Abstract
Primary induction of photo-oxidative (phox)-ER stress in cancer cells evoked immunogenic apoptosis (IA) associated with pre-apoptotic emission of calreticulin and ATP, and protective antitumor immunity. This IA subroutine involved “core” functions (e.g., PERK/PI3K-based modulation of secretory trafficking) rather than “private” ones (caspase-8 and eIF2α-phosphorylation) engaged by other IA inducers.
Keywords: photodynamic therapy, calreticulin, ATP, phox-ER stress, immunogenic apoptosis
Tremendous progress made in recent times has changed the notion that tumors are simply proliferating cancer cells organized into insular masses.1 Today, tumors are recognized as complex tissues composed of multiple cell types,1 including (innate) immune cells that have emerged as important definers of anti-tumor therapy response. Research has shown that, the immune system might play an important role in limiting formation of more than 80% of non-viral tumors.1 These trends have also heralded a change in anti-cancer therapeutics; oncologists now recognize that simply debulking the tumors via combination of surgery and radio-/chemo-therapy is seldom enough. Thus, the need to synergistically combine cancer cell killing (necrotic or apoptotic) with the activation of the anti-tumor immunity, has arose.2,3 Immunogenic apoptosis4 has all the major hallmarks of physiological apoptosis3 except that it can activate (rather than suppress) the immune system by emitting vital ‘immunogenic signals’, comprising damage-associated molecular patterns (DAMPs).2-5 Thus, cancer cells undergoing immunogenic apoptosis (or IA) have acquired the ability to communicate their antigenic memory to the immune system thereby leading to potent anti-tumor immunity.2,4 DAMPs that are vital for IA include surface-exposed calreticulin (ecto-CRT)2,4 and secreted ATP.2 IA tends to be stressor-dependent in that only selected chemotherapeutics induce it e.g., mitoxantrone, doxorubicin and oxaliplatin.2,4 This is because, reactive oxygen species (ROS)-based endoplasmic reticulum (ER) stress inducing agents are crucial for IA since ROS-based ER stress activates the specific danger signaling module required to emit immunogenicity-defining DAMPs.2,3 However, there are some technical glitches with these current inducers3 that limits the overall potential and applicability of IA.
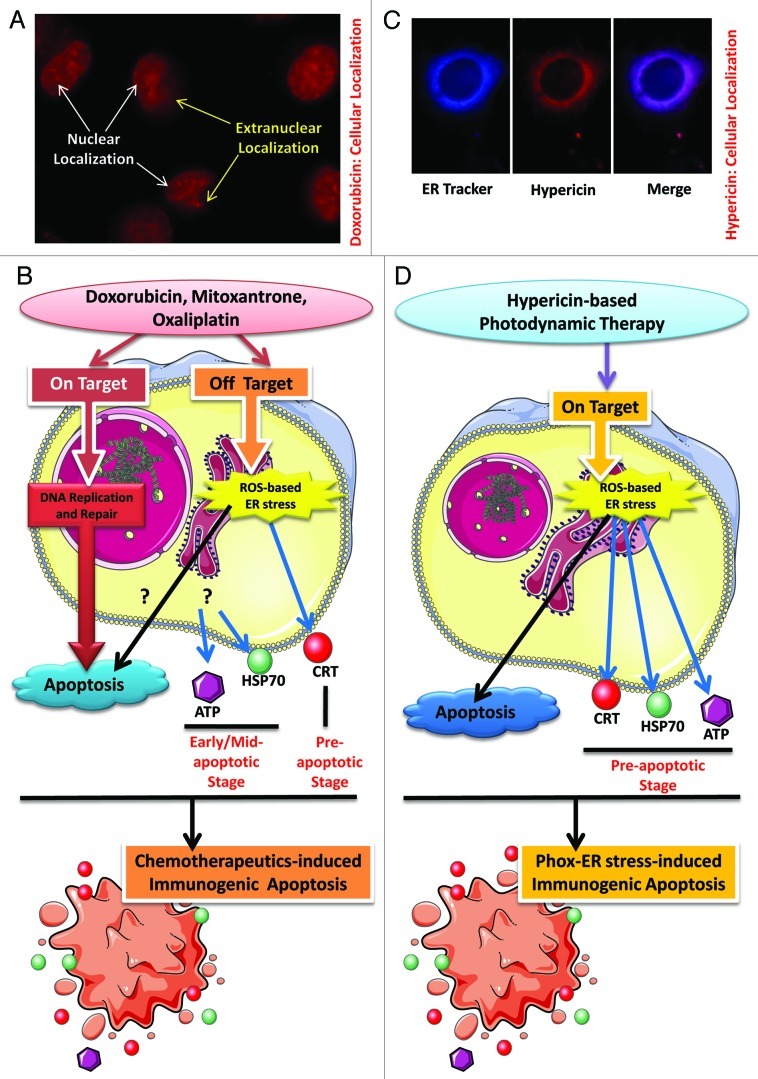
Current inducers of IA, like anthracyclines, mitoxantrone and oxaliplatin, are primarily DNA-targeting agents6 (Fig. 1A and B). Thus, not surprisingly bulk of their concentration localizes in the nucleus (site of ‘on target’ effects) and only a fraction of it localizes in extranuclear compartments (Fig. 1A) like the ER (site of “off target” effects). These advantageous off target effects are behind these agents’ ability to induce ROS-mediated ER stress and IA (Fig. 1B). However, this ROS-production is neither a primary effect of these inducers nor is pre-dominantly ER-directed.6,7 On the other hand, any attempts to increase the extranuclear concentrations (so as to accentuate the advantageous off target effects) of these inducers could prove to be “fatally” counterproductive since systemically speaking, these extranuclear concentrations are responsible for the known dose-limiting and ROS-dependent side effects of these compounds, like cardiotoxicity.7 Thus, we envisaged that the only way to improve this paradigm is by producing ROS as a primary/on target effect, predominantly directed at the ER.5,8
Figure 1. Immunogenic apoptosis subroutines. (A) Sub-cellular localization of doxorubicin. Human T24 bladder cancer cells incubated with 25 µM doxorubicin for 4 h show two distinct localizations—the “predominant” nuclear localization and the “residual” extranuclear localization. (B) Immunogenic apoptosis (IA) induced by DNA-damaging agents. Chemotherapeutics like doxorubicin, mitoxantrone and oxaliplatin exert two types of effects on the treated cells. A primary (on target) effect (result of nuclear localization) targets the DNA e.g., doxorubicin (or anthracyclines in general) intercalates into DNA and interferes with DNA replication, mitoxantrone inhibits topoisomerase II activity thereby disrupting DNA synthesis/repair and oxaliplatin acts as a coordination complex thereby inhibiting DNA synthesis. This “on target” effect is the main reason behind apoptosis induction by these agents. On the other hand their pro-oxidant effect, which results from their extranuclear localization, is responsible for the advantageous off target ROS-based ER stress which causes pre-apoptotic surface exposure of CRT and defines the dying cell’s immunogenicity. While this secondary effect is capable of engaging apoptotic pathways on its own, its overall contribution to the apoptosis observed after treatment with these agents is unknown. These processes are accompanied by early/mid apoptotic secretion of ATP and surface exposure of HSP70, both of which seem to be propelled as a result of general cellular stress. Overall, these processes lead to chemotherapeutics-induced IA. (C) Sub-cellular localization of hypericin. 150 nM of hypericin incubated with the T24 cancer cells for 16 h shows strong co-localization (merged image) with the ER Tracker Blue-White DTX dye. (D) Schematics of phox-ER stress-induced IA. Hypericin-based photodynamic therapy (PDT) causes ROS-based ER stress as a part of a predominant “on target” molecular effect (result of predominant ER localization). This leads to the pre-apoptotic emission of crucial DAMPs like secreted ATP and surface-exposed CRT, HSP70. Overall, this “on target” ROS-based ER stress (or phox-ER stress) boosts IA.
To this end, we recently made a significant conceptual and technical advancement by inducing bona fide IA2 by harnessing the production of ROS at the ER, with consequent ROS-based ER stress, via hypericin-based photodynamic therapy (Hyp-PDT).5 Hyp-PDT utilizes a drug/photosensitizer (hypericin) that pre-dominantly localizes in the ER5,8 (Fig. 1C). Once excited by visible light and in the presence of oxygen, hypericin generates ER-localized ROS8 that mediates SERCA2 loss-of-function, disruption of ER-Ca2+ homeostasis leading to ER stress and BAX/BAK-based mitochondrial apoptosis.5 Thus, Hyp-PDT provokes photo-oxidative ER stress (phox-ER stress) as a primary/on target event,5 which in cancer cells was found to cause emergence of various bona fide immunological signatures of IA2 (Fig. 1D) i.e. ecto-CRT,5,9 secreted ATP5 and surface-exposed heat shock protein 70 (ecto-HSP70).9 Here, all three of these DAMPs emerged in the pre-apoptotic stage (stage preceding phosphatidylserine exposure)5,9 (Fig. 1D). In fact, the kinetics of their emission was quicker than those reported previously for these three DAMPs. This makes phox-ER stress-induced IA (in our knowledge) the first cell death subroutine with three crucial DAMPs following overlapping kinetics to be pre-apoptotically emitted. Moreover, by prompting the primary production of ROS at the ER, we fostered the relative amounts of these DAMPs “exposed” to the extracellular space as compared with those caused by other IA inducers, which are prevalently DNA-damaging in nature.5,9 Thus, this form of “primary/on target” phox-ER stress-induced IA instigated a strong immunostimulatory pre-apoptotic stage before the immunosuppressive program that is innate to apoptosis,3 commences (post-phosphatidylserine exposure and caspase activation).
Phox-ER stressed dead/dying cancer cells were also found to establish a highly productive interface with dendritic cells (on the levels of phagocytosis and phenotypic/functional maturation);5,9 paving the way for IA that elicited a potent anti-tumor immune response.5 Our results in a CT26 prophylactic immunization model5 have been recently substantiated in a CT26 therapeutic model, where it was shown that CT26 tumor-bearing mice whose tumors were eradicated with Hyp-PDT resisted formation of new tumors when subsequently re-challenged with live CT26 cells.10 This further outlines the ability of Hyp-PDT to combine cancer cell killing with “revival/activation” of anti-tumor immunity within the same paradigm.
Moreover, we found that the ecto-CRT and immunogenicity incited by phox-ER stress were caspase(-8) independent5 thereby uncoupling cell death signaling and danger signaling, for the first time. In fact, the intracellular pathways controlling DAMP emission during phox-ER stress-induced IA, emerged predominantly from alterations of “house-keeping core functions”2 (e.g., secretory pathway, PERK-based/PI3K-based modulation of secretory trafficking)5 rather than the “private ones”2 instigated by other IA inducers, which are subject to genetic/post-translational variability (e.g., caspase-8 and eIF2α-phosphorylation).5 Thus, we have characterized for the first time that during IA, the danger signaling cascades mediating DAMP trafficking/emission have two components—the “core” ones which have a wide applicability (such that these pathways are common to most stimulus) and the “private” ones, whose existence is stimulus-dependent (and mainly applies to the chemotherapeutic agents but not to phox-ER stress).
Considering the promise offered by phox-ER stress-induced IA, it would be crucial in future to exploit this novel phenomenon for discovery of new immunogenic signals/DAMPs. It would be also vital to apply Hyp-PDT clinically for cancer treatment with the “added” context of anti-tumor immunity.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19750
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L, Kepp O, Kroemer G. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J. 2012;31:1062–79. doi: 10.1038/emboj.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 5.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1055–7. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 7.Vergely C, Delemasure S, Cottin Y, Rochette L. Preventing the cardiotoxic effects of anthracyclines: from basic concepts to clinical data. Heart Metab. 2007;35:1–7. doi: 10.1016/j.ancard.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Garg AD, Krysko DV, Vandenabeele P, Agostinis P. DAMPs and PDT-mediated photo-oxidative stress: exploring the unknown. Photochem Photobiol Sci. 2011;10:670–80. doi: 10.1039/c0pp00294a. [DOI] [PubMed] [Google Scholar]
- 9.Garg AD, Krysko DV, Vandenabeele P, Agostinis P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol Immunother. 2012;61:215–21. doi: 10.1007/s00262-011-1184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanovic R, Verwanger T, Hartl A, Krammer B. Low dose hypericin-PDT induces complete tumor regression in BALB/c mice bearing CT26 colon carcinoma. Photodiagnosis Photodyn Ther. 2011;8:291–6. doi: 10.1016/j.pdpdt.2011.04.003. [DOI] [PubMed] [Google Scholar]