Abstract
Using in vivo models of pancreatic ductal adenocarcinoma (PDAC), we demonstrated that mast cells migrate to the tumor site and provide a microenvironment that allows for tumor progression. These results indicate that targeting mast cells may be a promising novel therapy for PDAC.
Keywords: cell, pancreatic cancer, tumor microenvironment, inflammation, therapy target
Mast cells regulate adaptive immune responses via the release of cytokines and other immunomodulatory factors.1-3 These factors can promote immune suppression and may contribute to tumor progression progression. The important roles of mast cells have been reported in many human malignancies.4-8 However, the role of mast cells in human PDAC remains obscure. We hypothesized that mast cells in the tumor microenvironment are essential for PDAC tumorigenesis.
To test this hypothesis, we employed several in vivo animal models. First, we used a transgenic K-rasG12V spontaneous PDAC mouse model9 and observed an early influx of mast cells to the tumor microenvironment, which suggests that mast cells in the tumor microenvironment are essential for PDAC tumorigenesis. We then investigated the contribution of mast cells to PDAC tumorigenesis by using a mast cell-deficient mouse model (Kitw-sh/w-sh).10 We further assessed the clinical relevance by correlating mast cell infiltration with the survival of patients with PDAC. Our results indicate that mast cells play a key role in PDAC tumorigenesis and present a novel therapeutic target.
To measure mast cell influx during the development of PDAC, we utilized a transgenic K-rasG12V mouse model that developed CP, PanINs, and invasive PDAC.4 Pancreatic tissue was obtained at various stages and grouped by pathologic results: NP (n = 9) CP (n = 9), PanIN (n = 9), and PDAC (n = 4). Pancreatic tissues (n = 4) obtained from WT littermates served as controls. We determined when and how much mast cells infiltrate the PDAC tumor microenvironment. The influx of mast cells in CP persisted through the development of PanIN and PDAC. There was no significant difference in mast cell scores between CP and different stages (i.e., I-III) of PanIN and PDAC. While mast cells were evenly distributed in CP and PanIN lesions, they accumulated at the infiltrating edges of the tumor. Our results showed that mast cell infiltration was an early event and that mouse mast cells distributed into CP and mouse PanIN lesions but were rarely found in NP tissues. During the development of PDAC, mast cells accumulate to the tumor microenvironment. Using qRT-PCR analysis, we noted that during the progression from NP to CP to PanIN to PDAC, many cytokines, particularly mast cell-related cytokines and receptors (e.g., CXCL5, IL-8rb, IL1β, and tumor necrosis factor) were consistently upregulated in the microenvironment during PDAC development in K-rasG12V mice. These results suggest that mast cells migrate into the tumor microenvironment and may play a critical role in PDAC development.
To determine the contribution of mast cells to the tumorigenesis of PDAC, we compared tumor growth in mast cell-deficient Kitw-sh/w-sh mice (Kit−/− mice) and syngeneic C56BL/6 mice (WT mice). When Panc-02 PDAC cells, a tumorigenic murine PDAC cell line derived from a methylcholanthrene-induced tumor growing in a male C57BL/6 mouse, were orthotopically implanted in the pancreas, the growth of tumor was significantly suppressed in mast cell-deficient Kitw-sh/w-sh mice compared with syngeneic C56BL/6 mice; only 20% of Kit−/− mice had measurable tumors 28 days after implantation compared with 100% of WT mice (p = 0.033). Moreover, the Kit−/− mice lived significantly longer than the WT mice. Mast cells were found in the tumors of WT C57BL/6, and as expected, no mast cells were found in the pancreases of Kit−/− mice. Similar results were observed in a subcutaneous model with Panc-02 cells.
To further confirm the hypothesis that mast cells promote PDAC tumorigenesis, we employed a mast cell reconstitution mouse model. Bone marrow-derived mast cells from WT C56BL/6 mice were injected into mast cell-deficient Kit−/− mice and repopulated in the pancreatic tissues. Tumor growth was significantly increased in the Kit−/− mice repopulated with mast cells compared with the parental Kit−/− mice (p = 0.009). Mast cell reconstitution also increased the incidence of hemorrhagic ascites to 50%. The repopulation of mast cells was confirmed by both hematoxylin and eosin and toluidine blue staining. These data support the critical role of mast cells in PDAC progression.
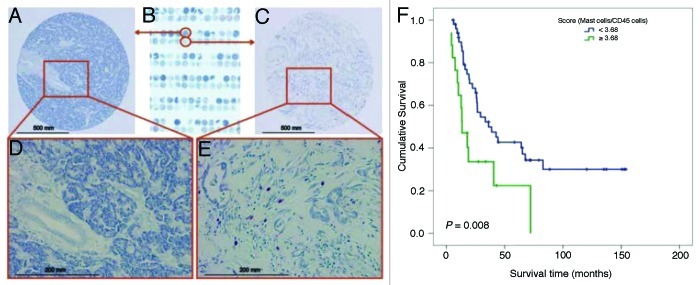
To determine the clinical relevance of mast cell influx in PDAC, we stained 67 pancreaticoduodenectomy specimens from a previously constructed tissue microarray with toluidine blue and counted the mast cells (Fig. 1A-E). Patients with mast cell scores < 3.68 survived significantly longer (median overall survival duration, 36.2 ± 9.4 mo) than did patients with mast cell scores ≥ 3.68 (median overall survival duration, 13.4 ± 3.4 mo; p = 0.008, high vs. low mast cell infiltration) (Fig. 1F). In addition, the incidence of recurrence was lower in patients with mast cell scores < 3.68 (67%) than in patients with mast cell scores ≥ 3.68 (100%; p = 0.003, Fisher’s exact test.)
Figure 1. Association of mast cell infiltration with survival of patients with PDAC. No mast cells were found in NP tissues. (A) 100x and (D) 400x. (B) A human PDAC tissue microarray was stained with toluidine blue. Mast cells were found in the human PDAC tumor microenvironment: (C) 100x and (E) 400x. Mast cells stained red-purple (metachromatic staining), and the background was blue (orthochromatic staining). (F) The mast cell score was correlated with survival in patients with PDAC. The mast cell score was normalized as the ratio of the number of mast cells to the percentage of pan-leukocytes (CD45-positive cells). The cutoff point of the mast cell score was set at 3.68. Upper curve, patients with mast cell scores < 3.68; lower curve, patients with mast cell scores ≥ 3.68. The p value was derived using the log-rank statistic.
In summary, we demonstrated that mast cells play a role in the development and progression of PDAC, a deadly disease with limited treatment options. We found (1) an early influx of mast cells in K-ras mutation-driven spontaneous PDAC, which mimics human PDAC, (2) the necessity of mast cells in vivo for PDAC tumor growth and (3) the clinical relevance of mast cells in PDAC. These findings indicate that mast cells are essential for PDAC progression and present a potential therapeutic target.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19612
References
- 1.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 3.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–23. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimbaldeston MA, Pearce AL, Robertson BO, Coventry BJ, Marshman G, Finlay-Jones JJ, et al. Association between melanoma and dermal mast cell prevalence in sun-unexposed skin. Br J Dermatol. 2004;150:895–903. doi: 10.1111/j.1365-2133.2004.05966.x. [DOI] [PubMed] [Google Scholar]
- 5.Grimbaldeston MA, Skov L, Baadsgaard O, Skov BG, Marshman G, Finlay-Jones JJ, et al. Communications: high dermal mast cell prevalence is a predisposing factor for basal cell carcinoma in humans. J Invest Dermatol. 2000;115:317–20. doi: 10.1046/j.1523-1747.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 6.Beer TW, Ng LB, Murray K. Mast cells have prognostic value in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:27–30. doi: 10.1097/DAD.0b013e31815c932a. [DOI] [PubMed] [Google Scholar]
- 7.Ribatti D, Crivellato E. The controversial role of mast cells in tumor growth. Int Rev Cell Mol Biol. 2009;275:89–131. doi: 10.1016/S1937-6448(09)75004-X. [DOI] [PubMed] [Google Scholar]
- 8.Galinsky DS, Nechushtan H. Mast cells and cancer--no longer just basic science. Crit Rev Oncol Hematol. 2008;68:115–30. doi: 10.1016/j.critrevonc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Ji B, Tsou L, Wang H, Gaiser S, Chang DZ, Daniluk J, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–82, 1082, e1-6. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]