Abstract
The elevated expression of PD-1, BTLA, and other co-inhibitory molecules on T cells from cancer patients has become an accepted signature for a state called T-cell “exhaustion” that has emerged almost as dogma in the field. However, here we propose that in some cases this “exhausted” T-cell phenotype may instead be an indicator of T cells that are in a more heightened state of T-cell activation more susceptible to negative regulation rather than being “exhausted.” This alternative interpretation fits in line with the view that CD8+ T-cell activation in cancer results from a continuum of signals regulating their differentiation towards potent effector cells.
Keywords: exhaustion, cancer, PD-1, BTLA, CD8+ T cells, tumor infiltrating lymphocytes
Biomarkers are especially attractive in immunology as a way of identifying a subpopulation of cells based on their differentiation, function, or activation state. One way of describing activation, as proposed in models of viral infection (e.g., by HIV or the lymphocytic choriomeningitis virus LCMV) is the concept of “exhaustion.”1 Exhausted T cells have been defined as a pool of dysfunctional cells resulting from chronic antigen stimulation, which are unable to respond further (cell progressively lose their ability to proliferate, secrete cytokines and exert cytotoxic functions), even after resting.2 Negative costimulatory markers associated with exhaustion include: PD-1, TIM-3, LAG-3 and the recently described B- and T-lymphocyte attenuator (BTLA).2,3 Such exhaustion markers have also been utilized to describe the state of tumor-infiltrating lymphocytes (TILs) and tumor-specific T cells in the peripheral blood.4,5 However, are these cells really exhausted? Is exhaustion conceptually the way we should view these cells?
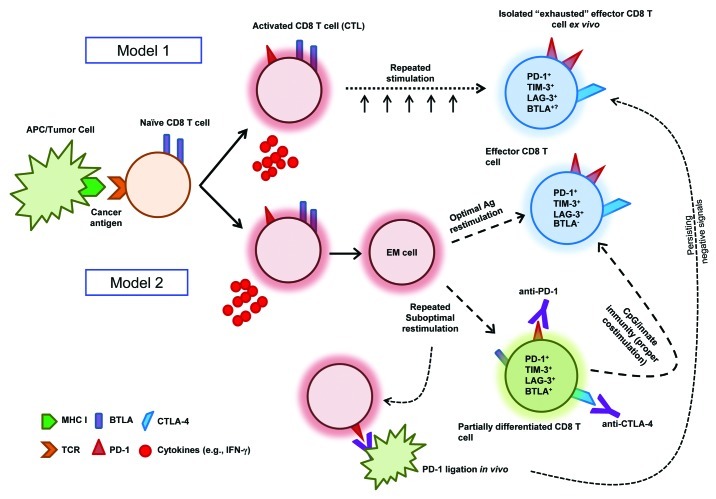
This question goes to the heart of how we view CD8+ T-cell activation and differentiation as well as of how these processes can be altered in cancer. High expression of PD-1 and other co-inhibitory molecules have been described as markers of dysfunctional and exhausted T cells following repeated antigenic stimulation, especially in cells isolated from the tumor microenvironment (Fig. 1, Model 1). However, many of these studies do not analyze additional phenotypic and functional attributes of these T cells that may be critical, especially their memory and differentiation status (i.e., the pathway toward fully differentiated cytotoxic T lymphocytes, CTLs). Furthermore, in vivo blockade of PD-1 and TIM-3 has been shown to restore antiviral and antitumor responses and T cells placed in culture ex vivo rapidly regain their full activity.6-8 In the field of tumor immunology, an alternative view of exhaustion is that of a state in which T cells are highly activated, yet incompletely differentiated, hence lacking the expected effector functions of cytokine secretion and potent CTL activity. Thus, these cells are really effector-memory T cells in a heightened state of activation resulting from repeated stimulation. The increased expression of co-inhibitory molecules could be interpreted as a normal immune regulatory mechanism preventing these cells from becoming more activated and hence potentially harmful. Recent work by Duraiswamy et al. supports this view. In an elegant gene expression analysis on sorted CD8+PD-1+/high T cells from healthy donors, they found that CD8+PD-1high T cells have a gene signature that does not overlap with that of “exhausted” T cells from HIV-infected patients, but rather with that of previously activated effector-memory cells that would normally be on their way to differentiating into effector cells.9 Thus, it is possible that PD-1high CD8+ T cells in cancer patients may not be necessarily exhausted and be simply activated effector-memory cells that persist without further differentiating; a state in which they have a lower proliferative potential and susceptible to more negative regulation by the immune system.9
Figure 1. Models of CD8+ T-cell differentiation in cancer: Balancing the “exhaustion” paradigm. Model 1 is based on viral infection, showing cytotoxic T lymphocyte (CTL) function upon activation. In this model, repeated stimulation results in terminal differentiation and exhaustion of these cells that can be observed ex vivo. Such a phenotype is marked by the expression of PD-1, TIM-3, LAG-3 and potentially BTLA. Model 2 describes an incomplete differentiation phenotype of CD8+ T cells in a tumor setting. In this case, if CTLs receive optimal antigen re-stimulation, they will downregulate BTLA and progress to terminally differentiated effector cells with a similar phenotype as described in Model 1. However, if CTLs receive suboptimal stimulation, they may only partially differentiate and retain BTLA expression, while simultaneously expressing PD-1, TIM-3, and LAG-3 and other co-inhibitory molecules. Ligation of PD-1 on these highly activated CD8+ T cells in vivo would result in a phenotype that may be mistaken as the “exhaustion” described in Model 1, due to negative signals that persist through the isolation and primary culture ex vivo. Eventually, this brake might be overcome by expanding T cells ex vivo (akin to releasing cells from anergy with IL-2) or by the use of PD-1 blocking antibodies when these cells come into contact with cells expressing PD-L1 or PD-L2 in vitro. An adjuvant such as CpG oligonucleotides (which is often given in combination with antigen-specific vaccines) could result in the activation of innate immune response and lead to proper T-cell co-stimulation, pushing them to complete their differentiation program toward CTLs while downregulating BTLA and PD-1 (these co-inhibitory molecules would become unnecessary due to the limited proliferative potential of highly differentiated CTLs).
Given these premises, it would make sense that CD8+ T cells isolated from tumors (which indeed are previously primed effector or effector-memory cells) exhibit a elevated state of activation due to repeated antigenic stimulation and/or the pro-inflammatory tumor microenvironment. These cells may be trying to differentiate into effector cells but are unable to do so due to a potential lack of proper co-stimulation or to the unfavorable cytokine milieu. Therefore, these cells would exhibit elevated levels of PD-1 expression as well as of other activation/co-inhibition markers like TIM-3 and LAG-3, but would not downregulate BTLA (which normally happens when T cells differentiate).10 This view is supported by work in melanoma TILs showing that tumor antigen-specific CD8+ T cells are enriched in the PD-1+ population.4 These seemingly “exhausted” CD8+ T cells are highly susceptible to negative regulation because they are trying to do what they are supposed to do: differentiate into effector cells. However, while the underlying reasons remain unclear, as these cells are only partially differentiated, they have lost some of their proliferative potential.
Another possible interpretation explaining the presence of seemingly exhausted CD8+ T cells in cancer is that PD-1+ cells might have recently been stimulated by PD-1 ligands (PD-L1 or PDL-2) in vivo, a scenario that appears very likely in cancer patients. This may occur frequently, especially when PD-1 becomes constitutively expressed following the hypomethylation of its locus in the genome.11 Thus, signaling via PD-1 may persist over protracted periods of time, even after cell isolation, resulting in the inhibition of T-cell activation and expansion after ex vivo re-stimulation. (Fig. 1). This can be mistakenly interpreted as T-cell exhaustion, a term implying a fundamental shift in the differentiation program of T cells, rather than an ongoing inhibitory signal that has persisted through isolation and sorting procedures. This view is supported by the fact that in many cases, the isolated T cells rapidly regain their ability to divide and perform effector functions after a few days or even a few hours in culture.
We and others have demonstrated that CD8+ TILs can be induced to proliferate ex vivo despite high levels of PD-1, BTLA, TIM-3 and other exhaustion-associated markers and that these TILs can mediate potent antitumor responses upon adoptive transfer into patients.12-14 Thus, either exhaustion does not exist in practice or it can be overcome through ex vivo expansion, much like T-cell anergy was classically shown to be overcome by IL-2-induced proliferation.15 The ability to activate and expand highly functional CD8+ TILs ex vivo for adoptive cell therapy also supports the view that negative co-stimulatory signals simply act on these T cells in vivo, curtailing their differentiation as a normal feedback mechanism “reigning in” highly activated T cells rather than being a process of “exhaustion.” Recent reports show that PD-L1 expression in melanoma is actually a positive rather than a negative prognostic indicator.16 The explanation for this paradox may be that highly activated (PD-1+, BTLA+ and TIM-3+) T cells penetrate into the tumor microenvironment releasing interferon γ (IFNγ) and other inflammatory cytokines that induce PD-L1 and PD-L2 expression in tumor cells as an “adoptive resistance” mechanism that attempt to control these highly activated TILs.17
Another case in point is a recent report on melanoma antigen (NY-ESO-1)-specific CD8+ T cells that were found to express higher levels of BTLA, PD-1 and TIM-3 than cytomegalovirus (CMV)-specific T cells from the same patient.18 In this setting, BTLA was suggested to be upregulated in tumor-specific CD8+ T cells to facilitate the induction of an exhausted phenotype. However, this interpretation is contradictory to the fact that all peripheral naïve CD8+ T cells constitutively express BTLA and, in fact, primed T cells downregulate BTLA as they differentiate toward and effector of effector-memory phenotype.19 Furthermore, in an elegant gene chip analysis of freshly-sorted MART-1-specific CD8+ T cells isolated from invaded lymph nodes by Speiser's group in Lausanne found that BTLA did not appear in an “exhaustion” gene signature using gene set enrichment analysis.5 Additional work by the Lausanne group compared the expression and function of BTLA in CD8+ T cells (from MART-1 peptide-vaccinated melanoma patients) at different stages of differentiation to virus-specific T cells at similar stages of differentiation.10 They found that BTLA was not upregulated by MART-1-specific CD8+ T cells, but rather not downregulated as a result of the normal T cell differentiation program seen in viral-specific T cells. Furthermore, when the same authors looked at another cohort of patients vaccinated with the MART-1 peptide, this time in together with CpG oligonucleotides (to activate innate immunity and stimulate Type I IFN production and maturation of dendritic cells, DCs), antigen-specific CD8+ T cells were found to often become BTLA-negative (presumably due to BTLA downregulation) and acquire more potent antitumor effector cell properties. This supports the view that primed MART-1-specific CD8+ T cells expressing BTLA from the first cohort were not exhausted but rather that CD8+ T-cell differentiation was defective. Thus, the co-expression of BTLA and PD-1 may simply mark highly activated T cells not proceeding through their normal course of differentiation in cancer patients. However, as implicated by the results of Speiser’s group, manipulating the system with activators of the innate immune system (e.g., CpG) may restore the CD8+ T-cell differentiation program (Fig. 1; Model 2).
Thus, CD8+ T cells from tumors and the periphery expressing PD-1, BTLA and other co-inhibitory molecules can be viewed as highly activated T cells that can be modulated through immunotherapy, and not as “exhausted” cells. This concept proposes “the antitumor immunity glass” as half-full, not half-empty. The decreased ex vivo proliferative capacity of these cells is arguably a normal consequence of an incomplete differentiation toward effector cells and not of an actual exhaustion. Therefore, it may be counterproductive to remove these supposedly exhausted cells as a means of improving antitumor immunity. These cells are simply doing what they have evolved to do in these settings: not indiscriminately proliferate, but balance proliferation with a differentiation program into effector CTLs that includes curtailing cell division rates. These cells may be the key antitumor effector-memory cells to target for improving anticancer immunotherapy. This concept is critical for the development of new and more refined immunotherapeutic combinations.14,20
In summary, we suggest that—as tumor immunologists—we keep an open mind towards our conceptual view of T-cell exhaustion when describing tumor antigen-specific T cells isolated from cancer patients expressing PD-1, BTLA, and other co-inhibitory molecules toward a view that keeps options open as to the true nature of these cells. More work needs to be performed to clearly define how T cells (especially CD8+ CTLs) normally differentiate (or don’t differentiate) in cancer patients. This will elucidate whether the supposedly exhausted phenotype that many are describing indeed represents a state in which T cells exhibit a high activation state, are more prone to negative regulation and have not completely differentiated (Fig. 1). We surmise that blocking PD-1 and other inhibitory molecules expressed on these so-called “exhausted,” previously tumor antigen-primed, CD8+ T cells, or activating innate immune signaling (e.g., with CpG or other TLR agonists), might rapidly unlock this blocked CTL differentiation pathway in cancer patients.
Grant Support
This work was supported by National Cancer Institute (NCI) grant R01-CA111999 to L.R., by The University of Texas MD Anderson Cancer Center Support Grant (P30-CA16672), and Award No. TL1RR024147 from the National Center for Research Resources to R.W. We also acknowledge support from a Melanoma SPORE Developmental Grant (P50-CA093459-05-DRP21) and a Team Science Award from the Melanoma Research Alliance (MRA) to L.R. Support from the Dr Miriam and Sheldon Adelson Medical Research Foundation (AMRF) and the Mulva Foundation are also greatly appreciated.
Glossary
Abbreviations:
- CMV
cytomegalovirus
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- LCMV
lymphocytic choriomeningitis virus
- PBMC
peripheral blood mononuclear cell
- TIL
tumor-infiltrating lymphocyte
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20823
References
- 1.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngblood B, Wherry EJ, Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS. 2012;7:50–7. doi: 10.1097/COH.0b013e32834ddcf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelosanto JM, Wherry EJ. Transcription factor regulation of CD8+ T-cell memory and exhaustion. Immunol Rev. 2010;236:167–75. doi: 10.1111/j.1600-065X.2010.00927.x. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8⁺ T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–60. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–30. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186:4200–12. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derré L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–67. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35:400–12. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu RFM-A, Forget M-A, Chacon J, Bernatchez C, Haymaker C, Chen JQ, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18:160–75. doi: 10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 16.Krönig H, Julia Falchner K, Odendahl M, Brackertz B, Conrad H, Muck D, et al. PD-1 expression on Melan-A-reactive T cells increases during progression to metastatic disease. Int J Cancer. 2012;130:2327–36. doi: 10.1002/ijc.26272. [DOI] [PubMed] [Google Scholar]
- 17.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37. [DOI] [PMC free article] [PubMed]
- 18.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–96. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7:e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakthivel P, Gereke M, Bruder D. Therapeutic intervention in cancer and chronic viral infections: antibody mediated manipulation of PD-1/PD-L1 interaction. Rev Recent Clin Trials. 2012;7:10–23. doi: 10.2174/157488712799363262. [DOI] [PubMed] [Google Scholar]