Abstract
Glycosylation is a key process impacting on many aspects of cellular interactions. We recently reported that a miRNA cluster controls glycosylation by directly targeting N-acetylgalactosamine transferases (GALNTs), resulting in increased tumor invasion and immunosuppression. Here we further discuss how defective glycosylation or GALNTs dysregulation may contribute to tumor progression
Keywords: miR-30d, GALNT, GalNAc-Ts, glycosylation, immunosuppression, miRNA, melanoma, metastasis
Our group has recently reported that the upregulation of the miR30b/30d cluster contributes to melanoma metastasis by enhancing cellular invasion and suppressing the host immune response.1 We showed that many of these effects are critically mediated by direct suppression of N-acetylgalactosamine transferases (GALNTs), enzymes responsible for initiating the cascade of mucin-type O-linked glycosylation. In particular, we demonstrated that GALNT7 silencing mimics the prometastatic effects of miR30b/30d overexpression. We also showed that glycosylation changes in the membrane of melanoma cells associated with miR-30d ectopic expression, strongly overlap with siGALNT7-induced glycomic profiles.
Our work, therefore, unravels an unexpected link between invasion and immune evasion, in which glycosylation plays a central role. This study raises numerous intriguing questions.
How does Defective Glycosylation Contribute to Tumor Progression?
The majority of cell-surface proteins are glycosylated. These post-translational modifications are essential for proper function of proteins and lipids. Alterations in cell-surface glycan structures have long been known to have a direct role in cancer etiology.2 Our work links tumor cell-surface glycans with the extracellular matrix and the immune system. We showed that miR-30d extensively controls the glycosylation pattern of transmembranal proteins in a GALNT7 silencing-dependent manner. We hypothesized that this aberrant glycosylation may affect key processes that support tumor progression and metastasis such as cell adhesion, motility, invasion and immune evasion (Fig. 1). This idea is supported by studies demonstrating that altered glycosylation may affect adhesion-motility equilibrium by impacting on the function of integrins such as α5β1 or α3β1, or influence cell-cell homotypic adhesion by modifying molecules, such as E-cadherin.3 An additional example is the aberrant O-glycosylation of MUC1 that facilitates attachment of cancer cells to normal lung tissue, which may support metastatic dissemination.4 Interestingly, it was also demonstrated how altered glycosylation patterns of key immune-related molecules such as MHC Class I and II on cancer cells influence peptide selection and/or recognition by T cells and therefore may contribute to tumor immune escape in the immediate microenvironment.5 Other studies have supported the idea that GALNTs dysregulation may alter the immune response or cell-cell/ECM contacts. For instance, inactivating mutations in GALNT12 associate with colon cancer,6 and mice deficient in Galnt1 display reduced lymphocyte homing and humoral immunity.7
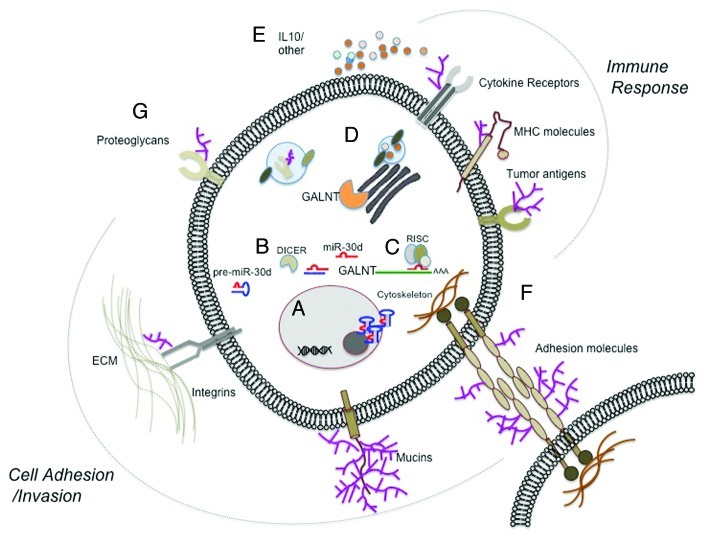
Figure 1. miR-30d overexpression and its targeting of UDP-GalNAc Transferases (GALNTs) lead to increased cell invasion and immunosuppression possibly by altering glycosylation patterns. Below are the sequential steps of this process: (A) Pre-miR30d is transcribed and processed in the nucleus and is transported to the cytoplasm. (B) pre-miR-30d is further processed by DICER and the mature form (miR-30d) is loaded into the RISC complex. (C) miR-30d suppresses GALNT translation and targets its mRNA for degradation. (D) GALNT levels in the Golgi are reduced and potentially lead to aberrant glycosylation of substrates and/or disrupted exocytosis of proteins and other membrane-bound proteoglycans. (E–G) Aberrant glycosylation may then affect both, the immune response (E) and the cellular interactions with the ECM or neighboring cells (F), thus cooperating for tumor progression. Additional effects on other receptors may lead to other metastasis-promoting processes (G).
Clinical cancer diagnostic markers are often glycoproteins, but most current diagnostic tests only measure the expression of the polypeptide. Clearly, given the long known alterations in glycans associated with cancer, it is highly likely that cancer markers that detect specific glycoforms of a protein will have much higher sensitivity and specificity for an accurate detection of cancer8 or to predict patient prognosis. In support of this approach, a fucosylated α-fetoprotein is now used as a diagnostic marker of primary hepatocarcinoma9 and fucosylated haptoglobin may be a better marker of pancreatic cancer than the haptoglobin polypeptide.10 Our findings imply the therapeutic benefit of detecting glycosylation changes in the tumor. It is highly plausible that specific glycosylation patterns associate with tumor aggressiveness and may potentially be targeted for better, more specific therapies.
How do GALNTs (and miR-30d) Actually Regulate IL10?
We found that GALNT7 silencing recapitulates miR-30b/30d’s ability to induce IL10 synthesis and secretion. However, the specific mechanisms involved remain unknown. Interestingly, in silico analyses predicted miR30b/30d binding sites in the 3′UTR of several suppressors of cytokine signaling (e.g., SOCS1 and SOCS3), inhibitors of the JAK/STAT signaling pathway, which is known to regulate IL10 transcription and was found activated in response to miR-30d upregulation. However, reporter assays failed to show direct or indirect regulation of miR-30d on these proteins. Alternative explanations may include defective glycosylation of specific GALNT7 substrates or that the reduced expression of GALNTs is altering cytokines trafficking from the Golgi.
How does IL10 Contribute to miR-30b/30d-induced Immune Suppression?
Using an in vivo model, we have shown that miR-30d overexpression associates with reduced recruitment of CD3+ cells but more FoxP3+ cells to the site of metastasis. Ex vivo, supernatants of miR-30d-transduced melanoma cells suppressed the expansion of CD4+ T-cells, mainly by inhibiting their proliferation and activation.
However, the specific silencing of IL10 incompletely rescued the suppressive effects of miR-30d on T cell activation, suggesting that other direct or indirect targets of miR-30d may exert immunomodulatory effects.
Which other Mechanisms may Contribute to miR-30d’s Immunesuppressive Behavior?
Gene expression profiling of melanoma cells transduced in vitro with miR-30d or immunohistochemical analysis of patients samples with high miR-30d levels revealed significant changes in other immune-related molecules such as CTLA4 and FOXP3. This is a surprising finding since these molecules are typically expressed by immune cells. Although their contribution to miR-30d’s effects needs to be further investigated, it is possible that the expression of these factors in cancer cells may enable non-immune cells to exert immunosuppressive effects in the metastatic site. This finding aligns with recent evidence supporting that tumors may behave as immune-like organs.
In sum, the impact of altered glycosylation on many aspects of metastatic dissemination makes this process and its regulation by miRNAs (and possibly other factors) an attractive avenue to further decipher tumor progression.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19535
References
- 1.Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20:104–18. doi: 10.1016/j.ccr.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143:672–6. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rambaruth ND, Dwek MV. Cell surface glycan-lectin interactions in tumor metastasis. Acta Histochem. 2011;113:591–600. doi: 10.1016/j.acthis.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clin Exp Metastasis. 2002;19:339–45. doi: 10.1023/A:1015590515957. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195:346–55. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 6.Guda K, Moinova H, He J, Jamison O, Ravi L, Natale L, et al. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc Natl Acad Sci U S A. 2009;106:12921–5. doi: 10.1073/pnas.0901454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenno M, Ohtsubo K, Hagen FK, Ditto D, Zarbock A, Schaerli P, et al. Initiation of protein O glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity. Mol Cell Biol. 2007;27:8783–96. doi: 10.1128/MCB.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taniguchi N. Toward cancer biomarker discovery using the glycomics approach. Proteomics. 2008;8:3205–8. doi: 10.1002/pmic.200890056. [DOI] [PubMed] [Google Scholar]
- 9.Oka H, Saito A, Ito K, Kumada T, Satomura S, Kasugai H, et al. Collaborative Hepato-Oncology Study Group of Japan Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378–83. doi: 10.1046/j.1440-1746.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 10.Miyoshi E, Nakano M. Fucosylated haptoglobin is a novel marker for pancreatic cancer: detailed analyses of oligosaccharide structures. Proteomics. 2008;8:3257–62. doi: 10.1002/pmic.200800046. [DOI] [PubMed] [Google Scholar]