Abstract
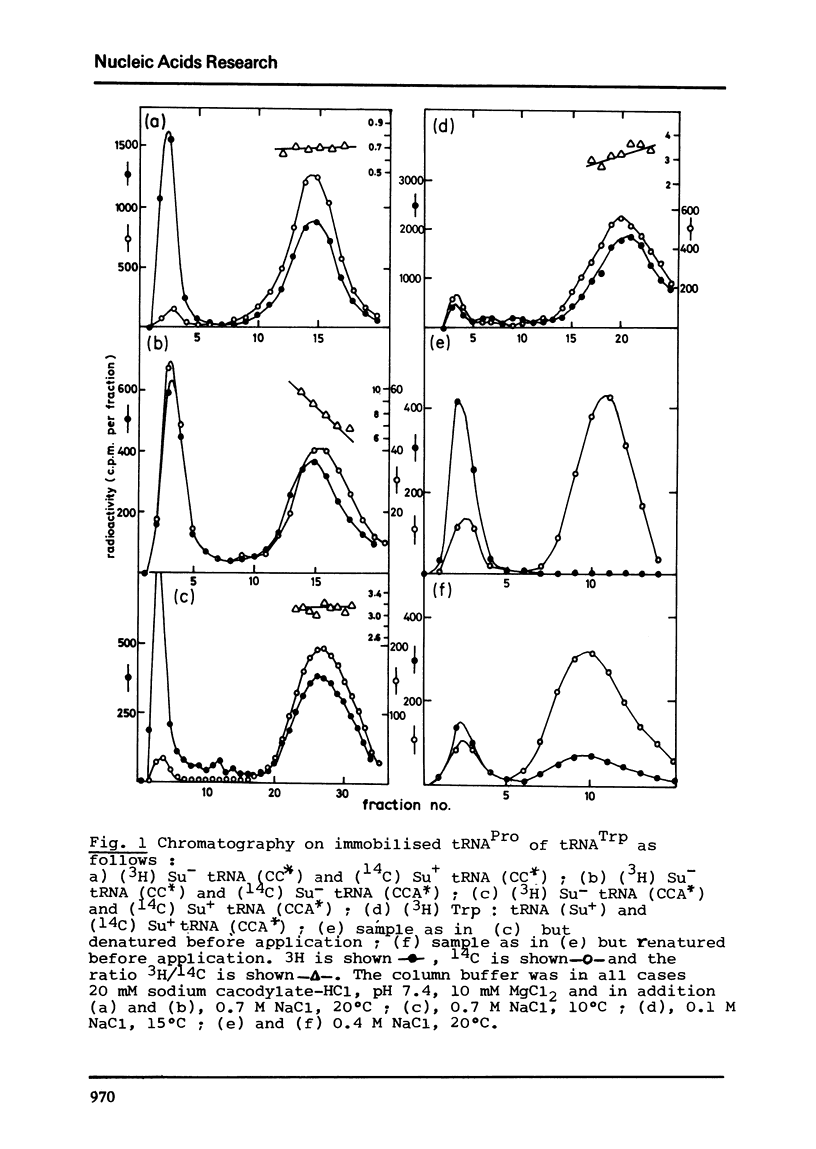
The association between Trp-tRNA and Pro-tRNA, which have complementary anticodon sequences, has been used as a probe of anticodon conformation. It is unaffected, however, by the base change in the D-stem present in UGA-suppressor Trp-tRNA. This does not support the hypothesis that UGA suppression depends upon a conformational change induced in the anticodon. The stable denatured form of wild-type Trp-tRNA no longer interacts with Pro-tRNA; the structure of the anticodon region must therefore be quite different in the denatured form.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckingham R. H., Danchin A. Fluorescence of tryptophanyl-tRNA(Trp) from E. coli; An interaction between the indole and tRNA and its dependence on tRNA conformation. FEBS Lett. 1973 Mar 1;30(2):236–238. doi: 10.1016/0014-5793(73)80659-3. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H., Danchin A., Grunberg-Manago M. Denaturation of UGA suppressor tRNA-Trp from E. coli. Biochem Biophys Res Commun. 1974 Jan;56(1):1–8. doi: 10.1016/s0006-291x(74)80307-4. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H., Danchin A., Grunberg-Manago M. The effect of an intramolecular cross-link on reversible denaturation in tryptophan transfer ribonucleic acid from Escherichia coli. Biochemistry. 1973 Dec 18;12(26):5393–5399. doi: 10.1021/bi00750a023. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Blumberg W. E. Binding constants from zone transport of interacting molecules. Biochemistry. 1973 Sep 11;12(19):3648–3662. doi: 10.1021/bi00743a013. [DOI] [PubMed] [Google Scholar]
- Eisinger J. Complex formation between transfer RNA'S with complementary anticodons. Biochem Biophys Res Commun. 1971 May 21;43(4):854–861. doi: 10.1016/0006-291x(71)90695-4. [DOI] [PubMed] [Google Scholar]
- Favre A., Buchingham R., Thomas G. tRNA tertiary structure in solution as probed by the photochemically induced 8-13 cross-link. Nucleic Acids Res. 1975 Aug;2(8):1421–1431. doi: 10.1093/nar/2.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartland W. J., Sueoka N. Two interconvertible forms of tryptophanyl sRNA in E. coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):948–956. doi: 10.1073/pnas.55.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J. N., Landy A., Zadrazil S., Smith J. D. The nucleotide sequences of tyrosine transfer RNAs of Escherichia coli. Eur J Biochem. 1970 Apr;13(3):461–483. doi: 10.1111/j.1432-1033.1970.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Takada C., Petre J. Complex formation between transfer RNAs with complementary anticodons: use of matrix bound tRNA. Biochem Biophys Res Commun. 1973 Aug 6;53(3):882–893. doi: 10.1016/0006-291x(73)90175-7. [DOI] [PubMed] [Google Scholar]
- Hall R. H. N6-(delta 2-isopentenyl)adenosine: chemical reactions, biosynthesis, metabolism, and significance to the structure and function of tRNA. Prog Nucleic Acid Res Mol Biol. 1970;10:57–86. doi: 10.1016/s0079-6603(08)60561-9. [DOI] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Högenauer G. Binding of UGA to wild type and suppressor tryptophan tRNA from E. coli. FEBS Lett. 1974 Mar 1;39(3):310–312. doi: 10.1016/0014-5793(74)80137-7. [DOI] [PubMed] [Google Scholar]
- Ishida T., Sueoka N. Effect of ambient conditions on conformations of tryptophan transfer ribonucleic acid of Escherichia coli. J Biol Chem. 1968 Oct 25;243(20):5329–5336. [PubMed] [Google Scholar]
- Ishikura H., Yamada Y., Nishimura S. The nucleotide sequence of a serine tRNA from Escherichia coli. FEBS Lett. 1971 Jul 15;16(1):68–70. doi: 10.1016/0014-5793(71)80688-9. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Rigler R., Ehrenberg M., Blomberg C. Allosteric mechanism for codon-dependent tRNA selection on ribosomes. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4248–4251. doi: 10.1073/pnas.72.11.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Hirst-Bruns M. E., Philipps G. R. Action of venom phosphodiesterase on transfer RNA from Escherichia coli. Biochim Biophys Acta. 1970 Sep 17;217(1):176–188. doi: 10.1016/0005-2787(70)90134-6. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Ofengand J. Assay for AA-tRNA recognition by the EFTu-GTP complex of Escherichia coli. Methods Enzymol. 1974;29:661–667. doi: 10.1016/0076-6879(74)29057-8. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Lührmann R., Gassen H. G. On the mRNA induced conformational change of AA-tRNA exposing the T-pse-C-G sequence for binding to the 50S ribosomal subunit. Biochem Biophys Res Commun. 1974 Feb 4;56(3):807–814. doi: 10.1016/0006-291x(74)90677-9. [DOI] [PubMed] [Google Scholar]
- Strigini P., Brickman E. Analysis of specific misreading in Escherichia coli. J Mol Biol. 1973 Apr 25;75(4):659–672. doi: 10.1016/0022-2836(73)90299-4. [DOI] [PubMed] [Google Scholar]
- Takasaki Y., Imahori K. CD and fluorescence studies of tRNAPhe from baker's yeast. J Biochem. 1973 Sep;74(3):513–517. doi: 10.1093/oxfordjournals.jbchem.a130271. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Imahori K. The conformation difference between tRNA Met f and formylmethionyl-tRNA Met f from E. coli. Biochem Biophys Res Commun. 1971 Oct 15;45(2):488–494. doi: 10.1016/0006-291x(71)90845-x. [DOI] [PubMed] [Google Scholar]



