Abstract
In a recent study we reported increased expression of IL-17A in the lung of patients with lung adenocarcinoma. Local blockade of IL-17A in the lung, in a model of lung cancer revealed enhanced anti-tumor immunity characterized by increased IFNγ, a diminished T-regulatory cell number and reduced tumor growth.
Keywords: IL-17A, tumor, lung, T-bet, T-regulatory cells
Lung adenocarcinoma, a non-small-cell lung cancer (NSCLC), is one of the most frequent types of lung tumor. It develops as a primary tumor and can metastasize in other organs. NSCLC commonly occurs in smokers, but around 15% of the affected patients are non-smokers. In the latter cases, it is believed that both genetic and environmental factors play a pathogenetic role.1 Early tumor recognition and therapeutical intervention are important for avoiding metastases and thereby extending life expectancy.
In an effort to look for potential targets for early tumor immunotherapy, we performed translational studies on experimental lung cancer and on lung tissue of patients suffering from lung adenocarcinoma. In these studies we found an increase of Th17 associated genes, especially IL-17A, in patients and alveolar-carcinoma bearing mice compared with healthy controls.2
IL-17A (IL-17A) is a recently re-discovered cytokine, produced by Th17 cells, that induces angiogenetic factors in NSCLC cells.3,4 It is induced by the cytokines transforming growth factor β (TGF-β), IL-6, IL-21 and IL-23.4,5 In our studies, we found that expression of T-bet, a transcription factor inducing the anti-tumor cytokine IFNγ, inversely correlated with IL-17A levels, while Foxp3, a transcription factor of the immunosuppressive T-regulatory cells, directly correlated with IL-17A levels in lung tumor tissue of patients. These data might indicate that IL-17A and the anti-tumor immune mediator T-bet mutually suppress each other resulting in advanced tumor growth by augmenting Foxp3-expressing regulatory T cells.
To further investigate the pathogenetic role of IL-17A we applied neutralizing anti-IL-17A antibody locally to the lungs of lung tumor-bearing mice. Anti-IL-17A treated mice showed reduced tumor growth in the lung and an increased survival. This was accompanied by increased IFNγ production by lung infiltrating CD4+ T cells.2 IFNγ has been recognized for its superior anti-tumor function by inhibiting proliferation and angiogenesis of tumor tissue and induction of tumor death. It is also known to improve antigen presentation to CD8+ T cells by upregulating MHCI expression.6 Thus anti-IL-17A antibody immunotherapy might be also relevant for patients suffering from lung cancer because it can block selectively angiogenesis and tumor growth locally.
To further analyze the role of IL-17A in lung adenocarcinoma we also compared the immunological responses in a murine lung adenocarcinoma model in the presence and absence of T-bet. We found that T-bet deficient mice have much more tumor in the lung compared with wild-type littermates indicating that T-bet might play a protective role. Anti-IL17A therapy in T-bet-deficient mice induced IFNγ expression by CD8+ T cells whereas there was no effect on wild-type mice treated the same way. These observations confirm previous data on different roles of T-bet in CD4+ and CD8+ T cells and might indicate that inhibition of IL-17A leads to an induction of T-bet-independent IFNγ production in CD8+ T-cells.7
Subsequently, the immunosuppressive component in the tumor environment after anti-IL-17A antibody treatment was analyzed. TGFβ, an immunosuppressive growth factor inducing both T-regulatory and Th17 cells, was found upregulated in T-bet deficient mice in the airways as compared with wild-type littermates. This result was consistent with an increased release of IL-17A by lung CD4+ T cells and an increased number of T-regulatory cells in the lungs of T-bet deficient mice. Anti-IL-17A antibody treatment did not alter this value in T-bet-deficient mice whereas the number of T regulatory cells was diminished after anti-IL-17A blockade in wild-type mice. TGFβ expression was not changed after anti-IL-17A blockade in mice. This is consistent with the finding that TGFβ production by the cell line used to induce experimental lung tumor was not influenced by increasing concentrations of IL-17A in culture.
By contrast, IL-6 was found to be upregulated in the supernatant of tumor cells after in vitro treatment with increasing concentrations of IL-17A. Consistently, anti-IL-17A antibody application reduced IL-6 levels in the airways of wild-type mice bearing tumor. This might result in an impairment Th17 cell development and decreased IL-17A production. We also demonstrated increased IL-6 levels in the supernatants of lung tumor infiltrating T-bet-deficient T-cells which may be an explanation for an increased IL17A expression in these mice. In this paper we further demonstrated that anti-IL-17A antibody treatment induced an increased proliferation of lung CD4+ T cells in wild-type mice bearing tumor which was associated with a decrease of T-regulatory cell number.2
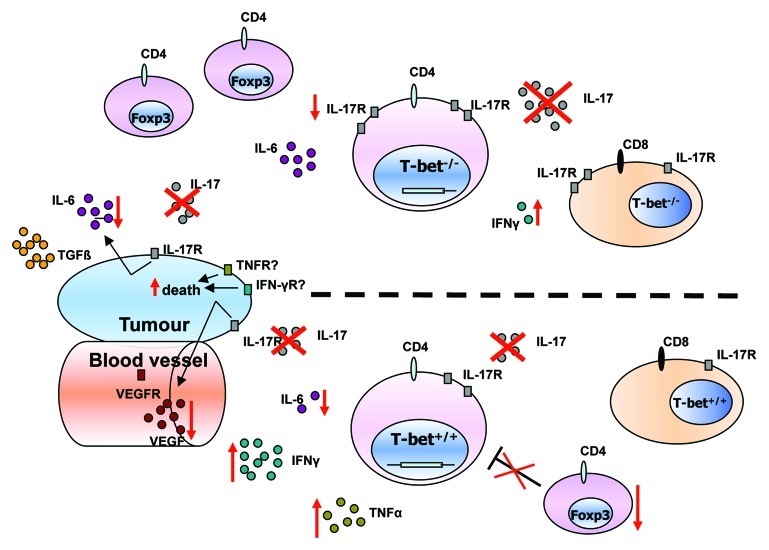
Finally, we found that T-bet inhibits IL-17R on tumor infiltrating lung CD4+ and CD8+ T cells and that in the presence of T-bet or Foxp3 there is no IL17R expression on these CD4+ T-cells (Fig. 1). These data might indicate that overexpression of T-bet might lead to a downregulation of both Th17 and T regulatory cells in tumor. Further experiments are underway to understand how T regulatory cells are controlled in lung tumor in the presence or absence of T-bet.
Figure 1. Effect of anti-IL-17A antibody treatment in the absence and presence of T-bet in experimental non-small-cell lung cancer. Immunological cytokine milieu in T-bet−/− (upper part) and wild-type (lower part) tumor bearing mice. The number of circles for each cytokine or rectangles for receptors, reflect their relative amount in T-bet−/− and wild-type mice. Red crosses represent the blockade of IL-17A. Red arrows indicate an upregulation or downregulation of cytokines, receptors or number of cells after IL-17A blockade. T-bet−/− mice show higher IL-17A, IL-6 and IL-17R expression and exhibit more T-regulatory cells whereas IFNγ levels are decreased. After IL-17A blockade there is an upregulation of IFNγ and TNFα secretion by CD4+ T-cells and a downregulation of IL-6 and Foxp3+ T cells in the lung of wild-type mice bearing tumor. In T-bet−/− mice IL-17A blockade led to a downregulation of IL-17R expression on CD4+ T-cells and an upregulation of IFNγ produced by CD8+ T-cells in the lung of mice bearing tumor.
The next challenge will be to transfer this knowledge into the clinic. In this endeavor it might be necessary to preselect ideal patients for this therapy based on the screening of their immune-relevant genes regulating Th1 and Th17 cells. Topical therapy appears to be advantageous to prevent systemic side effects of IL-17 A neutralization.
In any case, our results uncover novel aspects of the local immuno-regulatory network in NSCLC, identify a pathway for Th17-targeted immunotherapy and open new avenues for research.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19735
References
- 1.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(Suppl):191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::AID-CNCR2820751307>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 2.Reppert S, Boross I, Koslowski M, Türeci Ö, Koch S, Lehr HA, et al. A role for T-bet-mediated tumour immune surveillance in anti-IL-17A treatment of lung cancer. Nat Commun. 2011;2:600. doi: 10.1038/ncomms1609. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Xie Q, Cheng X, Diao X, Cheng Y, Liu J, et al. Role of interleukin-17 in lymphangiogenesis in non-small-cell lung cancer: Enhanced production of vascular endothelial growth factor C in non-small-cell lung carcinoma cells. Cancer Sci. 2010;101:2384–90. doi: 10.1111/j.1349-7006.2010.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–62. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Hwang ES. Transcriptional regulation of T helper 17 cell differentiation. Yonsei Med J. 2010;51:484–91. doi: 10.3349/ymj.2010.51.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 7.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]