Abstract
In the last decade several studies provided evidence that plasmacytoid dendritic cells (pDCs) infiltrate human neoplasms with poor prognosis. However, the role of tumor-associated pDCs remains controversial. Various studies indicate that pDCs play an immuno-suppressive role and facilitate tumor progression in both animal models and humans. In contrast, others found that the presence of activated tumor-associated pDCs results in tumor regression in mice. Given these findings, understanding pDC function in tumor biology is an important necessity and may pave the way for novel therapeutic strategies to fight malignancies.
Keywords: Plasmacytoid dendritic cells, toll-like receptors, inflammation
Introduction
Dendritic cells (DCs) are highly specialized antigen presenting cells (APCs) that recognize, process, and present “danger signals” to the adaptive immune system. DCs have been the subject of many studies and have recently been the focus of intense characterization.
Two main subpopulations have been identified: (1) non-lymphoid tissue migratory and lymphoid tissue–resident DCs and (2) plasmacytoid DCs (pDCs, also called natural interferon-producing cells (IPCs).1 pDCs originate in the bone marrow from both myeloid and lymphoid progenitors.2 The development and molecular regulation of pDCs has yet to be fully elucidated. FMS-like tyrosine kinase 3 ligand (Flt3L) is the main cytokine that induces the differentiation of common myeloid progenitor cells into both mDC and pDCs, but the E2–2 transcription factor is uniquely required for pDC differentiation.3 During steady-state conditions, mouse pDCs reside in lymphoid organs and blood, but also liver, lung, and skin, although their proliferation rate is very low.1,4 Human pDCs populate primary, secondary and tertiary lymphoid organs (aggregates/follicles), in addition to the liver and blood.5 They can migrate from the lymphoid organs toward T cell-rich areas of secondary lymphoid tissues through high endothelial venules (HEV) and toward the marginal zone of the spleen.6 In contrast, during pathological conditions, pDCs leave the bone marrow or the circulation and infiltrate into inflamed tissues where they can “sense” danger signals that lead to the release of large amounts of Type I interferons (IFNs).6,7 In this manner they generate protective immunity as Type I IFNs can activate myeloid DCs (mDCs), B, T, and NK cells.6,7 pDC chemotaxis is promoted by the expression of several molecules that allow their rolling from the circulation into the tissue. pDCs highly express CD62L, which when bound to its ligand, L-selectin (expressed on endothelial and other stromal cells6,8), facilitate the chemotaxis of pDCs. The repertoire of chemokine receptors on pDCs is expressed at greater amounts than on mDCs.6,7 CXCR3, upregulated by IFNγ signaling, binds to CXCL19 and CXCL10, and is required by pDCs to migrate into inflamed lymph nodes.9,10 Additional chemokine receptors expressed by pDCs are CCR1, CCR2, and CCR5, which can bind to CCL2, CCL3, CCL4, and CCL5.11 They also express CXCR4, implicated in pDC migration, and CCR7, which binds to the chemokines CXCL12 and CCL21.9,10 pDCs are also recruited into tissues in response to the release of the chemokine SDF-1/CXCL12, the CXCR4 ligand, which is expressed on dermal endothelial cells, HEV of lymph nodes, and in malignant cells.12 This suggests that pDCs can migrate to lymph nodes using CXCR4, and also explains their location in secondary lymphoid organs.13
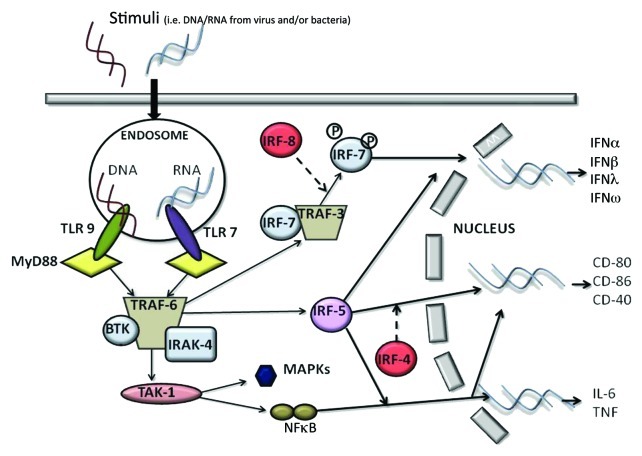
pDCs are highly specialized at sensing nucleic acids via the intracellular pattern recognition receptors TLR7 and TLR9 (Table 1).1,7 pDCs and mDCs have a different repertoire of TLR expression.1,7 Human and mouse mDCs can express TLR1, 2, 4, 5, 7, 8, while pDCs selectively express at high levels TLR7/8 and TLR9.14 TLRs are a family of receptors associated with the innate immune response. In particular, TLR7 recognizes single-stranded RNA enriched with guanosine or uridine from viruses, synthetic imidazoquinolines and guanosine analogs.15 On the other hand, TLR9 is activated by unmethylated CpG-ODN motifs typical of viruses and bacteria.7 These two receptors are very sensitive to different stimuli; TLR9 responds to DNA viruses, whereas TLR7 triggers ssRNA viruses.16 TLR7 and TLR9 recruit a cytoplasmic adaptor, myeloid differentiation primary response gene 88 (MyD88), which is able to assemble a multi-protein signal-transducing complex that induces interferon-regulatory-factor 7 (IRF-7) activation (Fig. 1).17
Table 1. Markers currently identified on pDCs.
| Marker | Structure/Function | Ligand | Effect of Activation |
|---|---|---|---|
|
BDCA-2/BDCA-4 |
Associated with FcεRly to form a signaling receptor complex |
ITAM |
Upon ligation, they inhibit TLR activation and release of Type I IFN. |
|
CD4 |
A glycoprotein expressed on the surface of T helper cells, monocytes, macrophages, and dendritic cells |
It recognizes the TCR-MHC class II complex and is required together with the CD3 zeta chain for the recognition of antigens |
Activation of pDCs |
|
CD123
76
|
The IL-3 receptor (70KD) is composed of a ligand specific α subunit and a signal transducing β subunit shared by the receptors for interleukin 3 (IL3), colony stimulating factor 2 (CSF2/GM-CSF), and interleukin 5 (IL-5). |
IL-3 |
Amplification of inflammation |
|
IL-T3
77
|
Characterized by its cytoplasmic ITIM domain |
Fc receptor |
Tolerance induction |
|
IL-T7
78
|
Characterized by its cytoplasmic ITIM domain and is also expressed on B, T, and NK cells |
IFN I |
Inhibition of release of Type I IFN (negative feedback) |
|
CD-11c
79
|
A heterodimeric integral membrane protein composed of an α chain and a β chain |
ICAM-2 and VCAM-1 |
Induces cell activation; It’s an adhesion receptor that is implicated in phagocytosis of latex beads and bacteria in the absence of complement. It plays an important role in the inflammatory response and can lead to the production of pro-inflammatory cytokines after an APC response. |
|
TLR-7 |
An intracellular endosomal pattern recognition receptor |
Single stranded RNA |
Upregulation of CD40, CD80, CD86, and CCr7. Induction of high levels of Type I IFN. Does not induce IL-12p70 production. |
| TLR-9 | An intracellular endosomal pattern recognition receptor | Unmethylated CpG dinucleotides from bacterial DNA | Upregulation of CD40, CD80, CD86, CD83, HLA-DR, and CCR7. Upregulation of Type I IFN, IL-6, TNFα, IL-8, and IP-10. Does not induce IL-10 secrection. |
Figure 1. The recognition of a stimulus by pDCs via TLR7 and/or TLR9 induces the activation of MyD88-dependent signaling pathways that lead to the expression of cytokines such as IL-6 and TNFα, co-stimulatory molecules such as CD80, and the synthesis/release of Type I IFN.
MyD88 also leads to TRAF-6-mediated NFκB and MAPKs activation, essential for the transcription of pro-inflammatory cytokines, chemokines and co-stimulatory molecules (Fig. 1 and Table 1).16
The exposure of pDCs to TLR7 or TLR9 ligands can lead to the production of Type I IFN and pro-inflammatory cytokines, such as TNFα, and chemokines, such as IL-8 (CXCL8).1,6,14 The constitutive expression of IRF7, which is different from mDCs where it must be induced, renders pDCs high producers of Type I IFN,11 which regulates T-cell immunity, leading toward a Th1 and cytotoxic T-cell polarization, activates mDCs, NK and B cells.6,7,11 Very importantly, IFNα modulates several aspects of the immune system, including pDC survival, mDC differentiation, modulation of Th1 and CD8+ T-cell responses, cross-presentation, upregulation of MHC and co-stimulatory molecules, activation of NK cells, and induction of primary antibody responses.18 However, a recent study found that Type I IFN negatively controls pDC turnover during steady-state conditions and viral infections.19
pDC activation can also lead to the production of IL-12p70, IL-1β and IL-6.20 Furthermore, recent discovery found that pDCs may mediate the release of IL-10,21 however another group22 showed that these cells do not directly produce IL-10.
Moreover, it was recently demonstrated that pDCs produce high amounts of Granzyme B,23 which is effective only in combination with perforins that are mainly produced by cytotoxic T lymphocytes (CTLs). This further connects pDCs to adaptive immunity. Additionally, it was also demonstrated that in the absence of an “efficient” adaptive CTL immunity, pDCs can behave as killing DCs due to the release of TNF-related apoptosis-inducing ligand (TRAIL) and to the induction of the expression of DR5, one TRAIL receptor, on the cell target.23,24
Plasmacytoid Dendritic Cells (pDCs) Phenotype
pDCs are a rare cell type representing only 0.5% of circulating cells in healthy individuals.7 They are round-shaped cells characterized by a prominent endoplasmic reticulum.6 Human pDCs are CD4+, CD45RA+, IL-3αR (CD123)+, immunoglobulin-like transcript factor (ILT)-3+, ILT-1low/-, SiglecH+, CD11clow/- cells (Table 1).6 Two additional surface markers for human pDCs are represented by BDCA-2 and BDCA-4, that correspond to the murine mPDCA-1, restricted to the peripheral blood and bone marrow-derived pDCs.6 BDCA-2 is a C-type lectin transmembrane glycoprotein that can internalize antigen for presenting to T cells. Some data show that triggering of BDCA-2 can potently inhibit in vitro induction of IFNα/β expression in pDCs by viruses.25 BDCA-4, instead, does not have a substantial effect on pDC function, but can be used for the purification of pDCs by magnetic selection (Table 1).
Mouse pDCs share most of the morphological and phenotypical features with the human counterpart, except for the identification as CD11clow/- Gr-1+/int B220+ 120G8+ cells.1,6,7 The recognition of the pDC surface markers is actually very important not only to distinguish pDCs from mDCs and other cell types but also for the isolation of these cells. To date an appropriate murine model to study the role of pDCs in the pathogenesis of various diseases is characterized by the recent established Bdca2-DTR26 and SiglecH-DTR models.27 These mouse models allow the study of pDCs in patho-physiological conditions through the depletion of pDCs by diphtheria toxin (DT) using the human diphtheria toxin receptor (DTR) that is driven by the BDCA2 promoter, as the mouse receptor for DTR binds several orders of magnitude more weakly to DT. However, many studies have also been conducted by using specific depleting antibodies, such as 120G8 Ab,28 BST-2 Ab,29 mPDCA-130 in vivo. All of these antibodies bind to the same surface marker (BST-2 or CD317). The antibody depletion models seem to be less specific than the DTR models, but still very efficient in pDC depletion, thus allowing the investigation of the role of pDCs during steady-state and pathological conditions. The caveat of Ab-mediated pDC depletion stands on the role of some molecules, such as BST-2, which is also expressed by stromal and other immune cells after an inflammatory stimulus.29
pDCs: Bridging the Gap between Innate and Adaptive Immunity
The production of Type I IFN by pDCs represents the bridge between the innate and the adaptive immune system. Type I IFN (IFNα and IFNβ) is an important component of innate immunity, especially during viral infections.1,6,7 Upon activation, in contrast to mDCs, pDCs produce high amounts of Type I IFN,1,6,7 which both amplifies its own production and induces the release of IL-12p70 from mDCs and NK cells.18,19,32 The activation of mDCs skews the immune environment toward a Th1-like bias, during which IFNγ production both facilitates Th1 differentiation,6,7 long-term T-cell immunity6 and a CTL-mediated response,31 as well as proliferation and survival of T cells.6,11
Moreover, through the production of IL-6 and Type I IFN, pDCs induce B cells to differentiate into plasma cells which are immunoglobulin (preferentially IgG and IgM) producing cells. In the process of activation of B cells, a key-role is played by the CD70 receptor expressed on pDCs, as it can induce the differentiation and the proliferation of IgG-producing B-cells.32
In addition, activated pDCs can undergo other important phenotypic changes that induces them to the change their phenotype toward a more mDC phenotype. The upregulation of MHC and T-cell costimulatory molecules enable pDCs to engage and activate naïve T cells.33-35 There have been many controversies regarding the role of pDCs to prime T-cells and cross-present antigens.35 The expression of MHC and T-cell costimulatory molecules is not as high as in mDCs and this is why pDCs are less efficient than mDCs at priming T cells.36 Moreover, the repertoire of antigens that can be presented by pDC-derived MHC molecules is more restricted than those of mDCs because not all of these antigens can reach the endocytic compartment in pDCs.37 However, some pDC receptors such as BDCA2, SiglecH and DCIR are able to bind antigens, mediate endocytosis, process and present it to T cells.38-41
Interestingly, activated pDCs can also promote Th2-like immune response,18 underlining their functional plasticity. There is evidence that IFNα stimulates the differentiation of pDCs into Th1-inducing pDCs, whereas in the absence of IFNα but in the presence of inflammatory signals stimulate differentiation in Th2-inducing pDCs.48 Moreover, some authors reported that CpG-activated pDCs exert a strong immune suppression and induce the differentiation of allogeneic CD4+CD25− T cells into CD4+CD25+ regulatory T cells in tumor conditions.42,43 Very interestingly, pDCs can directly or indirectly recruit Treg cells via PD-L1/PD-1 axis49,50 and through the release of immunosuppressive cytokines, such as IL-1043, and the membrane tolerogenic inducible co-stimulator ligand (ICOS-L).51
PDCs can also synthesize large amounts of functional indoleamine 2,3-dioxygenase (IDO), which requires autocrine release of Type I IFN, upon TLR9 and CD200R ligands stimulation.7 IDO-derived metabolites promote T-cell death44,46 and suppresses T-cell immunity in normal and pathological settings. In the same manner, reduced tryptophan amounts can lead to the release of regulatory cytokines, such as IL-10,47 associated with a tolerogenic environment.
Thus, in toto, these data suggests that pDCs represent a key effector cell in both innate and adaptive immunity regulation.48,52,53
Role of pDCs in Cancer
pDCs have been found in a variety of neoplasms although their function is still unknown. Solid tumors, such as head and neck, breast, ovarian, lung cancer, and skin tumors, are populated by pDCs that are in their non-active state.54 The mechanism that induces the recruitment of pDCs to the tumor site is not clear. However, cytokines such as CXCL10, CXCL12 and chemokines, such as CCL2, released by tumor and stromal tumor-associated cells, such as cancer–associated fibroblasts (CAFs), allow pDCs to migrate from the circulation to the injured tissue. Accordingly, Drobits et al., demonstrated that CCL2 produced in the inflamed skin of tumor-bearing mice facilitated pDC recruitment.23 In this study pDCs were cytotoxic and contributed to tumor regression.23 However, mice were treated with Imiquimod, a TLR7 ligand. Similarly, Liu et al.,55 demonstrated that the activation of pDCs via CpG could induce NK cell-dependent tumor regression in melanoma animal models. However, in our published data,57 the stimulation of lung tumor-bearing mice with CpG, a TLR9 ligand, did not lead to the same results as observed by Drobits23 and Liu et al.55 The activation of pDCs through CpG had the opposite effect as pDC activation increased the recruitment of Tregs and limited the inflammatory cell influx to the lung thereby establishing an immunosuppressive environment enabling tumor growth. In support, CCL2 is also able to recruit Treg to the tumor, implying a very poor prognosis for tumor patients.56 The discrepancy in these data could be a result of tissue-specificity that is very important in determining the tumor microenvironment, which in turn strongly influences immune cell phenotype. Moreover, in the absence of a specific stimuli, pDCs in the tumor mass have been associated with the development and maintenance of the immune-suppressive microenvironment.11
In addition, in humans, compared with healthy donors, circulating pDC numbers are reduced in tumor patients, although tumor masses show higher recruitment of these cells into the tumor site.5 Similar to mice, human pDCs in tumor masses are in their immature phenotype, although a thorough study has never been conducted onto the role of these cells in the human tumor microenvironment. However, it is clear from the various studies performed that pDCs play a fundamental role in the tumor microenvironment. In support of this, our data found that pDCs were highly recruited to the lung of tumor-bearing mice after CpG-ODN administration,57 and participated in lung tumor outgrowth associated with immune suppression. The specific depletion of pDCs decreased lung tumor burden with a concomitant Th1 and Th17 polarization that arrested tumor progression.57 In contrast, Liu et al.55 demonstrated that the activation of pDCs via CpG could induce NK cell-dependent tumor regression in melanoma animal models. Moreover, the activation of pDCs via the TLR7-dependent pathway induced melanoma regression in mice23 because of the transformation of pDCs into tumor-killing cells able to produce Granzyme B and TRAIL. Likewise, another group revealed that human pDCs can kill melanoma cells in vitro under imiquimod and IFN-α stimulation.24 While pDCs can produce high levels of Granzyme B, their role as cytotoxic immune cells remains to be determined because they lack the pore-forming perforin.54 On the other hand it has been proposed that under IL-3 and IL-10 exposure, pDCs release abundant Granzyme B, which in turn is capable of blocking T-cell proliferation, thus suggesting a new potential mechanism for tumor-immune evasion.54
Several mechanisms have been postulated for the immune-suppressive nature of tumor-associated pDCs: (1) release of tolerogenic factors; (2) ILT-7 expression; (3) PD-L1 expression; (4) Siglec H activity; and (5) induction of a Th2-like environment.
Tolerogenic factors produced by tumor cells, such as PGE2, can alter the Type I IFN signaling pathway.58 Tumor derived PGE2 and TGFβ act synergistically to block IFNα and TNFα secretion by pDCs.7,58 Opposite to IFNα and TNFα, IL-6 and IL-8 production are enhanced in PGE2- and TGFβ-treated pDC. Both IL-6 and IL-8 promote immune-cell survival and chemotaxis, but also enhance tumor cell proliferation and angiogenesis.59,60 Moreover, PGE2 is crucial for the secretion of other immunomodulatory factors such as SDF-1, the ligand for CXCR4, which is upregulated on both human pDCs and tumor environment.6,7 Thus, pDCs can be retained in the tumor tissue via PGE2-induced sensitization for SDF-1.12 In further support, PGE2- and TGFβ-mediated retention of pDCs in the tumor tissue is accompanied by the suppression of the lymph node-homing receptor, CCR7. In addition, PGE2-exposed pDCs are unlikely to present antigen/s and to prime T cells in the regional lymph nodes. Concomitantly, the suppression of CD40 expression and the overexpression of CD80/86 on pDCs enhances and even promotes Treg activation via the negative regulatory receptor cytotoxic T-lymphocyte antigen-4 (CTLA-4).61,62
Another potential mechanism for pDCs to favor tumor immune escape is the release of IDO-derived metabolites62 from both pDCs and tumor cells, implying on one side Treg differentiation and on the other Th cell apoptosis.45,46,62 Most human tumors overexpress IDO,52 explaining the elevated tryptophan catabolism in cancer patients. Interestingly, the activation of IDO in either cancerous cells or regulatory DCs can be sufficient to promote tumor immune escape.53
In addition, some cancer cells, such as lung cancer-derived cells, highly express ILT7L, which can bind to ILT7 that is on pDCs.63 ILT7L is induced by IFNγ and inhibits IFNα production from human pDCs, indicating that the ILT7L/ILT7 interaction between the cancer cells and pDCs may cause impairment of pDCs in the tumor microenvironment possibly leading to immunesuppression and poor prognosis of cancer patients as observed in clinical studies.63
Moreover, under tumor conditions pDCs can also affect mDC’s phenotype toward a more immature state, as already reported for human lung cancer.7,48,57 However, the mechanism is still not known.
To date pDCs can directly interact with Treg via the PD-1/PD-L1 axis.46 Moreover, antigen targeting to pDCs via Siglec-H inhibits Th cell-dependent immunity.41 Our published data showed that the administration of CpG increased SiglecH expression on pDCs recruited to the lung of tumor-bearing mice, further supporting their implication in the inhibition of Th cell expansion.57
In addition, pDCs activated by IL-3 and CD40 ligand (CD40L) promote the differentiation of naive CD4+ and CD8+ T cells into Th2 cells and anergic IL-10-producing CD8+ regulatory T cells, respectively.66 This state of anergy is mediated by IL-10, either directly (by interaction with cytotoxic T lymphocytes, CTLs) or indirectly (by inhibition of DCs).67 Because the tumor microenvironment is Th2-like, pDCs participate in this scenario by further augmenting immune suppression. Taken together, these effects may allow pDCs to establish a reduced inflammatory pattern, but at the same time to favor tumor progression/establishment, as observed in asthma,64 virus infection65 and cigarette smoke exposure.48
To note, the aforementioned studies describe the role of pDCs which are not activated by a specific stimulus. Therefore, the discrepancy about pDC’s role in cancer may rely on the stimulation/activation of pDCs. In support, the activation of pDCs via Imiquimod or CpG results in tumor regression.23,55
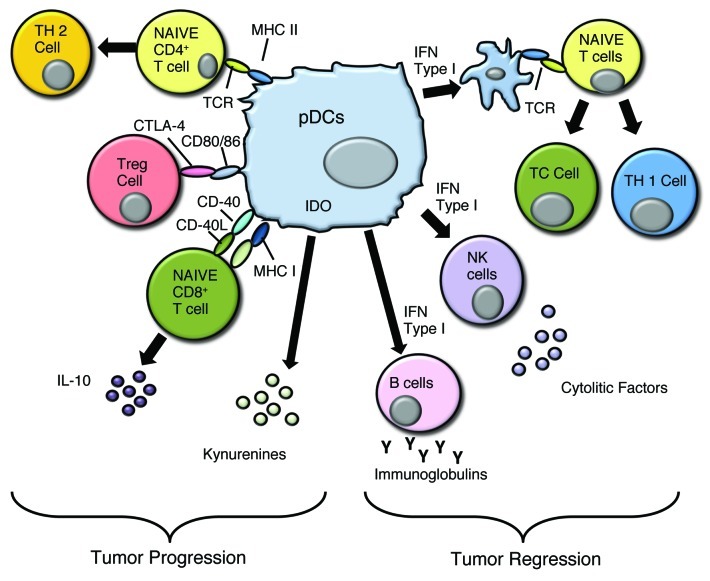
It seems obvious then that the activation of pDCs at the tumor site is a limiting step in tumor regression (Fig. 2).
Figure 2. pDCs can favor both tumor progression and tumor regression. The mechanisms that allow tumor cell proliferation are due to the induction of a Th2-like microenvironment, activation of Treg via CTLA4/CD80 or CD86, and IL-10 production that can modulate the immune fate of cytotoxic lymphocytes, such as CD8+ T cells. Tumor regression is achieved by Type I IFN dependent and independent activation of DCs, NK cells and B cells while promoting a Th1-polarizing environment.
pDCs and Cancer Immunotherapy
Until now, cancer immunotherapy has been focused primarily on DCs ability to enhance T-cell responses. This has been the grounds for many of the anti-cancer vaccines,68 which are based on the injection of TLR ligands and on tumor-derived antigens to increase the adaptive immune response. One example is CpG-ODN, which induced anti-tumor activity because it increased the number of activated pDCs at the vaccine site in melanoma models.55 Similarly, imiquimod-activated pDCs favored tumor regression due to their killing activity in a mouse model of skin cancer.23 However, several studies have found that tumor-derived pDCs are in their tolerogenic or inactive phenotype due to the tumor microenvironment. In the absence of a specific stimulus, pDCs facilitate tumor progression.
The recruitment of pDCs into the tumor site is due to the chronic latent inflammation. However, the activation of these cells depends on the type of tumor, and given that pDCs act differently depending on the microenvironment they encounter, it is very important to understand the role of endogenous stimuli that might activate pDCs allowing a Th1 and T cytotoxic polarization or in an opposite way, a Th2 and/or suppressive immunity. pDCs are activated by nucleic acids. It was postulated that DNA deriving from dying tumor cells can bind to LL-37, the endogenous protein that facilitates the recognition of self-DNA to TLR9.70 Indeed, the exogenous administration of LL-37 into the tumor site was considered as a potential anti-tumor agent because it can activate pDCs.69,70 However, an immuno-suppressive environment can condition the pDC phenotype resulting in tumor outgrowth. In breast cancer and advanced stage ovarian cancer,70 large numbers of pDCs are correlated with malignancy progression. In these latter cases pDCs induced IL-10-producing Treg and expressed high levels of IDO (Fig. 2).
The inhibitory immune activity of pDCs could be due to the absence of an appropriate stimulus in the tumor microenvironment, but may also be actively sustained by the production of inhibitory factors such as TGFβ, VEGF and IL-10.11 Angiogenesis is crucial for tumor growth because cancerous cells need oxygen and nutrients to proliferate. Indeed, pDCs may play a role in tumor angiogenesis as pDCs produce TNFα and CXCL8, both of which are implicated in angiogenesis.72 On the other hand, the activation of pDCs in some models is essential to become tumor-killing cells, however the mechanism by which tumor-associated pDCs can circumvent the suppressive environment and switch from inactive toward an active anti-tumor phenotype is still unknown.
pDCs are largely known for their anti-viral activity and induction of adaptive immunity, which significantly differs from their activity in tumors. This is largely due to viral elements that activate innate immune cells via TLR-dependent pathways.6,7 In contrast, tumors, which derive from host tissues, are incapable of activating innate immune cells. Therefore, the main hypothesis for the anti-tumor activity of pDCs was based on the activation of pDCs by TLR ligands resulting in an enhanced inflammatory cascade that improved T-cell immunity and recruitment to the tumor site. Once pDCs traffick into the tumor microenvironment, they interact with tumor cells and/or immune cells so that their capability to produce high amounts of Type I IFN is apparently blocked and immune-suppression prevails. Instead, when properly activated by TLR ligands, pDCs might provide abundant Type I IFN to induce the activation of surrounding innate and adaptive immune cells. Although the latter evidence is still limited to melanoma models, it could prove of potential therapeutic value for other malignancies.
The capacity to recognize tumor antigens could be a decisive strategy for immunotherapy. Antigen-pulsed pDCs can stimulate specific primary and memory autologous CD4+ and CD8+ T-cell immune responses in vitro31 and prime functional T-cell responses in vivo as shown after vaccination of mice with CpG or virus-activated pDCs.72 The use of autologous pDCs for cancer immunotherapy is difficult because of the scarcity of these cells in the circulation and the possible functional alterations of pDCs harvested from tumor-bearing patients. Aspord et al., proposed that pDCs loaded with melanoma-derived peptides (MelA, GP100, TYR and MAGE-3) primed T cells which showed an efficient killing activity of tumor cells.73 This is an interesting immunotherapeutic strategy to fight cancer, however the concept of pDCs as APCs still needs to be clarified and the role of the tumor microenvironment on the pDC phenotype should be taken into account. Aspord et al., assumed that pDCs cross-present antigens more rapidly than do mDCs.73 However, the difference in using mDCs or pDCs in cancer vaccination or immunotherapy still remains to be elucidated. It is not clear what would be the benefit to use one or the other type of DCs to prime T cells against tumor cells. Indeed, an interesting study proved that the immunizations with a mixture of matured pDCs and mDCs resulted in increased levels of antigen-specific CD8+ T cells and enhanced antitumor response compared with immunization with each DCs subset alone.74 Moreover, Kalb et al.,24 and Drobits et al.,23 found a killing activity of pDCs on melanoma cells that was dependent on TRAIL and Type I IFN. It is to note though that these effects were investigated on melanoma cells that, compared with other models of cancerous cells, were more susceptible to pDC-derived killing activity, even independently of the adaptive immunity. In addition, intratumoral stimulation of pDCs with TLR7 and TLR9 agonists has been successfully used in the clinic to treat basal cell carcinoma.75 So far no killing activity of pDCs has been proven on other cancerous cells other than melanoma cells. Thus properly activated pDCs are endowed with anti-tumor activity, but the tumor microenvironment represents the limiting step that subverts the potential therapeutic use of pDC activity in cancer.71
In conclusion, a potential anti-tumor strategy will be developed only after understanding the biology of pDCs. Importantly, it will be necessary to understand the correlation between the prognosis and tumor-associated pDCs. Moreover, their phenotype plays a pivotal role at inducing cytotoxic or immune suppressive pathways. Once the role of these cells in the different types of tumors is recognized, it will be possible to develop a potential anti-tumor therapeutic strategy based on one hand on the elimination of pDCs from the tumor masses, or on the other hand to potentiate their activity as tumor killing cells. Thus, the study of pDC biology and understanding the nature of pDCs associated with several neoplasms could pave the way for new therapeutic possibilities.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the University of Salerno for providing the financial support FARB in favor of A.P. R.S. is supported by the University of Salerno Fellowship.
Glossary
Abbreviations:
- TLRs
Toll-like receptors
- MyD88
myeloid differentiation factor
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- PAMPs
pathogen-associated molecular patterns
- DAMPsl
danger-associated molecular patterns
- DCs
dendritic cells
- pDCs
plasmacytoid dendritic cells
- Treg
T regulatory cells
- IDO
indoleamine-2, 3-dyoxigenase
- IFN I
interferon Type I
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20171
References
- 1.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1:442–50. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 2.Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010;31:270–7. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33:905–16. doi: 10.1016/j.immuni.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–8. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 5.Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–28. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 6.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 7.Sorrentino R, Morello S, Pinto A. Role of plasmacytoid dendritic cells in lung-associated inflammation. Recent Pat Inflamm Allergy Drug Discov. 2010;4:138–43. doi: 10.2174/187221310791163062. [DOI] [PubMed] [Google Scholar]
- 8.Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–28. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 9.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167:1862–6. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 10.Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Brière F, et al. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003;198:823–30. doi: 10.1084/jem.20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann N Y Acad Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 12.Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 13.Penna G, Volcano M, Sozzani S, Adorini L. Differential migration Behavior and chemokine production by myeloid and plasmacytoid Dendridic cells. Hum Immunol. 2002;36:1164–71. doi: 10.1016/S0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett. 2008;121:1–6. doi: 10.1016/j.imlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 16.Gilliet M, Cao W, Liu Y-J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch I, Caux C, Hasan U, Bendriss-Vermare N, Olive D. Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol. 2010;31:391–7. doi: 10.1016/j.it.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 19.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med. 2011;208:2367–74. doi: 10.1084/jem.20110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasius AL, Colonna M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 2006;27:255–60. doi: 10.1016/j.it.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Kassner N, Krueger M, Yagita H, Dzionek A, Hutloff A, Kroczek R, et al. Cutting edge: Plasmacytoid dendritic cells induce IL-10 production in T cells via the Delta-like-4/Notch axis. J Immunol. 2010;184:550–4. doi: 10.4049/jimmunol.0903152. [DOI] [PubMed] [Google Scholar]
- 22.Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J Immunol. 2006;177:7551–8. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- 23.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, et al. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–85. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalb ML, Glaser A, Stary G, Koszik F, Stingl G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-α-mediated antitumor reactivity. J Immunol. 2012;188:1583–91. doi: 10.4049/jimmunol.1102437. [DOI] [PubMed] [Google Scholar]
- 25.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, et al. BDCA-2 a novel plasmacytoid cell-specific tipe II C-type of lectin, mediates antigen capture and is a potent inhibitor of interferon a/B induction. J Exp Med. 2001;194:1823–34. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–66. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, et al. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–71. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Asselin-Paturel C, Brizard G, Pin JJ, Brière F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–77. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 29.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–5. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 30.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–5. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood. 2010;115:3051–7. doi: 10.1182/blood-2009-08-239145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–50. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 34.Björck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–6. doi: 10.1182/blood.V98.13.3520. [DOI] [PubMed] [Google Scholar]
- 35.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–61. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Wilson NS, Villadangos JA. Regulation of antigen presentation and cross-presentation in the dendritic cell network: facts, hypothesis, and immunological implications. Adv Immunol. 2005;86:241–305. doi: 10.1016/S0065-2776(04)86007-3. [DOI] [PubMed] [Google Scholar]
- 37.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–34. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol Rev. 2005;207:191–205. doi: 10.1111/j.0105-2896.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 39.Jaehn PS, Zaenker KS, Schmitz J, Dzionek A. Functional dichotomy of plasmacytoid dendritic cells: antigen-specific activation of T cells versus production of type I interferon. Eur J Immunol. 2008;38:1822–32. doi: 10.1002/eji.200737552. [DOI] [PubMed] [Google Scholar]
- 40.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–53. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 41.Loschko J, Heink S, Hackl D, Dudziak D, Reindl W, Korn T, et al. Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J Immunol. 2011;187:6346–56. doi: 10.4049/jimmunol.1102307. [DOI] [PubMed] [Google Scholar]
- 42.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassner N, Krueger M, Yagita H, Dzionek A, Hutloff A, Kroczek R, et al. Cutting edge: Plasmacytoid dendritic cells induce IL-10 production in T cells via the Delta-like-4/Notch axis. J Immunol. 2010;184:550–4. doi: 10.4049/jimmunol.0903152. [DOI] [PubMed] [Google Scholar]
- 44.Lee SM, Lee YS, Choi JH, Park SG, Choi IW, Joo YD, et al. Tryptophan metabolite 3-hydroxyanthranilic acid selectively induces activated T cell death via intracellular GSH depletion. Immunol Lett. 2010;132:53–60. doi: 10.1016/j.imlet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 48.Sorrentino R, Gray P, Chen S, Shimada K, Crother TR, Arditi M. Plasmacytoid Dendritic Cells Prevent Cigarette Smoke and Chlamydophila pneumoniae—induced Th2 Inflammatory Responses. Am J Respir Cell Mol Biol. 2010;43:422–31. doi: 10.1165/rcmb.2009-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–61. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 50.Tokita D, Mazariegos GV, Zahorchak AF, Chien N, Abe M, Raimondi G, et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85:369–77. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 51.Puccetti P, Fallarino F. Generation of T cell regulatory activity by plasmacytoid dendritic cells and tryptophan catabolism. Blood Cells Mol Dis. 2008;40:101–5. doi: 10.1016/j.bcmd.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 52.Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 53.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–21. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 54.Vermi W, Soncini M, Melocchi L, Sozzani S, Facchetti F. Plasmacytoid dendritic cells and cancer. J Leukoc Biol. 2011;90:681–90. doi: 10.1189/jlb.0411190. [DOI] [PubMed] [Google Scholar]
- 55.Liu C, Lou Y, Lizée G, Qin H, Liu S, Rabinovich B, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–75. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erreni M, Mantovani A, Allavena P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011;4:141–54. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorrentino R, Morello S, Luciano A, Crother TR, Maiolino P, Bonavita E, et al. Plasmacytoid dendritic cells alter the antitumor activity of CpG-oligodeoxynucleotides in a mouse model of lung carcinoma. J Immunol. 2010;185:4641–50. doi: 10.4049/jimmunol.1000881. [DOI] [PubMed] [Google Scholar]
- 58.Bekeredjian-Ding I, Schäfer M, Hartmann E, Pries R, Parcina M, Schneider P, et al. Tumour-derived prostaglandin E and transforming growth factor-β synergize to inhibit plasmacytoid dendritic cell-derived interferon-α. Immunology. 2009;128:439–50. doi: 10.1111/j.1365-2567.2009.03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 60.Voorzanger N, Touitou R, Garcia E, Delecluse HJ, Rousset F, Joab I, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–505. [PubMed] [Google Scholar]
- 61.Kurtz J, Raval F, Vallot C, Der J, Sykes M. CTLA-4 on alloreactive CD4 T cells interacts with recipient CD80/86 to promote tolerance. Blood. 2009;113:3475–84. doi: 10.1182/blood-2008-01-133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–8. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 63.Tsukamoto N, Okada S, Onami Y, Sasaki Y, Umezawa K, Kawakami Y. Impairment of plasmacytoid dendritic cells for IFN production by the ligand for immunoglobulin-like transcript 7 expressed on human cancer cells. Clin Cancer Res. 2009;15:5733–43. doi: 10.1158/1078-0432.CCR-09-0171. [DOI] [PubMed] [Google Scholar]
- 64.de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smit JJ, Lindell DM, Boon L, Kool M, Lambrecht BN, Lukacs NW. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One. 2008;3:e1720. doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bratke K, Klein C, Kuepper M, Lommatzsch M, Virchow JC. Differential development of plasmacytoid dendritic cells in Th1- and Th2-like cytokine milieus. Allergy. 2011;66:386–95. doi: 10.1111/j.1398-9995.2010.02497.x. [DOI] [PubMed] [Google Scholar]
- 67.Battaglia M, Gianfrani C, Gregori S, Roncarolo MG. IL-10-producing T regulatory type 1 cells and oral tolerance. Ann N Y Acad Sci. 2004;1029:142–53. doi: 10.1196/annals.1309.031. [DOI] [PubMed] [Google Scholar]
- 68.Lesterhuis WJ, Aarntzen EH, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, et al. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66:118–34. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Hurtado P, Peh CA. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J Immunol. 2010;184:1425–35. doi: 10.4049/jimmunol.0902305. [DOI] [PubMed] [Google Scholar]
- 70.Chuang CM, Monie A, Wu A, Mao CP, Hung CF. Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum Gene Ther. 2009;20:303–13. doi: 10.1089/hum.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Schlecht G, Garcia S, Escriou N, Freitas AA, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 2004;104:1808–15. doi: 10.1182/blood-2004-02-0426. [DOI] [PubMed] [Google Scholar]
- 73.Aspord C, Charles J, Leccia MT, Laurin D, Richard MJ, Chaperot L, et al. A novel cancer vaccine strategy based on HLA-A*0201 matched allogeneic plasmacytoid dendritic cells. PLoS One. 2010;5:e10458. doi: 10.1371/journal.pone.0010458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lou Y. liu C, Kim GJ, Liu Y-J, Hwu P., Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells ie induction of antigen-specific antitumour immune responses. J Immunol. 2007;178:1534–41. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- 75.Lonsdorf AS, Kuekrek H, Stern BV, Boehm BO, Lehmann PV, Tary-Lehmann M. Intratumor CpG-oligodeoxynucleotide injection induces protective antitumor T cell immunity. J Immunol. 2003;171:3941–6. doi: 10.4049/jimmunol.171.8.3941. [DOI] [PubMed] [Google Scholar]
- 76.Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies.Muñoz L, Nomdedéu JF, López O, Carnicer MJ, Bellido M, Aventín A, Brunet S. Sierra J.Haematologica. 2001;86:1261–9. [PubMed] [Google Scholar]
- 77.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–58. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 78.Cho M, Ishida K, Chen J, Ohkawa J, Chen W, Namiki S, et al. SAGE library screening reveals ILT7 as a specific plasmacytoid dendritic cell marker that regulates type I IFN production. Int Immunol. 2008;20:155–64. doi: 10.1093/intimm/dxm127. [DOI] [PubMed] [Google Scholar]
- 79.Sadhu C, Ting HJ, Lipsky B, Hensley K, Garcia-Martinez LF, Simon SI, et al. CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J Leukoc Biol. 2007;81:1395–403. doi: 10.1189/jlb.1106680. [DOI] [PubMed] [Google Scholar]