Abstract
PGE2 is the key factor needed for MDSCs development, accumulation and functional stability. PGE2 initiates an EP2/EP4-mediated positive feedback between COX2 and PGE2 in monocytic precursors, redirecting dendritic cell differentiation to MDSCs. COX2- or EP2/EP4- blockade abrogates MDSC functions and their CXCR4-CXCL12-mediated attraction to cancer environment, providing convenient immunotherapeutic targets.
Keywords: COX2, PGE2, human, cancer, myeloid-derived suppressor cells, immune dysfunction, immunotherapy, transplantation
Myeloid-derived suppressor cells (MDSCs)1 are critical mediators of tumor-induced immune dysfunction and cancer progression.2 MDSCs represent a heterogeneous population of immature myeloid cells (iMC) involving precursors of macrophages, granulocytes, and dendritic cells (DC) capable of immunosuppression,1,3 and using diverse suppressive factors, including indoleamine 2,3-dioxigenase (IDO1), IL-10, arginase 1 (ARG1), inducible nitric oxide synthase (iNOS, NOS2), nitric oxide (NO), and reactive oxygen species (ROS), to suppress immune responses at the tumor sites.1
In analogy to the heterogeneous mechanism of MDSC function, the induction of MDSC can be triggered by multiple factors with nominally-opposing functions, including interleukin-1β (IL-1β), IL-6, IL-10, TLR-ligands, macrophage colony stimulating factor (M-CSF) and vascular endothelial growth factor (VEGF), or prostaglandin E2 (PGE2).1 PGE2, a ubiquitous cancer-associated inflammatory mediator produced by cancer cells, stroma, and infiltrating myeloid cells (reviewed in ref. 4), was previously shown to prevent the development of functional DCs in the human system,5 and to promote MDSC accumulation in cancer-bearing mice.6
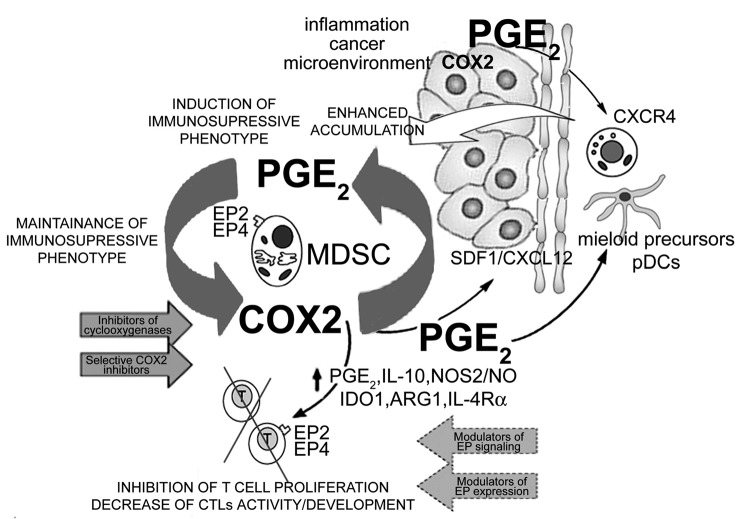
Our two recent reports7,8 demonstrate that PGE2 is both required and sufficient to redirect the differentiation of human dendritic cells into monocytic MDSCs (see Figure 1). It also mediates the induction of MDSC-associated suppressive factors by additional MDSC-inducing stimuli, in a mechanism involving the establishment of a positive feedback loop between PGE2 and cyclooxygenase (COX)-27, the key regulator of PGE2 production.4
Figure 1. Positive COX2-PGE2-EP2/EP4-mediated feedback loop in the biology of cancer-associated MDSCs. Inflammation (IL-1β, TLR ligands, IFN-γ) and/or cancer-produced PGE2 or PGE2 inducers drive the early induction of COX2 in local myeloid cells (monocytes, macrophages, immature DCs), promoting their production of suppressive factors (IDO1, IL-10, ARG1, NOS2 and PGE2 itself), and acquisition of suppressive functions. The EP2- and EP4-dependent signals are also critical in the induction and persistence of functional CXCR4 on monocytic cells and for the production of CXCL12/SDF-1 in cancer environment. These processes are further amplified by the de novo-produced endogenous PGE2, now produced at high levels by MDSCs themselves, thereby creating a positive feedback loop, leading to accumulation of MDSCs in cancer environment. In addition to inducing other suppressive factors, PGE2 also directly suppresses CTL development and functions, acting via EP2 and EP4 receptors. The key role of the EP2- and EP4-mediated COX2-PGE2 feedback to control multiple aspects of MDSC function provides for convenient targets to control MDSC-associated immune dysfunction in cancer immunotherapy.
We observed7 that the frequencies of MDSCs in peritoneal ascites from ovarian cancer patients strongly correlate with local expression of COX2 and production of PGE2. The ability of cancer microenvironment to induce MDSCs phenotype and functions in differentiating myeloid precursors (monocytes) ex vivo is abolished by inhibitors of cyclooxygenases (such as indomethacin) or selective inhibitors of COX2. Moreover, the presence of synthetic PGE2 (or agonists of the two PGE2 receptors, EP2 or EP4) during the GM-CSF- and IL-4-driven monocyte differentiation is sufficient to redirect the development of DCs into CD1a-CD14+CD80-CD83- MDSC that produce IL-10, IDO1, ARG1, NOS2 and IL-4Rα, and suppress the proliferation and development of CD8+ T cells into granzyme B (GrB)high CTLs.7
PGE2 (and other diverse MDSC-inducing factors, such as IL-1β, IFNγ or LPS) induce high levels of COX2 in differentiating MDSCs, initiating a positive feedback loop, enhancing the production of endogenous PGE2 and stabilizing the suppressive functions of MDSCs.7 Importantly from the therapeutic standpoint, the positive feedback between active COX2 and autocrine production of endogenous PGE2 proved to be essential for the MDSC stability. Even short-term (overnight) treatment of the fully-developed MDSCs isolated from cancer patients using COX2 inhibitors or EP2 and EP4 antagonists abrogated endogenous production of PGE2, suppressed the expression of IL-10, IDO1, NOS2 and endogenous COX2, and reversed the CTL-suppressive functions of cancer-isolated MDSCs.7
Cancer-associated MDSCs uniformly expressed high levels of CXCR4,8 known to be present on blood MDSCs in cancer-bearing individuals,9 and showed strong migratory responsiveness to CXCL12.8 While cancer environment induced high levels of CXCR4 on differentiating MDSCs, the CXCR4 expression and responsiveness of the ascites-isolated MDSCs to CXCL12 (recombinant or present in cancer ascites) were blocked by their pre-treatment with COX2 inhibitors.
Apart from driving the development of CXCR4+ MDSCs and promoting their stability, PGE2 proved to be the key factor promoting the production of CXCL12/SDF-1 (CXCR4 ligand) in cancer microenvironment. The concentrations of CXCL12 in cancer ascites samples were strongly correlated with the local production of PGE2, while the ability of the ascites cells to produce CXCL12 and attract CXCR4+ MDSCs was blocked by COX2 inhibition.8
The dependence of human cancer-associated MDSCs on undisturbed COX2 activity helps to circumvent the obstacles to effective targeting the MDSC-associated immuno-suppression imposed by their multi-factorial origin and multiple mechanisms of suppressive function,1 helping to design effective immune therapies of advanced ovarian cancer and potentially other malignancies. Our current data also facilitates the generation of large numbers of MDSCs for the immunotherapy of autoimmune, and inflammatory diseases, or transplant rejection. The current data and the previously-documented role of PGE2 in the induction, recruitment and functions of Tregs, and its inhibitory impact on the development, attraction, and functions of type-1 immune cells (CTLs, Th1 and NK cells; reviewed in ref. 4), suggest that PGE2-targeting is an important element of effective cancer immunotherapies.
Glossary
Abbreviations:
- ARG1
arginase 1
- COX
cyclooxygenase
- CXCR4
C-X-C chemokine receptor type 4
- CXCL12
C-X-C chemokine receptor ligand 12 (also SDF-1)
- CTL
cytotoxic lymphocyte
- DC
dendritic cell
- EP
prostanoid receptor, E-series of prostaglandin receptor
- GrB
granzyme B
- iMC
immature myeloid cells
- IDO
indoleamine 2,3-dioxigenase
- iNOS
inducible nitric oxide synthase (also NOS2)
- IL
interleukin
- MCSF
macrophage colony stimulating factor
- MDSC
myeloid-derived suppressor cells
- NK
natural killer
- NO
nitric oxide
- PGE2
prostaglandin E2
- Th
T helper
- VEGF
vascular endothelial growth factor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19681
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–53. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 4.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 6.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 7.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, et al. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130:1109–19. doi: 10.1002/ijc.26123. [DOI] [PubMed] [Google Scholar]