Abstract
Chimeric antigen receptors (CARs) possess fixed specificity for a single antigen and require empirical testing in T cells. To address this, we have developed a novel, adaptable immune receptor strategy that allows for the rapid generation and testing of T cells of nearly infinite antigen specificity.
Keywords: universal, T cell, biotin, chimeric, immune, receptor, avidin, adoptive, immunotherapy, platform
Adoptive cell transfer (ACT) therapy using bioengineered T cells continues to show significant promise in the treatment of cancer. To this end, investigators at academic and government centers have tested the concept of chimeric antigen receptors (CARs) in advanced cancer. A CAR is a single unit immune receptor of fixed specificity generally comprised of an extracellular antigen-specific antibody fragment coupled to intracellular T cell-signaling domains.1 In recent trials, dramatic eradication of refractory chronic lymphocytic leukemia, where all tumor cells express CD19, was achieved by CD19-specific CAR T cell therapy, where all tumor cells express CD19.2,3 Despite these encouraging results, significant challenges still exist to widespread CAR application. For instance, other tumors are often heterogeneous in antigen expression, differing among individuals, but also in the same patient. Additionally, cancer cells can lose antigen expression by a process of immune-editing, contributing to tumor relapse following initially-effective specific therapy. Targeting a single antigen with CAR therapy may accordingly result in initial tumor regression, but ultimately select for the outgrowth of antigen-loss variants. To facilitate broad clinical application of CARs, scientists have proposed the establishment of a panel of bioengineered T cells with different specificities, custom-made for each individual.4 Here, each new CAR must be individually created, empirically-tested and produced under clinical-grade conditions; a process that is both technically and economically challenging. The creation of a standardized, distributable immune receptor platform that can be easily tailored for specific antigen-targeting and is amenable to rapid preclinical screening and clinical application would markedly increase accessibility of ACT therapy.
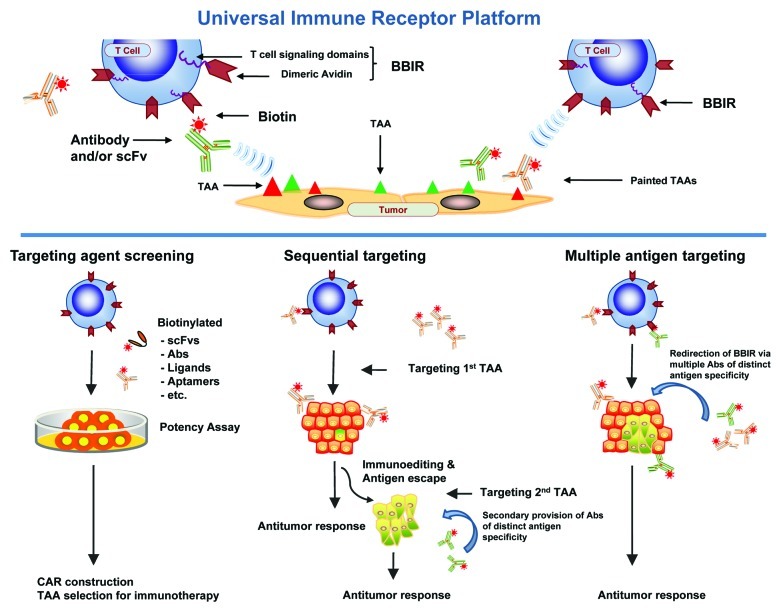
In our recent study, a technological strategy was designed to overcome restrictions of current gene-engineered cellular therapy which is restricted in antigen specificity, patient accessibility, and tumor type.5 Here, we outfitted primary human T cells with a universal immune receptor redirected against biotinylated antigen-specific molecules (biotin binding immune receptor; BBIR). BBIR T cells specifically recognized and were activated by various biotinylated molecules, including scFvs and antibodies, that were either immobilized on a plate, specifically bound to immobilized antigen or bound to antigen-expressing tumor cells (Fig. 1, upper). Redirection of BBIR T cells against protein antigens was dependent upon intermediate interaction with bound biotinylated antigen-binding molecules; non-binding biotinylated molecules had no effect. Importantly, addition of soluble biotin to cultures at physiological levels found in human serum had no inhibitory effect on the specific immunoactivation of BBIR T cells. Furthermore, soluble biotin alone did not cause antigen-independent activation of BBIRs, indicating the need for immobilization and BBIR cross-linking.
Figure 1. Schematic of the universal immune receptor platform. (Upper) Schematic of biotin binding immunoreceptor (BBIR) comprised of a dimeric form of chicken avidin protein fused to the T cell signaling domains interacting with a biotinylated tumor associated antigen (TAA) specific molecule. Biotinylated antigen-specific molecules are either pre-targeted to antigen or co-administered with BBIR T cell to enable redirection of BBIRs against a chosen antigen(s). (Lower) Schematic representation of in vitro and in vivo application of BBIR platform. (Left) BBIR platform allows for rapid in vitro screening of candidate targeting agents (scFvs, ligands, aptamers, etc.) for future application, e.g., CAR construction. (Middle) BBIR engineered T cell strategy for sequentially targeting antigens. If antigen escape and tumor recurrence occurs after primary antigen targeting, BBIR T cells can be consecutively redirected against a different TAA by secondary administration of an antibody of distinct specificity. (Right) BBIR platform allows for simultaneous targeting multiple TAAs to efficiently attack tumors with highly heterogeneous TAA expression.
BBIR T cells were immunoreactive against tumor-associated antigens (TAAs) expressed on the cell surface, as demonstrated by their production of Th1 cytokines and cytolytic activity when stimulated with ovarian cancer cells painted with a biotinylated anti-EpCAM antibody. A notable secondary benefit to the BBIR platform was its applicability for rapid screening of scFvs to be used in CAR construction (Fig. 2, lower). Here, a biotinylated anti-mesothelin scFv permitted BBIR T cell redirection to mesothelin-expressing cancer cells and predicted its utility in a CAR construct.6,7
Importantly, the BBIR platform allowed T cells to generate an immune response against variable TAAs either simultaneously or sequentially (Fig. 1, lower). When tested against a panel of cancer cell lines that express varying TAAs, including mesothelin, folate binding protein (FRα) and/or EpCAM, binding of biotinylated specific antibodies to TAA on the respective tumor enabled specific immune-recognition of various cancer cells with non-overlapping antigen expression. The flexibility in antigen-specificity afforded by BBIR allowed sequential redirection from one antigen to another antigen of distinct specificity. For example, BBIRs could be redirected from first targeting and eliminating a subset of EpCAM-expressing tumor cells to additionally targeting and killing residual tumor cells expressing FRa, which had survived the first wave of EpCAM targeting. These observations highlight the universality and versatility of the BBIR platform.
Consistent with CAR technology, enhanced effector function and increased survival by antigen-stimulated BBIR T cells was observed when CD28 costimulation was incorporated into the BBIR construct. Inc. costimulation distinguishes the BBIR system from conventional bispecific-antibodies, where stimulation through the TCR/CD3 alone can result in anergy, or antigen-induced cell death.8,9 While BBIRs do require an intermediate biotinylated molecule for redirected antigen specificity, incorporation of costimulatory endodomains into BBIR vectors successfully resolved this issue. Consistent with their in vitro effector profile, BBIRs also exerted antigen-specific anti-tumor activity in vivo in a xenograft model of large, established human cancer when mice received biotinylated EpCAM, but not control, antibody indicating that introduction of antigen-specific biotinylated antibody induced anti-tumor activity of BBIRs in vivo.
The BBIR platform represents the first “universal immune receptor” approach for the targeting of gene-modified T cells to diverse and multiple antigens via interaction with antigen-bound biotinylated molecules, either simultaneously or sequentially. Though validated with biotinylated antibodies as antigen targeting molecules, the platform is also permissive to application with virtually any biotinylated molecule including but not limited to ligands, receptors, oligonucleotides, aptamers, and/or single chain TCRs. BBIR affinity allows for sequential targeting and resistance to soluble biotin blockade, however generation of fully-human universal immune receptors incorporating alternative intermediate binding domains of higher affinity or valence is feasible and actively under investigation. Coupled with the recent demonstration that CAR redirected allogeneic T cells can be used as universal “off-the-shelf” effectors,10 universal immune receptors as we have described stand to standardize and improve access to adoptive immunotherapy for cancer. In conclusion, universal immune receptors represent a bold, new platform for the rapid screening and generation of redirected T cells with function against virtually any antigen for which a specific targeting agent exists, and thus holds potential for widespread application.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19730
References
- 1.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Kochenderfer JN. Personalized cell transfer immunotherapy for B-cell malignancies and solid cancers. Mol Ther. 2011;19:1928–30. doi: 10.1038/mt.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbanska K, Lanitis E, Poussin M, Lynn R, Gavin BP, Kelderman S, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T cell antigen receptor. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-3890. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergan L, Gross JA, Nevin B, Urban N, Scholler N. Development and in vitro validation of anti-mesothelin biobodies that prevent CA125/Mesothelin-dependent cell attachment. Cancer Lett. 2007;255:263–74. doi: 10.1016/j.canlet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected Antitumor Activity of Primary Human Lymphocytes Transduced With a Fully Human Anti-mesothelin Chimeric Receptor. Mol Ther. 2011 doi: 10.1038/mt.2011.256. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guinan EC, Gribben JG, Boussiotis VA, Freeman GJ, Nadler LM. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261–82. [PubMed] [Google Scholar]
- 9.Daniel PT, Kroidl A, Kopp J, Sturm I, Moldenhauer G, Dörken B, et al. Immunotherapy of B-cell lymphoma with CD3x19 bispecific antibodies: costimulation via CD28 prevents “veto” apoptosis of antibody-targeted cytotoxic T cells. Blood. 1998;92:4750–7. [PubMed] [Google Scholar]
- 10.Marcus A, Waks T, Eshhar Z. Redirected tumor-specific allogeneic T cells for universal treatment of cancer. Blood. 2011;118:975–83. doi: 10.1182/blood-2011-02-334284. [DOI] [PubMed] [Google Scholar]