 1B). In these experiments the effect of VEGF on DC differentiation was also determined, but addition of VEGF at concentrations of up to 200ng/ml did not result in suppressed MoDC differentiation in our hands (data not shown).
Figure 1. The tumor-derived factors IL-10, IL-6, VEGF, TGF-β and PGE-2 inhibit differentiation of MoDC; the inhibitory effects of IL-6 alone can be prevented by pharmacological STAT3 inhibiton. (A) Expression (in percentage positive cells) of the monocytic marker CD14 and the DC marker CD1a by MoDC differentiated with or without the indicated suppressive factors. Shown are mean ± SD from 5 experiments. * = p < 0.05 compared with GM4 differentiation. (B) FACS analysis of surface expression of the markers CD1a, CD14, CD40, HLA-DR and B7-H4 on MoDC, differentiated in the presence or absence of suppressive factors. Markers indicate fluorescence of IgG isotype controls and mean fluorescence intensities are listed. Results are representative of three experiments. (C) Representative western blot analyses of pSTAT3, STAT3 and β-actin expression in MoDC, differentiated in the presence or absence (-) of the indicated suppressive factors. (D) Expression of CD14 and CD1a by DC, differentiated with or without suppressive factors and in the presence or absence of the JAK2/STAT3 inhibitor AG490. Shown are mean ± SD from six experiments. * = p < 0.05 compared with the paired condition without AG490. (E) Representative western blot analyses of pSTAT3, STAT3 and β-actin expression in MoDC differentiated in the presence of IL-6 and treated with the STAT3 inhibitor AG490 (the latter two conditions shown for two separate donor MoDC).
Whereas PGE2 and TGFβ induced only marginal STAT3 phosphorylation, IL-10 and IL-6 were strong STAT3 activators (Figure 1C). To determine whether activation of STAT3 was responsible for the observed DC suppression, phosphorylation of STAT3 was blocked by addition of the small-molecule inhibitor AG490 at a maximum non-toxic dose of 10 μM during differentiation. As shown in Figure 1D, blocking activation of STAT3 did not abrogate the suppressive effects of IL-10, PGE2 or TGFβ on CD1a+ MoDC differentiation. DC differentiated in the presence of AG490 and IL-10 did show a slight but nevertheless significant reduction in the percentage of CD14+ cells. The most prominent effects of AG490 were seen in DC differentiated in the presence of IL-6, resulting in a partial block of differentiation inhibition as reflected by both significantly decreased percentages of CD14+ cells and significantly increased percentages of CD1a+ cells. This improved differentiation by STAT3 inhbition was accompanied by enhanced T cell stimulatory ability of the DC in an allogeneic MLR (data not shown). As shown in Figure 1E, MoDC differentiated in the presence of IL-6 had elevated levels of phosphorylated STAT3 (pSTAT3) that could be decreased by addition of AG490 to the differentiation cultures, consistent with modulation of the JAK2/STAT3 pathway.
STAT3 inhibition cannot completely prevent tumor induced suppression of MoDC differentiation
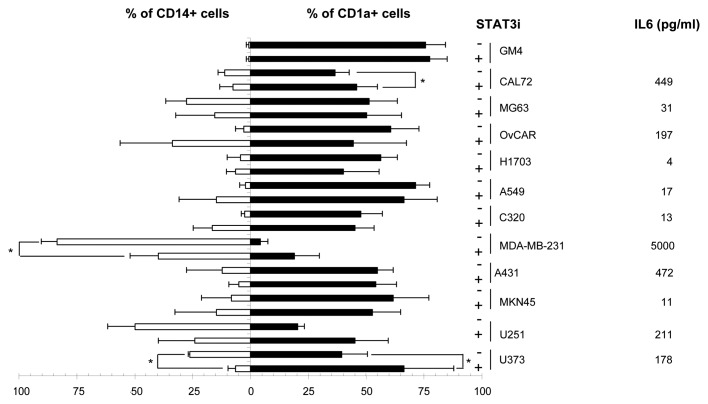
To determine whether inhibition of STAT3 could prevent suppression of MoDC differentiation induced by tumor-derived supernatants, AG490 was added to CD14+ monocytes prior to the addition of 30% v/v of supernatant derived from a panel of 11 tumor cell lines representing 8 different tumor types. As shown in Figure 2 by lower CD1a and residual CD14 expression levels, the majority of the tumor-derived supernantants (TDSN) indeed suppressed DC differentiation, although to varying degrees. Quantification of IL-6 levels in the TDSN revealed that 6 out of 11 TDSN tested contained levels of IL-6 exceeding 100pg/ml (see Figure 2). Blocking STAT3 by AG490 only significantly improved DC differentiation in 3/11 TDLN-conditioned cultures. Of note, these particular TDLN did contain relatively high levels of IL-6, but even so, blocking of STAT3 did not suffice to completely abrogate their suppressive effects. Of note, STAT3 inhibition did not abrogate the suppressive effects of most of the tested TDSN, and in some cases even surprisingly resulted in increased percentages of CD14+ cells (OvCAR, A549 and C320).
Figure 2. STAT3 inhibition only partially interferes with MoDC-inhibitory effects of tumor-derived supernatants and in a minority of tested cell lines only. Expression of CD14 and CD1a (in % positive cells) by DC differentiated with or without tumor supernatants and in the presence or absence of AG490 (STAT3i). IL-6 levels in the tested supernatants are listed. * = p < 0.05 compared with the paired condition without AG490. Shown are mean ± SD from three experiments.
Combined inhibition of STAT3 and p38 MAPK consistently prevents tumor-mediated suppression of MoDC differentiation
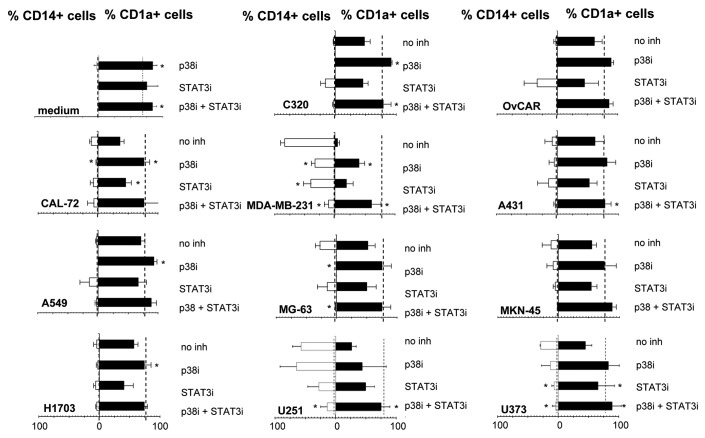
To determine whether the TDSN effects could be countered by interference in the p38 MAPK pathway, the p38 inhibitor SB203580 was also tested at a maximum non-toxic dose level, i.e., 10 μM. As shown in Figure 3, inhibition of p38 MAPK significantly increased the percentage of CD1a+ DC even during normal DC differentiation. Similarly, in all TDSN-conditioned cultures p38 inhibition resulted in improved DC differentiation. On the whole, p38 inhibition had a more profound effect than STAT3 inhibition. Most importantly, combined p38 and STAT3 inhibition effected optimal DC differentiation in the presence of all tested TDSN and most effectively prevented their inhibitory effects, regardless of tumor type. Also, for the three TDSN where blocking STAT3 gave an unexpected increase in the percentage of CD14+ cells (Figure 2), this effect was abrogated by combined STAT3 and p38 inhibition.
Figure 3. Combined STAT3 and p38 MAPK inhibition consistently prevents suppression of human MoDC differentiation by supernatants from a panel of tumor cell lines. Eleven supernatants derived from the indicated tumor cell lines representing 8 different tumor types were added during differentiation. The percentages of CD1a+ and CD14+ cells after normal DC differentiation with GM-CSF and IL-4 are indicated by the dotted lines. Shown are mean ± SD from 3 experiments. * = p < 0,05 compared with differentiation without inhibitors.
Combined STAT3/p38 inhibition enhances T cell stimulatory and Th1 induction capacity of MoDC
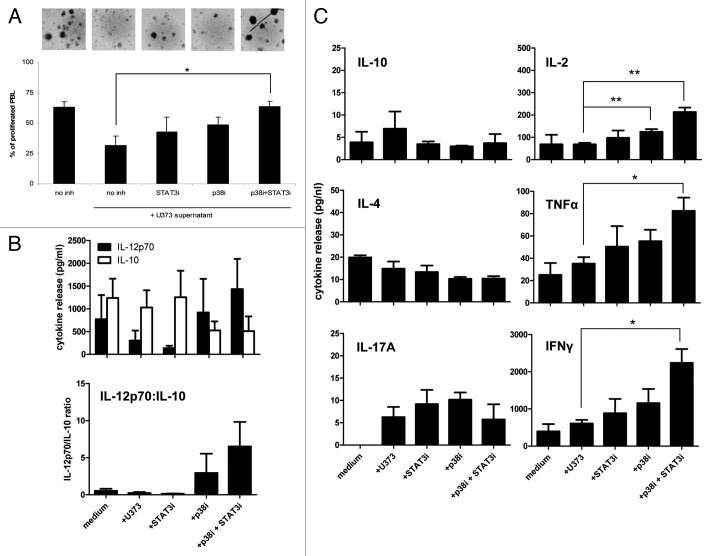
To determine the added value of p38 inhibition in terms of DC functionality, we selected the U373 glioma supernatant with relatively high IL-6 levels, the DC-suppressive effects of which were already efficiently countered by STAT3 inhibition alone (Figure 2 and Figure 3). As shown in Figure 4A, additional inhibition of p38 nevertheless led to a significant increase in T cell priming capacity of the DC in an allogeneic MLR. Similarly, combined STAT3/p38 inhibition resulted in considerably higher IL-12p70:IL-10 release ratios upon CD40L stimulation (Figure 4B), suggestive of an enhanced capacity of the U373-conditioned DC to induce a cell-mediated immune response. This was confirmed by the observation of significantly elevated levels of released Th1 cytokines (IL-2, IFNγ, and TNFα) in the MLR cultures which included MoDC differentiated in the presence of both inhibitors (Figure 4C, right panels). Simultaneous slight decreases in IL-4 and IL-10 release were consistent with a true Th1/Th2 switch, effected by combined STAT3/p38 inhibition (Figure 4C, left panels). Of note, very similar effects on T cell activation were observed when the U251 glioma supernatant was used (data not shown), which did require combined STAT3/p38 inhibition to abrogate its inhibitory effects on DC differentiation.
Figure 4. Blocking both the STAT3 and the p38 MAPK pathway improves the T cell stimulatory functions of human MoDC differentiated in the presence of U373 glioma supernatant. (A) Capacity of DC differentiated in the presence of U373 supernatant with or without the STAT3 and/or p38 MAPK inhibitors to stimulate allogeneic T-cell proliferation, means ± sd are shown, n = 4; *p < 0.05 between indicated conditions. Photographic inserts show proliferating T cell aggregates for the different conditions (magnification 400x). (B) Secretion levels of IL-12p70 and IL-10 by MoDC differentiated in the presence or absence of U373 supernatant with or without the STAT3 and/or p38 MAPK inhibition; expressed in pg/ml or as IL-12p70:IL-10 ratios. Shown are mean ± SD from 4 experiments (C) Secretion of Th1/Th2/Th17 cytokines at day four in the MLR cultures described under (A). Shown are mean ± SD from four experiments; *p < 0.05 between the indicated conditions.
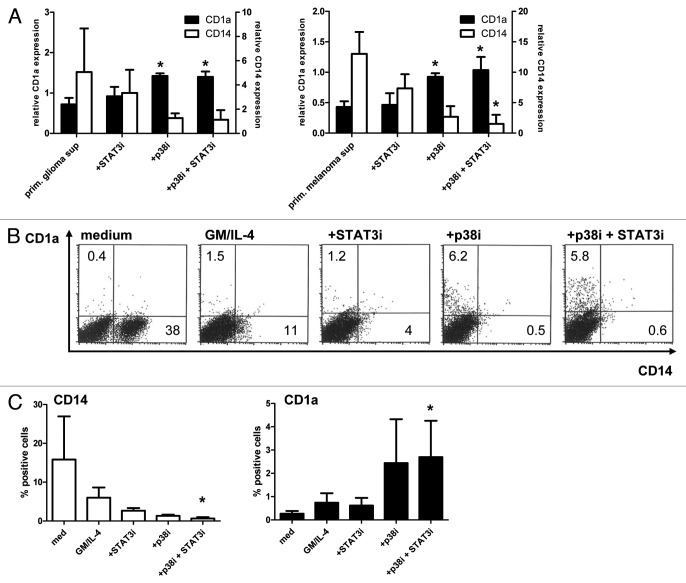
A dominant role of p38 over STAT3 in primary tumor-mediated inhibition of DC differentiation
To comfirm the DC-stimulatory effects of combined STAT3/p38 inhibition in primary tumors, TDSN were generated from human primary glioma and metastatic melanoma cultures and tested on MoDC differentiation in the presence or absence of the p38 and/or STAT3 inhibitors (see Figure 5A). Both for the glioma and the melanoma supernatants, inhibition of p38 MAPK improved DC differentiation. Combining p38 MAPK inhibition with STAT3 inhibition did result in a slightly lower percentage of CD14 expressing cells compared with inhibition of p38 MAPK alone. Indeed, STAT3 inhibition seemed to affect CD14 expression without inducing CD1a+ DC differentiation.
Figure 5. Combined p38 MAPK and STAT3 inhibition blocks the suppressive effects of supernatants from primary tumor supernatants and improves the differentiation of monocytic DC precursors in melanoma cultures from metastatic lesions. (A) Relative CD14 and CD1a expression levels on MoDC differentiated in the presence of primary glioma (n = 4) or melanoma supernatants (n = 3) in the presence or absence of STAT3 and p38 MAPK inhibitors (percentages of medium controls were set at 1). Shown are the means ± SD; *p < 0.05 compared with condition without inhibitors. (B,C) Single-cell suspensions from human metastatic melanomas were cultured for 6 d without additives or with GM-CSF and IL-4 with or without additional STAT3 and/or p38 MAPK inhibitors. (B) FACS analyses of CD14 and CD1a expression (percentages listed) from a representative experiment and (C) mean ± SD from 3 independent experiments with different melanoma samples are shown. *p < 0.05 compared with the medium condition.
To establish effects of STAT3 and/or p38 inhibition on DC differentiation in the tumor microenvironment, single-cell suspensions from three metastatic melanoma lesions were cultured for six days after which CD14 and CD1a expression was determined by FACS analysis. In Figure 5B results from a representative experiment are shown, while average percentages of positive cells are shown in Figure 5C. GM-CSF and IL-4 were added to induce DC differentiation, but only resulted in the disappearance of a CD14+ cell population, which was further reduced by either STAT3 or p38 inhibition. Of note, DC differentiation was demonstrable by CD1a expression only in the presence of the p38 inhibitor, which reached significance in combination with STAT3 inhibition (Figure 5C).
Discussion
DC development and activation can both be frustrated by inhibitory factors commonly associated with cancer development, such as VEGF, TGFβ, IL-10, IL-6 or prostanoids (of the latter most notably PGE2).18–22 These tumor-derived factors are known to be produced at high concentrations by tumor cells, and thus provide a mechanism by which tumor cells exert systemic suppression on the innate and adaptive immune system. Hyper-activation of STAT3 in tumor cells leads to production of these factors, the transcription of which is under the control of STAT3.8 From several mouse studies it has been suggested that signaling pathways downstream of receptors of many of these tumor-derived factors in turn converge on the transcription factor STAT3, resulting in STAT3 activation in tumor-conditioned DC. In this way, a “feed-forward loop” between tumor cells and DC is formed, reinforcing a cycle of immune suppression and tumor growth.8 These observations suggest a crucial and generalized role for STAT3 activation in tumor-induced inhibition of DC-development.9,10,23 Blocking activation of STAT3 might thus be expected to provide a generally applicable approach to overcome cancer-related DC suppression. In human models, however, so far little evidence has been obtained for the involvement of STAT3 in tumor induced suppression of DC development. In this study we therefore determined whether inhibition of the transcription factor STAT3 could abrogate suppression in human DC differentiation from monocytes induced by a panel of major tumor-derived suppressive factors, namely IL-6, IL-10, PGE2 and TGFβ. All tested tumor-derived factors interfered with DC differentiation and functionality as expected and all factors induced STAT3 expression and activation. Beside having an effect on DC differentiation as determined by CD1a and CD14 expression, addition of tumor-derived factors also resulted in reduced levels of costimulatory molecules and increased expression levels of B7-H4, a molecule that has been shown to inhibit T-cell responses.24 Accordingly, reduced MLR activity was observed for all DC suppression-related conditions, which could be normalized by STAT3 inhibition in the case of IL-6 only (data not shown). Indeed, also in terms of phenotype, only the inhibitory effects on MoDC differentiation of IL-6 could be overcome by inhibition of STAT3 through the use of the JAK2/STAT3 inhibitor AG490. Bharadwaj et al. showed that soluble factors derived from a pancreatic cancer cell line inhibited MoDC differentiation and that these effects could be partially prevented by addition of the JAK2/STAT3 inhibitor AG490 to the MoDC differentiation cultures.25 Even though the observed counteracting effects of AG490 were modest, like us, they linked these effects to IL-6 mediated suppression. Indeed, the IL-6/JAK2/STAT3 axis is emerging as a major mediator of inflammation, angiogenesis and tumor cell survival,8 and now also appears to be involved in tumor induced inhibition of human DC differentiation. Of note, the involvement of this axis may also depend on the type of DC precursor. In a human CD34+ DC precursor model we previously observed a block in DC differentiation by glioma-derived supernatants and were able to identify IL-6 as the responsible factor.26 Whereas this inhibition could not be counteracted by STAT3 inhibition, the effects of the same glioma supernatants on DC differentiation from CD14+ monocytes could be modulated by STAT3 inhibition (see U251 and U373 in Figure 2).
As tumors secrete a mixture of suppressive factors, it is not unexpected that sole inhibition of the IL-6/STAT3 pathway proved insufficient to overcome general tumor-mediated DC suppression. This was clearly demonstrated by the effects of STAT3 inhibition on MoDC differentiation in the presence of TDSN from a panel of 11 human tumor cell lines representing eight different tumor types. A partial block of suppressive effects by STAT3 inhibition was observed for only three out of the 11 cell lines tested. Although these supernatants contained high levels of IL6, DC suppression induced by these supernatants could not completely be overcome by STAT3 inhibition. Furthermore, the phenotype of DC differentiated in the presence of 3 other supernatants that contained relatively high levels of IL6 were not affected by addition of AG490. This clearly indicates the involvement of additional factors and down-stream signaling pathways in cancer–related inhibition of MoDC differentiation.
Another possible important pathway involved in suppression of DC is the p38 MAPK pathway. MAPK are intracellular signaling molecules involved in cytokine signaling and synthesis. Various cellular stress signals can activate p38 MAPK, e.g., inflammatory cytokines, TLR ligands, and growth factors.27 Inhibition of p38 can block the synthesis of pro-inflammatory factors like TNFα, IL-1β, prostaglandins and IL-6,27–29 and p38 inhibitors have therefore been clinically tested in patients with inflammatory bowel diseases.30 In seeming contrast, DC treatment with the p38 MAPK inhibitor SB203580 has been also reported to result in a decreased release of the immunosuppressive cytokine IL-10; for instance, binding of LPS to TLR4 or engagement of the lectin-like receptor DC-ASGPR resulted in IL-10 release which could be inhibited by p38 inhibition.31,32 Beside these effects during activation of DC, the p38 MAPK pathway has also been implicated in the differentiation of DC. Exposure to LPS was reported to arrest DC development but this was prevented by p38 inhibition, leading to restored cytokine production and recovered activity of ERK and NF-κB.33 Wang et al. demonstrated that MoDC generated from myeloma patients were phenotypically and functionally defective as a result of high production of IL6 and that blocking p38 in the progenitor cells could partly overcome this.16 The latter observation is in line with our own, i.e., that p38 inhibition can alleviate the suppressive effects on DC differentiation of soluble factors derived from a wide range of solid tumor types. Indeed, inhibition of p38 on the whole had a more profound influence than STAT3 inhibition in this regard. This apparent dominance of p38 over STAT3 inhibition in countering DC suppression was confirmed in short-term cultures of primary melanoma suspensions. In addition, we found that combined STAT3 and p38 interference had a profound effect on the T cell priming capacity of modulated DC, resulting in Th1-skewed responses. The latter observation is in keeping with findings by others, showing decreased release of PGE2 and IL-10 and increased release of IL-12 by p38-inhibited DC, resulting in effective Th1 skewing.34,35
Our findings thus point to favorable effects of p38 MAPK inhibition in terms of breaking cancer-imposed DC suppression, particularly in combination with STAT3 inhibition, and suggest its utility in support of cancer immunotherapy. Small-molecule p38 inhibitors have been clinically tested but were unfortunately demonstrated to have an unacceptable safety profile.36 Moreover, caution is warranted in the clinical application of systemic p38 MAPK inhibition in the treatment of cancer, as p38 MAPK signaling has been shown to control quiescence and dormancy of disseminated micrometastases.37 Intradermal administration of p38-activating lentiviruses combined with a vaccine was even shown to afford superior immune activation and tumor protection in a mouse model.38 Thus, the effects of p38 signaling and inhibition thereof on tumor control appear to be highly context dependent. Our observations in melanoma suspensions from metastatic lesions suggest that p38 inhibition, alone or combined with STAT3 inhibition, might be particularly effective in the confines of the tumor microenvironment. A previous study showed disturbed DC differentiation in such metastatic melanoma lesions to be IL-10 dependent.39 Apart from ensuring proper DC differentiation from monocytic precursors, p38 inhibition might also prevent conversion of monocytes to MDSC and trans-differentiation of DC to endothelial cells in aid of angiogenesis in the tumor.40,41 In addition, p38 inhibition in the tumor microenvironment might also prevent T cell suppression by Tregs induced by IL-10-modulated tolerogenic DC (i.e., Tr1).42 Thus, targeted delivery of p38 inhibitory therapeutics to the tumor microenvironment might be the way to go. Alternatively, identification of upstream suppressive factors or downstream signaling elements might offer druggable targets with more selective effects on DC suppression. We were unable to link p38 signaling to DC suppression by a specific suppressive factor (i.e., IL6, IL10, TGFβ, or PGE2, data not shown). This suggests that there is another as yet unidentified p38-modulating suppressive factor common to the tested tumor-derived supernatants, or that a combination of suppressive factors in the supernatants targets p38 signaling. Perhaps identification of elements signaling downstream from p38 might open the way to more selective inhibition of DC suppression without undue risk of the reported side effects of clinical p38 inhibition or of collateral tumor outgrowth.36,37
In conclusion, combined interference in both the STAT3 and the p38 MAPK pathways may be applicable in a wide range of solid tumor types to counter DC suppression and induce Th1 responses in tumor-conditioned microenvironments in support of immunotherapeutic approaches.
Materials and Methods
Cells and culture conditions
The human cancer cell lines U373 and U251 (glioma), CAL-72 and MG-63 (osteosarcoma), A549 and H1703 (non-small cell lung cancer), C320 (colon cancer), MDA-MB-231 (breast cancer), OvCAR (ovarian cancer), A431 (skin cancer) and MKN-45 (stomach cancer) were cultured in DMEM (Lonza, 12614F) supplemented with 10% fetal calf serum (FCS, Hyclone, SV30160.03), 100 IU/ml penicillin (Astellas Pharma, RVG 53418) and 100 μg/ml streptomycin (Riemser, N2) at 37°C in a 5% CO2 humidified atmosphere. Tumor-derived supernatants were harvested from after 72 h of culture of monolayers.
MoDC were generated as described previously.43 In short, peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood of normal human volunteers by density centrifugation over Lymphoprep (Fresnius, 1114547). CD14+ monocytes were isolated from PBMC by CD14 MACS (Miltenyi Biotec, 120–000–305). After washing, the cells were differentiated for 5–7 d in IMDM (Lonza, BE12–722F) supplemented with 10% FCS, 100 IU/ml penicillin and 100 μg/ml streptomycin, 100 ng/ml GM-CSF (Bayer, NDC58468–0180–2) and 1000 U/ml IL-4 (R&D, 204-IL/CF). Suppressive factors were added at the following optimized18 concentrations: 20 ng/ml IL-10, 50 ng/ml IL-6, 100 ng/ml PGE-2 and 150 ng/ml TGFβ. To suppress DC differentiation, 5% of 20x concentrated supernatant26 or 30% of unconcentrated supernatant derived from the different tumor cell lines was added during differentiation. To inhibit STAT3 or p38 MAPK signaling, 10 μM AG490 (Invitrogen, PHZ1204) or 10 μM SB203580 (Promega, V1161) were respectively administered 30 min. before the tumor supernatant was added to the CD14+ monocytes. After 6 d of differentiation, DC phenotype was determined by FACS analysis. The cytokine secretion profile of the DC was determined with the human inflammation CBA kit (BD, 551811) after CD40L stimulation as described.44
Primary glioma tumors were brought directly into culture after surgery of the patient (as described by Lamfers et al.45), while melanoma suspensions from surgically removed metastatic lesions were similarly cultured upon cryopreservation; see paragraph below for details.46 Supernatants were collected within the first five passages of these tumor cells, by plating a confluent concentration of cells and allowing them to secrete factors for 72 h.
Antibodies and flow cytometry
PE- or FITC-labeled antibodies directed against human CD14 (BD, 345784), CD1a (BD, 555807), CD83 (Beckman Coulter, PNIM2218U), CD86 (BD, 555657), CD40 (Beckman Coulter, PNIM1936U), BDCA3 (Miltenyi Biotec, 130–090–513), CD80 (BD, 557226) and HLA-DR (BD, 347401) were used for flowcytometric analyses, with non-human specific IgG1 antibodies as controls (BD, 345815/345816). Antibody staining was performed in PBS (Braun Melsungen, 362–3140) supplemented with 0.1% BSA (Roche Diagnostics, 10735094001) and 0.02% sodium-azide (Merck, 1066880100) for 30 min at 4°C. Stained cells were analyzed on a FACScalibur (BD Biosciences) using Cell Quest software (BD, version 6.0).
Western blot analysis
Western blot analysis was used to assess the activation state of STAT3 essentially as described.26 Cellular lysates of MoDC differentiated with the different tumor-derived factors were dissolved in 6x Laemmli sample buffer with 2-mercaptoethanol (Merck, 8057400250) and boiled at 95°C for 5 min. Samples and Smartladder molecular weight marker (Eurogentec, MW-1700–02) were electrophoresed through a denaturing 7% sodium dodecyl sulfate-polyacrylamide gel and protein bands were electroblotted onto a PVDF protein membrane (BioRad, 1620176). Proteins were detected using anti-(p)STAT3 antibody (Cell Signaling 9131S and 9132) and HRP-conjugated goat anti-rabbit IgG (Dako, P0448), all used at a 1 in 2500 dilution. As a loading control, blots were incubated with mouse anti-β-actin (Sigma, A5441), at a 1 in 10000 dilution and HRP-conjugated rabbit anti-mouse IgG (Dako, P026002), at a 1 in 2500 dilution. Blots were developed with enhanced chemoluminescence (GE Healthcare, RPN2132) onto ECL hyperfilm (GE Healthcare, 28906837).
Mixed leukocyte reaction and T cell cytokine profiling
Mixed leukocyte reaction (MLR) was performed with 5–7 d-differentiated immature MoDC that were added as stimulator cells to round-bottom, 96-well, tissue-culture plates (Greiner, 650180) at indicated graded doses. As responder cells, 105 peripheral blood lymphocytes (PBL) labeled with 3μM 5(6)-Carboxyfluorescein (CFSE, Sigma Aldrich, 21888) per well were used, which were obtained from PBMC after monocyte depletion by MACS using anti-human CD14 microbeads (Miltenyi Biotec, 120–000–305) and following the manufacturer’s instructions. Stimulation of PBL was performed in triplicate. Unstimulated PBL were used as negative controls. Cells were cultured in IMDM (Lonza, BE12–722F) supplemented with 10% human serum (Sanquin, K1147), 100 IU/ml penicillin and 100 μg/ml streptomycin for up to 9 d. Starting at day 5, samples were taken daily from each well and T cell proliferation was determined with FACS analysis. To determine the levels of T cell cytokines, supernatants were taken at day 4 and analyzed by flow cytometry using the Th1/Th2/Th17 CBA kit (BD, 560484).
Metastatic melanoma cultures
Melanoma samples were collected from advanced-stage patients, who were enrolled in an IRB-approved clinical study of autologous whole-cell vaccination at the VU University medical center between 1987 and 1998, in accordance with the Helsinki Declaration of 1975.46 Cell suspensions were processed and cryopreserved within 24 h of surgical removal as described by Vermorken et al.47 The cells were thawed and resuspended in IMDM with 10% FCS, 100 IU/ml penicillin and 100 μg/ml streptomycin and seeded in 48-well plates at a concentration of 1.106/ml. GM-CSF, IL-4, AG490 or SB203580 were added as described above in the “Cells and culture conditions” paragraph. Six days later the cells were harvested, stained for CD1a and CD14, and analyzed by FACS.
Statistical analyses
Paired Student’s t-tests (two-tailed) were performed to determine significant differences between conditions. Values of p < 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by the Netherlands Organization for Scientific Research (NWO Vidi grant 917–56–321).
Glossary
Abbreviations:
- MoDC
monocyte-derived dendritic cell
- STAT3
signal transducer and activator of transcription 3
- MAPK
mitogen-activated protein kinase, IL, interleukin
- PGE2
prostaglandin-E2
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20365
References
- 1.Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–9. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012 doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenberg JJ, Fehres CM, van Cruijsen H, Oosterhoff D, de Gruijl TD. Cross-talk between tumor and myeloid cells: how to tip the balance in favor of antitumor immunity. Immunotherapy. 2011;3:77–96. doi: 10.2217/imt.10.95. [DOI] [PubMed] [Google Scholar]
- 4.Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, et al. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23:3308–16. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- 5.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 6.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–8. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nefedova Y, Cheng P, Gilkes D, Blaskovich M, Beg AA, Sebti SM, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J Immunol. 2005;175:4338–46. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 11.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–6. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J, Qian J, Yang J, Wang S, Freeman ME, 3rd, Yi Q. Critical roles of Raf/MEK/ERK and PI3K/AKT signaling and inactivation of p38 MAP kinase in the differentiation and survival of monocyte-derived immature dendritic cells. Exp Hematol. 2005;33:564–72. doi: 10.1016/j.exphem.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107:2432–9. doi: 10.1182/blood-2005-06-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Hong S, Yang J, Qian J, Zhang X, Shpall E, et al. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients’ monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006;108:4071–7. doi: 10.1182/blood-2006-04-016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res. 2009;15:4382–90. doi: 10.1158/1078-0432.CCR-09-0399. [DOI] [PubMed] [Google Scholar]
- 18.Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, et al. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol. 2002;168:4333–43. doi: 10.4049/jimmunol.168.9.4333. [DOI] [PubMed] [Google Scholar]
- 19.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66. [PubMed] [Google Scholar]
- 20.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 21.Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J. 2004;18:1439–41. doi: 10.1096/fj.03-0969fje. [DOI] [PubMed] [Google Scholar]
- 22.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–75. [PubMed] [Google Scholar]
- 23.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–54. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 24.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–61. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 25.Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007;67:5479–88. doi: 10.1158/0008-5472.CAN-06-3963. [DOI] [PubMed] [Google Scholar]
- 26.van Cruijsen H, Oosterhoff D, Lindenberg JJ, Lougheed SM, Fehres C, Weijers K, et al. Glioblastoma-induced inhibition of Langerhans cell differentiation from CD34(+) precursors is mediated by IL-6 but unaffected by JAK2/STAT3 inhibition. Immunotherapy. 2011;3:1051–61. doi: 10.2217/imt.11.107. [DOI] [PubMed] [Google Scholar]
- 27.Schindler JF, Monahan JB, Smith WG. p38 pathway kinases as anti-inflammatory drug targets. J Dent Res. 2007;86:800–11. doi: 10.1177/154405910708600902. [DOI] [PubMed] [Google Scholar]
- 28.Mögel I, Baumann S, Böhme A, Kohajda T, von Bergen M, Simon JC, et al. The aromatic volatile organic compounds toluene, benzene and styrene induce COX-2 and prostaglandins in human lung epithelial cells via oxidative stress and p38 MAPK activation. Toxicology. 2011;289:28–37. doi: 10.1016/j.tox.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Yang WS, Park YC, Kim JH, Kim HR, Yu T, Byeon SE, et al. Nanostructured, self-assembling peptide K5 blocks TNF-α and PGE₂ production by suppression of the AP-1/p38 pathway. Mediators Inflamm. 2012 doi: 10.1155/2012/489810. Epub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng YJ, Li YY. The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J Dig Dis. 2011;12:327–32. doi: 10.1111/j.1751-2980.2011.00525.x. [DOI] [PubMed] [Google Scholar]
- 31.Bogunovic D, Manches O, Godefroy E, Yewdall A, Gallois A, Salazar AM, et al. TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer Res. 2011;71:5467–76. doi: 10.1158/0008-5472.CAN-10-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–21. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J, Qian J, Wang S, Freeman ME, 3rd, Epstein J, Yi Q. Novel and detrimental effects of lipopolysaccharide on in vitro generation of immature dendritic cells: involvement of mitogen-activated protein kinase p38. J Immunol. 2003;171:4792–800. doi: 10.4049/jimmunol.171.9.4792. [DOI] [PubMed] [Google Scholar]
- 34.Harizi H, Limem I, Gualde N. CD40 engagement on dendritic cells induces cyclooxygenase-2 and EP2 receptor via p38 and ERK MAPKs. Immunol Cell Biol. 2011;89:275–82. doi: 10.1038/icb.2010.94. [DOI] [PubMed] [Google Scholar]
- 35.Yang Z, Zhang X, Darrah PA, Mosser DM. The regulation of Th1 responses by the p38 MAPK. J Immunol. 2010;185:6205–13. doi: 10.4049/jimmunol.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dambach DM. Potential adverse effects associated with inhibition of p38alpha/beta MAP kinases. Curr Top Med Chem. 2005;5:929–39. doi: 10.2174/1568026054985911. [DOI] [PubMed] [Google Scholar]
- 37.Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA. ERK1/2 and p38α/β signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res. 2011;17:5850–7. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escors D, Lopes L, Lin R, Hiscott J, Akira S, Davis RJ, et al. Targeting dendritic cell signaling to regulate the response to immunization. Blood. 2008;111:3050–61. doi: 10.1182/blood-2007-11-122408. [DOI] [PubMed] [Google Scholar]
- 39.Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO. Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumor lesions. Am J Pathol. 2004;165:1853–63. doi: 10.1016/S0002-9440(10)63238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Du H, Li Y, Qu P, Yan C. Signal transducer and activator of transcription 3 (Stat3C) promotes myeloid-derived suppressor cell expansion and immune suppression during lung tumorigenesis. Am J Pathol. 2011;179:2131–41. doi: 10.1016/j.ajpath.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu J, Zhao J, Liu K, Zhao J, Yang H, Huang Y, et al. MAPK/ERK1/2 signaling mediates endothelial-like differentiation of immature DCs in the microenvironment of esophageal squamous cell carcinoma. Cell Mol Life Sci. 2010;67:2091–106. doi: 10.1007/s00018-010-0316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adler HS, Steinbrink K. MAP kinase p38 and its relation to T cell anergy and suppressor function of regulatory T cells. Cell Cycle. 2008;7:169–70. doi: 10.4161/cc.7.2.5312. [DOI] [PubMed] [Google Scholar]
- 43.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–35. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 44.van de Ven R, de Jong MC, Reurs AW, Schoonderwoerd AJ, Jansen G, Hooijberg JH, et al. Dendritic cells require multidrug resistance protein 1 (ABCC1) transporter activity for differentiation. J Immunol. 2006;176:5191–8. doi: 10.4049/jimmunol.176.9.5191. [DOI] [PubMed] [Google Scholar]
- 45.Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–42. [PubMed] [Google Scholar]
- 46.Baars A, Claessen AM, van den Eertwegh AJ, Gall HE, Stam AG, Meijer S, et al. Skin tests predict survival after autologous tumor cell vaccination in metastatic melanoma: experience in 81 patients. Ann Oncol. 2000;11:965–70. doi: 10.1023/A:1008363601515. [DOI] [PubMed] [Google Scholar]
- 47.Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345–50. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]