Abstract
CpG oligonucleotides stimulate via TLR9 and enhance anti-tumor immunity, an effect attributed to the activation of NK and CD8+ T cells. Our recent work demonstrates that CpG ODN also induce monocytic myeloid-derived suppressor cells to mature into M1 macrophages, further aiding tumor elimination. This provides insight into the mechanism through which CpG promote tumor regression.
Keywords: CpG ODN, CpG oligonucleotides, TLR9, immunotherapy, myeloid derived suppressor cells, myeloid-derived suppressor cells, toll like receptor, tumor microenvironment, tumor-associated macrophages
The innate immune system is activated via engagement of Toll-like receptors. Members of the TLR family recognize “pathogen associated molecular patterns” (PAMPs) expressed by infectious pathogens.1 For example, the unmethylated CpG motifs present at high frequency in bacterial DNA trigger via TLR9. CpG ODN mimic the immunostimulatory activity of bacterial DNA and are being used to enhance the host’s immune response to cancer.2 In pre-clinical and clinical studies, the protection conferred by CpG ODN is improved by intra-tumoral injection: systemic administration is considerably less effective.3 Studies show that intra-tumoral delivery of CpG ODN improves local dendritic cell function, promotes the entry of macrophages into the tumor site, and enhances the activation of tumor-specific CD8+ CTL and NK cells.4
Unfortunately, immunotherapy alone is rarely successful in eliminating large established tumors. The tumor microenvironment plays a role in this failure, as large tumors contain regulatory T cells and myeloid-derived suppressor cells (MDSC) that interfere with the lytic activity of CTL and NK cells.5 MDSC are particularly problematic, as they typically constitute a majority of all tumor infiltrating cells. Two distinct subpopulations of MDSC have been identified. Both are Gr-1+ and CD11b+ with granulocytic MDSC being Gr-1hi, Ly6g+, and Ly6cInt whereas monocytic MDSC (mMDSC) are Gr-1int, Ly6glo, and Ly6chi. Although both subsets suppress T and NK cell responses through the production of arginase-1 and/or inducible NO synthase (iNOS), mMDSC show greater suppressive activity on a per cell basis. Thus, an effective means of blocking the immunosuppressive activity of mMDSC would represent an important advance in efforts to improve the efficacy of tumor immunotherapy.6 To examine the effect of CpG ODN on MDSCs, we used the murine CT26 colon cancer model.7 The effect of injecting CpG vs. control ODN into large tumors (> 1 cm diameter) was monitored. As previously reported, CpG treatment significantly slowed tumor growth and increased the frequency of NK and CD8+ T cells infiltrating the tumor. Of interest, CpG treatment also led to a dramatic reduction of the number of tumor infiltrating mMDSCs (Fig. 1A3). This effect persisted for 4 d and required the intra-tumoral injection of CpG ODN (control ODN had no effect on MDSCs, nor did systemically administered CpG ODN). The observed effect was limited to the site of CpG administration: when mice had tumors in two different locations, MDSC numbers were significantly reduced only at the CpG-treated site.
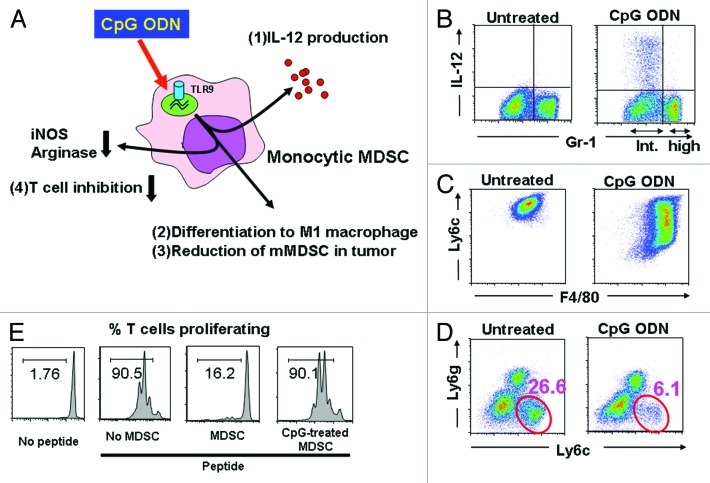
Figure 1. Effect of CpG ODN on tumor associated MDSC. (A) Monocytic MDSC express TLR9 and respond to CpG stimulation by (1) producing IL-12, (2) differentiating into M1 macrophages, (3) reducing the number of mMDSC at the tumor site and (4) losing their ability to suppress T cell function. (B) MDSC were cultured with CpG ODN for 8 h and analyzed for the expression of Gr-1 and intracytoplasmic IL-12. Note that IL-12 was produced by the Gr-1int population of mMDSC but not the Gr-1hi population of granulocytic MDSC. (C) mMDSC were cultured with CpG ODN for 48 h and analyzed for the expression of the MDSC marker Ly6c and the macrophage marker F4/80. Note that CpG stimulation decreased the expression of Ly6c while increasing the expression of F4/80. (D) CT26 colon cancer bearing mice were injected intra-tumorally with CpG ODN. Three days later, the number of tumor-infiltrating CD11b+ Ly6c+ mMDSC had fallen significantly. (E) Purified mMDSC were treated with CpG ODN and then cultured with CFSE labeled CD8+ HA-specific Tg T cells in the presence of HA peptide. CD8 T cell proliferation was monitored by CFSE dilution. Note that CpG treatment inhibited the suppressive activity of the MDSC.
Further analysis showed that murine mMDSC expressed TLR9, consistent with the hypothesis that CpG treatment was directly affecting mMDSC activity. Purified mMDSC treated in vitro with CpG ODN rapidly increased their production of Th1 cytokines (most notably IL-6, IL-12 and TNFα; Figure 1A1). More importantly, CpG-treated mMDSC lost their ability to suppress CD8+ T cells (Fig. 1A4). Functional analysis showed that CpG treatment reduced the expression of NO and arginase-1 by mMDSC, allowing tumor-specific CTL to remain active.
Explaining this change in activity was the observation that CpG ODN treatment induced mMDSC to differentiate into M1 macrophages. When highly purified mMDSC were stimulated with CpG ODN in vitro, their expression of the MDSC markers Ly6c and Gr-1 decreased whereas their expression of the macrophage marker F4/80 increased (Fig. 1C). Indeed, the M1 macrophages into which CpG-treated MDSC differentiated expressed tumoricidal activity such that transferring CpG-treated mMDSC into tumor bearing mice significantly slowed disease progression. M1 macrophages are characterized by their (1) expression of multiple TLRs, (2) production of proinflammatory cytokines (such as IL-12) when stimulated, and (3) ability to protect the host from infectious pathogens and tumors.8 This constellation of activities was exhibited by CpG-treated mMDSC. The precise mechanism underlying this differentiation of mMDSC into M1 macrophages is the topic of ongoing study.
Thus, these recent findings extend our understanding of the pleiotropic effects of CpG ODN on the immune system. In additional to previously described impact on NK and T cells, CpG DNA was found to stimulate mMDSC to lose their suppressive activity and mature into tumoricidal M1 macrophages. Consistent with preclinical studies from our laboratory and elsewhere, two Phase I clinical trials involving the intra-tumoral delivery of CpG ODN to treat malignant skin tumors yielded promising results. Hofmann et al. used this strategy to induce complete or partial tumor remission in half of all subjects, whereas Molenkamp et al. and Brody et al. showed that intratumoral CpG administration (alone or combined with radiation therapy) could induce systemic tumor regression by improving the generation of tumor-specific CD8+ T cells.9-11 Our recent report provides insight into a previously unappreciated mechanism by which CpG ODN contributes to tumor regression, and indicates that intra-tumoral injection represents the optimal route for ODN delivery.
Glossary
Abbreviations:
- iNOS
inducible nitric oxide synthase
- MDSC
myeloid-derived suppressor cells
- mMDSC
monocytic MDSC
- ODN
phosphorothioate oligodeoxynucleotide
- PAMP
pathogen associated molecular patterns
- TLR
toll like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19731
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–58. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 3.Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdörfer B, et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892–9. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 4.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 6.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–9. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann MA, Kors C, Audring H, Walden P, Sterry W, Trefzer U. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520–7. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- 10.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–32. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molenkamp BG, Sluijter BJ, van Leeuwen PA, Santegoets SJ, Meijer S, Wijnands PG, et al. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14:4532–42. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]