Abstract
TG4010 is a therapeutic cancer vaccine based on a viral vector, a modified vaccinia of Ankara (MVA), expressing MUC1 as well as interleukine 2. Today the clinical development is focused on advanced non-small cell lung cancer in combination with first line chemotherapy. Potential biomarkers predictive of activity have been identified.
Keywords: TG4010, biomarker, cancer vaccine, non-small cell lung cancer
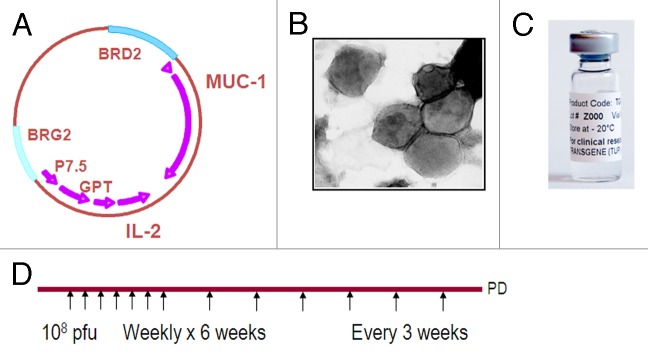
Several immunotherapy drugs are today tested in this disease in advanced stages of development.1 TG4010 (Transgene SA) is one of these candidates. This targeted immunotherapy product is based on an attenuated virus, the modified vaccinia of Ankara, that has been genetically modified to express the tumor antigen MUC1 as well as interleukine 2. TG4010 is intended to be administered sub-cutaneously at the dose of 108 pfu weekly for six weeks and then every three weeks (Fig. 1).
Figure 1. (A) Schematic map of TG4010 viral genome. (B) Electron microscope view of TG4010 showing the viral particles with their membrane. (C) Pharmaceutical formulation of TG4010. (D) Schedule of administration of TG4010 in current clinical trials.
A good safety profile and early signs of clinical and biological activity have been observed in Phase I.2 A biological proof of activity when given in monotherapy has been obtained in prostate cancer patients at the stage of first rising PSA following local treatment.3 In metastatic renal cell carcinoma combination with interferon α and interleukine 2 proved to be feasible and showed encouraging overall survival.4 Regarding NSCLC, Phase II trials have studied TG4010 mainly in combination with first line chemotherapy in patients with advanced stage NSCLC.
A first Phase II has assessed the combination of TG4010 with cisplatin and vinorelbine. This study met its primary endpoint based on response rate and raised the hypothesis that TG4010 could improve the outcome in patients receiving first line chemotherapy.5
To test this hypothesis a larger randomized study was initiated.6 This open-label study recently published in The Lancet Oncology enrolled 148 patients with stage IV NSCLC, 74 received cisplatine and gemcitabine plus TG4010 and 74 received the same chemotherapy alone. The primary objective was to show an improvement of progression-free survival at 6 mo. A biomarker program was also associated to the study in order to be able to identify the population of patients with the best response to chemo-immunotherapy.
With 43.2% of patients progression free at 6 mo in the experimental arm this second Phase II in NSCLC met its statistical endpoint too. In the control arm 35.1% of patients remained free of progression at 6 mo. Interestingly the magnitude of the improvement was more important on response rate: 41.9% vs. 28.4%. Furthermore the outcome in terms of survival of responding patients in the experimental arm was better than in the control arm (23.3 vs. 12.5 mo). This observation is similar to the better duration of response observed in melanoma patients receiving dacarbazine plus ipilimumab as compared with dacarbazine alone.7 Regarding overall survival the median survival in both arms were similar, 10.7 mo vs. 10.3 mo however a late separation of the curves, classic with immunotherapy products showed a better long-term survival for the experimental arm. Conversely more patients died early in the experimental arm.
The biomarker program associated to the study put in evidence a close association between the patients who died early in the experimental arm and a high level at baseline of triple positive CD16+CD56+CD69+ lymphocytes corresponding to a phenotype of activated NK cells (aNK).
When observing the 101 (75%) of patients with a normal level of aNK cells at baseline the effect of the addition of TG4010 to chemotherapy was substantial: median overall survival was 17.1 mo vs. 11.3 mo, percentage of patients progression free at 6 mo was 56.3% vs. 37.7% and finally response rate was 54.2% against 28.3% in the control arm. In the 37 (25%) patients with a high level of aNK cells at baseline results were opposite: overall survival was 5.3 mo vs. 10.3 mo, 6 mo progression free survival was 19.0% vs. 31.3% and response rate was 19.0% vs. 31.3%. Of interest, the effect of the level of aNK at baseline influenced only the outcome in the experimental arm but not in the control arm. This makes the baseline level of aNK a possible predictive marker for the selection of patients expected to benefit from the addition to TG4010 to their chemotherapy and of course raises the question of the underlying mechanism involved in this observation. The most plausible explanation is coming from the recent findings regarding the regulatory effect the NK cells exert on the players of the adaptive immune response. Depending on their level of activation the NK cells can enhance the development of a cellular immune response against an antigen but can also dampen this same response when they are stimulated beyond a given threshold.8 If this observation made with TG4010 is confirmed this could possibly apply to other situations in oncology where the NK cells are already activated by the presence of the tumor and a treatment like a therapeutic vaccine or an oncolytic virus known by essence to stimulate further the NK cells. In order to both validate prospectively the predictive value of aNK cells when using TG4010 in combination with first line chemotherapy of advanced NSCLC and demonstrate a meaningful improvement of survival with this combination the next step of the development will be a phase IIB/III. The Phase IIB part of the study will aim at validating the biomarker and combining TG4010 with current chemotherapies not yet tested in combination with TG4010. The Phase III part of the study is then powered to show a gain of survival and support registration of TG4010 in this indication.
The tumor antigen MUC1 being expressed on many frequent epithelial tumors, a success in NSCLC would open the door to developments of TG4010 in other indications and settings characterized by an expression of MUC.
Glossary
Abbreviations:
- NSCLC
non-small cell lung cancer
- MUC1
mucine number 1
- CD
cluster of differentiation
- PD
progressive disease
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19863
References
- 1.Ujhazy P, Carbone D. Summary of presentations from the 11th targeted therapies for lung cancer meeting: immunotherapy and vaccines for treatment of lung cancer. J Thorac Oncol. 2011;6(Suppl 4):S1815–7. doi: 10.1097/01.JTO.0000407570.29900.a5. [DOI] [PubMed] [Google Scholar]
- 2.Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5:690–9. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 3.Dreicer R, Stadler WM, Ahmann FR, Whiteside T, Bizouarne N, Acres B, et al. MVA-MUC1-IL2 vaccine immunotherapy (TG4010) improves PSA doubling time in patients with prostate cancer with biochemical failure. Invest New Drugs. 2009;27:379–86. doi: 10.1007/s10637-008-9187-3. [DOI] [PubMed] [Google Scholar]
- 4.Oudard S, Rixe O, Beuselinck B, Linassier C, Banu E, Machiels JP, et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol Immunother. 2011;60:261–71. doi: 10.1007/s00262-010-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Lévy E, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008;3:735–44. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 6.Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12:1125–33. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]