Abstract
Summary: The mating pheromone a-factor secreted by Saccharomyces cerevisiae is a farnesylated and carboxylmethylated peptide and is unusually hydrophobic compared to other extracellular signaling molecules. Mature a-factor is derived from a precursor with a C-terminal CAAX motif that directs a series of posttranslational reactions, including prenylation, endoproteolysis, and carboxylmethylation. Historically, a-factor has served as a valuable model for the discovery and functional analysis of CAAX-processing enzymes. In this review, we discuss the three modules comprising the a-factor biogenesis pathway: (i) the C-terminal CAAX-processing steps carried out by Ram1/Ram2, Ste24 or Rce1, and Ste14; (ii) two sequential N-terminal cleavage steps, mediated by Ste24 and Axl1; and (iii) export by a nonclassical mechanism, mediated by the ATP binding cassette (ABC) transporter Ste6. The small size and hydrophobicity of a-factor present both challenges and advantages for biochemical analysis, as discussed here. The enzymes involved in a-factor biogenesis are conserved from yeasts to mammals. Notably, studies of the zinc metalloprotease Ste24 in S. cerevisiae led to the discovery of its mammalian homolog ZMPSTE24, which cleaves the prenylated C-terminal tail of the nuclear scaffold protein lamin A. Mutations that alter ZMPSTE24 processing of lamin A in humans cause the premature-aging disease progeria and related progeroid disorders. Intriguingly, recent evidence suggests that the entire a-factor pathway, including all three biogenesis modules, may be used to produce a prenylated, secreted signaling molecule involved in germ cell migration in Drosophila. Thus, additional prenylated signaling molecules resembling a-factor, with as-yet-unknown roles in metazoan biology, may await discovery.
INTRODUCTION
The secreted Saccharomyces cerevisiae mating pheromone a-factor is a 12-mer peptide that is unusual among extracellular signaling molecules in that it is prenylated and carboxylmethylated, making it highly hydrophobic (5). Mature a-factor is synthesized by yeast cells of the MATa haploid mating type and is derived from a precursor with an N-terminal extension and a C-terminal CAAX motif (“C” is cysteine, “A” is often an aliphatic amino acid, and “X” is any residue) (37, 184, 185, 207). “CAAX processing” comprises an ordered series of posttranslational biochemical reactions that result in prenylation of the cysteine residue of the CAAX motif, endoproteolysis of the AAX tripeptide, and carboxylmethylation of the prenylated cysteine. Historically, the a-factor precursor served as a valuable model for the discovery and functional analysis of the enzymes that mediate these CAAX-processing reactions (17, 19, 41, 99, 279).
In this review, we discuss in detail the three modules comprising the biogenesis pathway of a-factor, including (i) C-terminal CAAX processing, (ii) two sequential N-terminal cleavage steps, and (iii) export by a nonclassical mechanism mediated by the ATP binding cassette (ABC) transporter Ste6. We also discuss the interaction of a-factor with its receptor, Ste3, on the surface of cells of the opposite mating type, MATα. The small size of a-factor and its hydrophobicity, due to the prenyl and methyl posttranslational modifications, present unique challenges as well as distinct advantages for biochemical analysis. We discuss how these properties can be exploited for experimental analysis and how they can be applied to the discovery of novel signaling molecules. As an example, we provide data for L-factor, a previously uncharacterized pheromone secreted by the fungal species Saccharomycodes ludwigii. The a-factor-like pheromones of other yeasts, both Ascomycetes and Basidiomycetes, are also briefly covered here.
The enzymes that perform the steps of a-factor biogenesis are interesting in their own right. They are conserved from yeast to mammals, and are important for the maturation and proper function of a wide variety of proteins that play many different cellular roles in all eukaryotes. In particular, we discuss how a-factor studies led to the discovery of the zinc metalloprotease Ste24 and its mammalian homolog ZMPSTE24, which cleave prenylated a-factor in yeast and the prenylated C-terminal tail of the nuclear scaffold protein lamin A in mammalian cells, respectively (24, 94, 203, 255). Mutations in the lamin A gene affecting the ZMPSTE24 processing site result in the aberrant and persistent prenylation of lamin A, which leads to the premature-aging disorder Hutchinson-Gilford progeria syndrome (HGPS) (78, 87). Likewise, mutations that alter the ZMPSTE24 gene and diminish cleavage of the prenylated lamin A tail cause a spectrum of premature-aging-related disorders (2, 19, 20, 190, 279). Cleavage of the lamin A tail by ZMPSTE24 may also be important for normal human aging (178, 208, 217).
Recent intriguing evidence discussed here suggests that the entire a-factor pathway, including all three a-factor biogenesis modules, appears to be used in the Drosophila embryo to produce a prenylated, secreted signaling molecule that serves as an attractant in germ cell migration (213, 214). This finding raises the possibility that a molecule resembling a-factor may also contribute to mammalian germ cell migration and suggests that additional prenylated a-factor-like signaling molecules with roles in other, as-yet-unknown metazoan processes await discovery.
THE YEAST MATING PHEROMONES a-FACTOR AND α-FACTOR DEFINE DISTINCT PARADIGMS FOR THE BIOGENESIS AND SECRETION OF SIGNALING MOLECULES
The Saccharomyces cerevisiae peptide mating pheromones a-factor and α-factor are small peptide signaling molecules, secreted by haploid cells of opposite mating types (MATa and MATα, respectively), that promote mating and diploid formation in yeast (Fig. 1). The pheromone secreted by one haploid cell type binds to a specific receptor on the surface of the opposite cell type to stimulate a G-protein-coupled mating response pathway. Upon activation of this pathway, MATa and MATα cells undergo G1 cell cycle arrest, cell-cell fusion, and nuclear fusion to form a MATa/MATα diploid. The topics of the yeast mating pathway, mating type determination, cell-cell signaling, and intracellular signaling during mating have been reviewed extensively (13, 14, 41, 67, 86, 110, 153, 157, 175, 246, 268).
Fig 1.
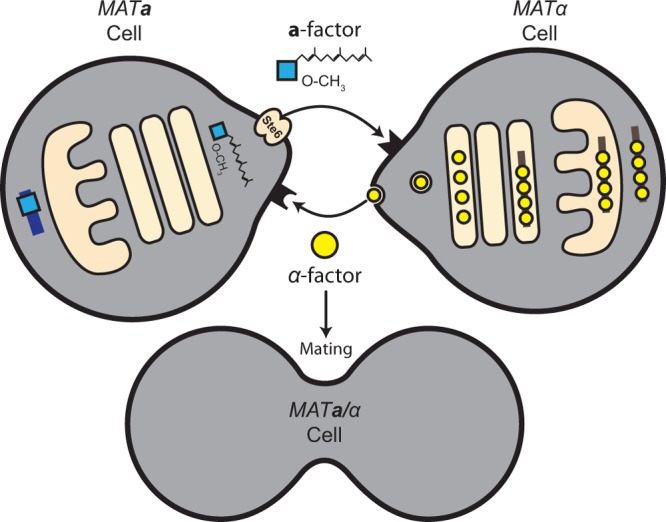
Overview of the biogenesis and secretion of a-factor and α-factor. MATa cells (left) secrete a-factor, a farnesylated and carboxylmethylated peptide. The a-factor precursor undergoes a series of biogenesis steps prior to export, including farnesylation, carboxylmethylation, and several proteolytic cleavages, to yield mature a-factor. These steps occur in the cytosol or on the cytosolic face of intracellular membranes. Export of a-factor occurs via a “nonclassical” secretory mechanism, mediated by the ABC transporter, Ste6. MATα cells (right) secrete α-factor, which is synthesized as a precursor containing multiple tandem copies of α-factor. The α-factor precursor traverses the classical secretory pathway and undergoes glycosylation in the ER (irregular tan shape), followed by proteolytic processing in the Golgi apparatus (tan ovals) to yield mature α-factor that travels in vesicles to the plasma membrane and is secreted. During the response of haploid yeast to mating pheromones, cells undergo morphological alterations, becoming polarized toward one another and exhibiting distinct projections, as shown. Such cells are termed shmoos. The mating machinery is localized to the shmoo tip; during mating, cells fuse at these shmoo tips to form MATa/MATα diploid cells.
The yeast mating pheromones, a-factor and α-factor, are both synthesized as precursors (36 and 38 amino acids long for the functionally redundant MFA1 and MFA2 gene products, respectively, and 165 and 120 amino acids for the MFα1 and MFα2 gene products, respectively). The precursors encoded by MFA1/MFA2 and MFα1/MFα2 undergo multiple steps of posttranslational modification and proteolytic cleavage prior to their secretion (41, 97, 153, 245, 246). The study of the very different biogenesis pathways of the a-factor and α-factor precursors has provided the opportunity for cell biologists to identify novel posttranslational processing enzymes and to investigate distinct secretory mechanisms, as discussed below. In general, the enzymes that mediate pheromone biogenesis in yeast also perform important functions in mammalian cells. Many of the genes encoding these enzymes were first discovered through yeast screens for mating-defective sterile (ste) mutants, facilitating the identification of their mammalian counterparts.
Although the a-factor and α-factor pheromones have analogous functions in stimulating signaling through their interaction with a cell surface receptor on the opposite cell type, these two secreted pheromones differ significantly from one another in their chemical properties. Whereas the a-factor 12-mer peptide is highly hydrophobic, due to its C-terminal farnesyl lipid and carboxylmethyl modifications, α-factor is a hydrophilic and unmodified 13-mer peptide. Importantly, the a-factor and α-factor precursors exemplify two different paradigms for how cells carry out the biogenesis and secretion of signaling molecules (Fig. 1), as discussed in detail in the sections below.
Biogenesis of α-Factor: an Overview
The hydrophilic α-factor pheromone uses the “classical” secretory pathway for its biogenesis and secretion (Fig. 1). Secreted mature α-factor is derived from one of two similar precursors encoded by the MFα1 and MFα2 genes (152, 154). The MFα1 precursor is 165 amino acids long and is the better studied of the two. It contains an N-terminal signal sequence, a “pro” region, and four tandem copies of the α-factor 13-mer, separated by spacers that contain cleavage sites for multiple proteases (154). The MFα1 precursor undergoes posttranslational translocation across the endoplasmic reticulum (ER) membrane, followed by signal sequence cleavage and N-linked glycosylation on three asparagine residues on its “pro” region in the ER lumen (48). Upon vesicular transport from the ER to the Golgi apparatus, the glycan chains of the MFα1 precursor are remodeled in the Golgi lumen and three proteolytic cleavage steps occur within the MFα1 spacers, mediated by the Kex1, Kex2, and Ste13 enzymes, to yield four copies of the mature unmodified α-factor 13-mer (81, 127–129). Secretory transport vesicles that contain the fully processed α-factor bud from the Golgi apparatus and fuse with the plasma membrane (PM) to release α-factor to the external milieu (Fig. 1). The α-factor biogenesis pathway has been extensively reviewed previously (97, 246).
The α-factor precursor has been an important model molecule for dissection of the classical secretory pathway both in vivo and in vitro (12, 48, 227, 234). Notably, α-factor is particularly advantageous for in vitro studies of vesicular transport due to the fact that it is posttranslationally translocated into the ER, rather than cotranslationally translocated, and can thus be translated and radiolabeled in vitro and subsequently added to microsomes or permeabilized cells (12, 107, 227). The reconstruction of the α-factor precursor vesicular transport step from the ER to the Golgi apparatus in vitro, using gently lysed spheroplasts and exogenously added radiolabeled α-factor precursor, represented an important breakthrough that permitted the development of functional assays to purify and measure the activity of many important secretory components (12, 227). These include molecules that regulate transport vesicle formation and vesicle coat proteins (124).
Significantly, studies of the α-factor proteolytic processing enzymes, and in particular Kex2, have also had an impact on mammalian cell biology. Kex2 was the first discovered member of an enzyme family called the proprotein convertases (PPCs), also known as the kexins or furin proteases (97, 216). Kex2 is the prototype member of this family of highly conserved subtilisin-like serine proteinases. The PPCs have multiple regulatory functions, including mediating key roles in the posttranslational processing of several mammalian hormone precursors (216, 240, 241). Thus, from the several examples above, it is evident that studies using yeast α-factor as a model molecule have led to an understanding of many different cell biological processes shared by all eukaryotes.
Biogenesis of a-Factor: an Overview
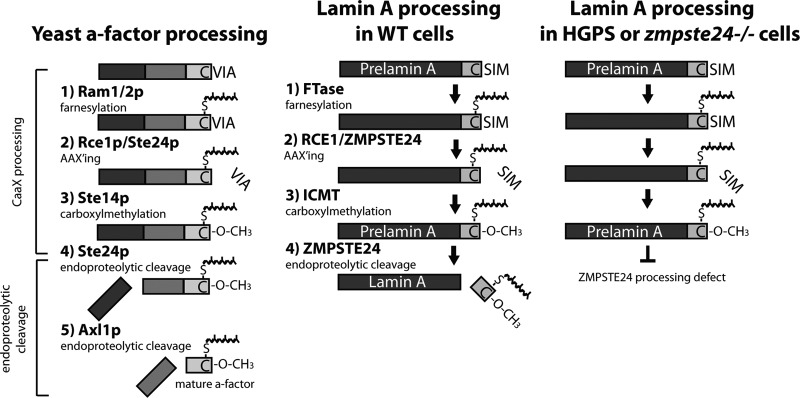
In contrast to α-factor, a-factor does not use the classical secretory pathway (Fig. 1) (150, 179, 181). The precursor and mature forms of a-factor encoded by MFA1 are 36 and 12 amino acids long, respectively, and are shown in Fig. 2. The detailed biogenesis pathway of a-factor is shown in Fig. 3. The a-factor precursor undergoes a sequential series of six steps during biogenesis, which involve three separate modules. These are the three C-terminal CAAX-processing steps (prenylation, proteolysis, and carboxylmethylation [steps 1 to 3]), two sequential N-terminal proteolytic processing events (steps 4 and 5), and export using an alternative “nonclassical” export mechanism mediated by the ATP binding cassette (ABC) transporter Ste6 (step 6) (116, 181, 183, 201, 253, 279) (Fig. 3).
Fig 2.
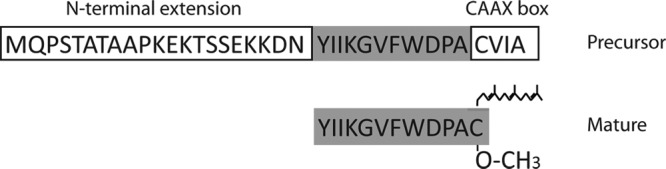
Comparison of the precursor and mature a-factor molecules. Mature a-factor and the precursor encoded by MFA1 are shown. The a-factor precursor undergoes C-terminal modification of its CAAX motif, followed by proteolytic cleavages that remove the N-terminal extension.
Fig 3.
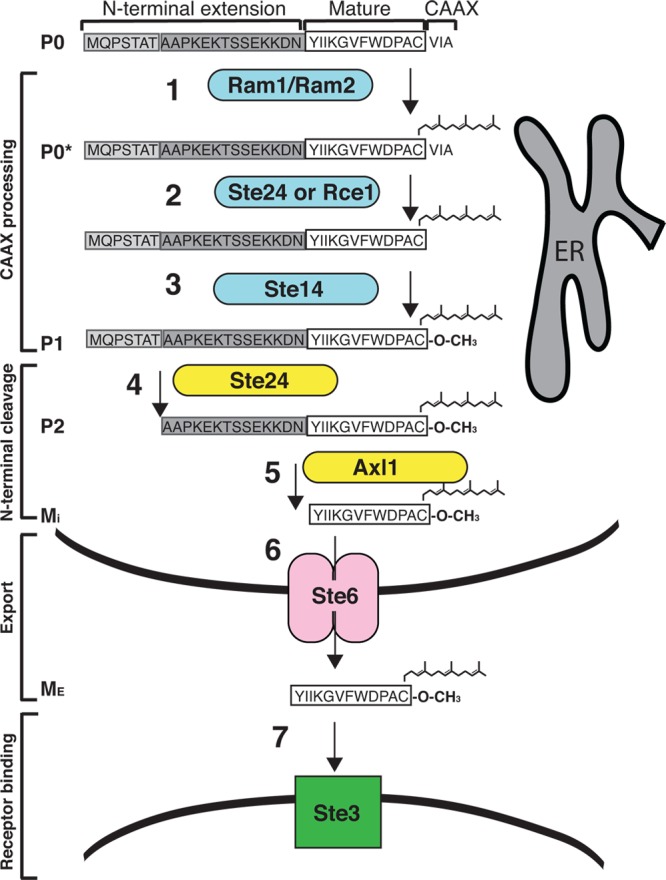
Model for the biogenesis of a-factor. The modules (CAAX processing, N-terminal cleavage, export, and receptor binding), biosynthetic intermediates (P0, P0*, P1, P2, MI, and ME), and enzymes (colored shapes) involved in the maturation of a-factor are indicated here and discussed in the text. Postprenylation CAAX modifications and the first step of N-terminal processing are carried out by integral membrane proteins (Rce1, Ste14, and Ste24) localized in the ER membrane, presumably with their active sites facing the cytosol. It is not known how a-factor is subsequently shuttled to the plasma membrane, where it is transported out of the cell by the ABC transporter protein Ste6. Extracellular a-factor binds to the receptor Ste3 on the cell surface of MATα cells. It should be noted that the cellular location of Axl1 and its site of a-factor processing have not been firmly established.
As already noted, the classical secretory pathway per se is not directly involved in a-factor processing, as first suggested by the lack of an N-terminal hydrophobic signal sequence in the a-factor precursor and the finding that extracellular a-factor activity and mating can still be detected in secretion-defective (sec) mutants at the nonpermissive temperature, albeit at a decreased level (150, 179). Thus, although many of the enzymes that mediate a-factor processing are located in the ER membrane (Fig. 3), a-factor is at no point translocated across the ER membrane, in contrast to α-factor and other secreted signaling molecules. Instead, a-factor transport across the membrane occurs after the completion of biogenesis, rather than preceding it. While the processing of a-factor is completely unaffected in temperature-sensitive sec mutants, the somewhat decreased levels of mating and a-factor observed in sec mutants are likely due to an indirect effect on the trafficking of the a-factor transporter Ste6 (58).
In the sections below, we discuss the role of a-factor studies in the CAAX processing field and review in detail each “module” of the a-factor biogenesis pathway. Like α-factor, a-factor has served as an important model molecule for the study of conserved cell biological processes, distinct from those involved in α-factor biogenesis.
YEAST a-FACTOR AS A “PROTOTYPE” FOR THE IDENTIFICATION OF CAAX-PROCESSING ENZYMES
Yeast a-factor holds an important place in the field of protein prenylation. Our current understanding of CAAX processing can be traced to the discovery of prenylation as a common posttranslational modification, shared by two unrelated classes of proteins: fungal mating pheromones and Ras oncoproteins, in yeast and mammalian cells. Identification of the genes encoding CAAX-processing enzymes, through genetic screens in S. cerevisiae, played a key role in this field. Elucidation of the CAAX-processing pathway provided an early example of the commonality of cell biological processes in eukaryotic organisms ranging from yeast to humans (60).
Obscure Fungal Pheromones Lead to the Discovery of Protein Prenylation and Carboxylmethylation
The original scientific report of prenylation as a novel protein modification that exists in nature came from the structural analysis of what we now know to be “a-factor-like” pheromones secreted by several obscure species belonging to a class of fungi called the Basidiomycetes. Note that S. cerevisiae belongs to a different class of fungi, called the Ascomycetes. In a landmark discovery in 1979, a pheromone (rhodotorucine A) secreted from the red yeast Rhodosporiduim toruloides was shown by mass spectrometry to comprise an 11-amino-acid-long peptide containing a novel lipid modification. This modification was the 15-carbon isoprenoid farnesyl, covalently linked to the C-terminal cysteine residue of rhodotorucine A by a thioether linkage (131–133). Prior to this study, farnesyl-cysteine had not been known as a biological entity.
Additional secreted farnesylated pheromones were subsequently identified in other fungi, including the jelly fungi Tremella mesenterica (tremerogens A-10 and a-13) (187, 228, 229) and Tremella brasiliensis [factors A(Ia) and A(Ib)] (120, 121). Importantly, at least two of these mating factors, tremerogens A-10 and A(Ia), were also found to be α-carboxylmethylated on the same cysteine residue that was farnesylated, thus heralding the discovery of a second novel posttranslational modification, carboxylmethylation (120, 228, 229). The discovery of two new types of protein modifications, farnesylation and α-carboxylmethylation, on a single C-terminal cysteine residue provided the starting point for our current understanding of CAAX processing.
Studies of S. cerevisiae a-Factor and Ras Connect Prenylation and Carboxylmethylation of Cysteine to the CAAX Motif
Discovery of the RAM1 gene suggests an unexpected link between a-factor and Ras proteins through their common CAAX motif.
The knowledge that proteins can be prenylated and carboxylmethylated might have remained buried in the literature, had it not been for a series of intriguing observations with yeast calling attention to the “CAAX motif,” which we now know directs the addition of these modifications. A key finding was an unanticipated genetic connection between two otherwise unrelated yeast proteins, a-factor and Ras. For activity, both proteins were shown to share the requirement for a gene called RAM1 (so named because it is involved in Ras and a-factor maturation) (207). It was also pointed out in this early study that both a-factor and Ras proteins also share a C-terminal CAAX motif, although at the time, the role of this motif was not known.
The knowledge that Basidiomycete pheromones are farnesylated and carboxylmethylated on a C-terminal cysteine (120, 228, 229), together with the finding that a specific set of human proteins incorporate a compound derived from mevalonate (170, 235a), led investigators to examine whether mammalian Ras proteins are also farnesylated and carboxylmethylated. Ultimately, both yeast and mammalian Ras proteins, yeast a-factor, and the mammalian nuclear scaffold protein lamin B (all derived from precursors with a C-terminal CAAX motif) were shown to contain a farnesylated and carboxylmethylated C-terminal cysteine residue, just like tremerogens from the Basidiomycetes fungi (5, 60, 61, 88, 106, 232, 249, 274). Furthermore, the ram1 mutant was shown to be biochemically defective for prenylation (233). These findings solidified what is now common knowledge, namely, that in all eukaryotes the CAAX motif directs a series of modifications: prenylation, endoproteolyis of the AAX, and carboxylmethylation of the farnesylated cysteine residue of the CAAX motif (23, 99, 113, 274, 281).
CAAX proteins can be farnesylated or geranylgeranylated, depending on the identity of the AAX residues.
CAAX processing is a common posttranslational modification, as approximately 2% of proteins encoded in the genomes of yeast and other eukaryotes terminate with a CAAX motif, and many of these have been directly demonstrated to be prenylated (23, 99, 232, 274, 281). The C-terminal residue, X, of the CAAX motif dictates the type of prenyl group that is added to a protein: generally, if X is any amino acid except Phe or Leu, the 15-carbon isoprenoid moiety farnesyl is added by farnesyltransferase (FTase), while if X is Phe or Leu, the 20-carbon isoprenoid geranylgeranyl is added by protein geranylgeranyltransferase type I (GGTase I) (23, 50, 161). FTase and GGTase I are heterodimeric enzymes that share a β subunit (Ram2) but contain distinct α subunits, Ram1 and Cdc43, respectively (91, 198). Geranylgeranylation can also be carried out at other C-terminal motifs (CC or CXC), which are present on Rab proteins, and in these cases is mediated by geranylgeranyltransferase type II. GGTase II is encoded by the BET2 and BET4 genes (125, 198).
The prenylation field has benefited from the study of fungal pheromones and yeast a-factor in particular.
In retrospect, the trail of evidence which led to the discovery of prenylation and the CAAX-processing pathway provided an early example that clues drawn from different organisms and types of proteins (secreted fungal pheromones, mammalian oncoproteins, and mammalian nuclear scaffold proteins) can be effectively pieced together to inform researchers about common eukaryotic pathways. In terms of a-factor studies, as will become clear in the sections below, several fields have benefited from the knowledge of a-factor biogenesis. These include postprenylation CAAX processing, ABC transporter function, and mammalian nuclear lamin A processing. It is likely that other areas of research, as yet unknown, will also benefit from our knowledge of a-factor. An early hint that this is so comes from the studies of germ cell migration in Drosophila discussed at the end of this review.
BIOGENESIS PATHWAY OF a-FACTOR
Structures of the Precursor and Mature Forms of a-Factor
The two similar versions of the S. cerevisiae a-factor precursor encoded by the homologous genes MFA1 and MFA2 are functionally redundant and differ by only a few residues (37, 184). Most studies of a-factor biogenesis have been carried out with the MFA1-encoded precursor (Fig. 2, top). The mature bioactive form a-factor is a farnesylated and carboxylmethylated peptide, 12 amino acids long, with a molecular mass of 1.6 kDa, as determined by mass spectrometry (5) (Fig. 2, bottom). The a-factor precursor is itself quite short compared to most proteins (36 amino acids for Mfa1 and 38 amino acids for Mfa2). The mature portions of the a-factor precursors encoded by MFA1 ((Fig. 2, shaded) and MFA2 are flanked by a hydrophilic N-terminal extension and the C-terminal CAAX motif.
To the extent that it has been examined, the Mfa2 precursor has been found to undergo the same biogenesis pathway as Mfa1 (56). It is worth noting that the MFA2 mRNA transcript, which is unstable, has served as a valuable model molecule for studying mRNA processing and stability, which has led to the identification of a number of mRNA processing factors (239).
The a-Factor Biogenesis Pathway Involves Three Modules: C-Terminal CAAX Processing, N-Terminal Cleavage, and Nonclassical Export
The a-factor biogenesis pathway, beginning with the precursor and culminating in mature secreted a-factor, is shown in Fig. 3 along with the a-factor biosynthetic intermediates and the cellular machinery that mediates each step of biogenesis. As noted above, the a-factor biogenesis pathway can be conveniently viewed as involving three distinct and ordered modules (116): module 1, C-terminal CAAX modification of the a-factor precursor (comprised of enzymatic steps 1 to 3); module 2, proteolytic removal of the N-terminal extension (proteolytic steps 4 and 5); and module 3, export, mediated by the ABC transporter Ste6 (step 6). Also indicated in Fig. 3 is the interaction of extracellular a-factor with its receptor, Ste3, on the surface of MATα cells (step 7). A variety of approaches, including genetic, biochemical, and cell biological studies, have revealed the identities, cellular locations, and biochemical features of the enzymes that mediate a-factor biogenesis, as summarized below and discussed in detail in the following sections.
Briefly, in module 1 (C-terminal CAAX processing), a cytosolic farnesyltransferase consisting of two subunits, Ram1 and Ram2, recognizes the CAAX motif of the a-factor precursor (P0), and mediates the first posttranslational processing step, namely, farnesylation (108, 207, 233) (Fig. 3, step 1). Subsequently, either of the two ER-bound proteases, Rce1 or Ste24, mediates the endoproteolysis or “AAXing” of the three C-terminal amino acids of the CAAX motif (VIA for a-factor) (Fig. 3, step 2). Rce1 and Ste24 are partially functionally redundant enzymes with regard to proteolysis of the CAAX motif (33, 34, 254, 255). Finally, the isoprenyl cysteine carboxyl methyltransferase (ICMT), Ste14, adds a carboxylmethyl group to the farnesylcysteine of a-factor (Fig. 3, step 3), resulting in the fully C-terminally modified a-factor precursor (P1) (112–114).
In module 2, N-terminal processing occurs in two sequential steps. The zinc metalloprotease Ste24 cleaves the P1 form of a-factor between residues T7 and A8 to yield the P2 intermediate (Fig. 3, step 4) (94, 237, 254, 255). Subsequently, a second cleavage event, mediated by another zinc metalloprotease, Axl1, takes place between residues N21 and Y22 (Fig. 3, step 5), yielding mature intracellular a-factor (MI) (1, 94). The reason that N-terminal processing occurs in two steps is not understood, but it is clear that Axl1 cannot cleave without prior proteolysis by Ste24 (116). Notably, Ste24 plays two distinct a-factor biogenesis roles, first in C-terminal AAXing (Fig. 3, step 2) and second in the first step of N-terminal processing (Fig. 3, step 4). The dual roles of Ste24 are discussed in detail in the sections below.
In module 3, a-factor is exported from the cell via a nonclassical secretory mechanism. Ste6, a member of the ATP binding cassette (ABC) transporter superfamily (26, 150, 179, 181), carries out this export step, resulting in mature extracellular a-factor (ME) (Fig. 3, step 6). After export, secreted a-factor diffuses through the external milieu to interact with the G-protein-coupled receptor Ste3 on the surface of MATα cells (Fig. 3, step 7), stimulating the mating responses that ultimately lead to cell and nuclear fusion (98, 105, 246).
The a-factor pheromone from S. cerevisiae and similar pheromones from other fungi (discussed below) are unique among signaling molecules in two ways: first, they are prenylated, and second, they utilize an ABC transporter for export. The connection between these two unique properties remains only partially understood. An interesting possibility, discussed further at the end of this review, is that other signaling molecules, aside from fungal pheromones, also exist and use similar conserved molecular machinery.
Identification of the a-Factor Biosynthetic Intermediates
Several key experimental approaches have been effectively utilized in the investigation of the a-factor biogenesis pathway. These include genetic studies to identify mating-defective sterile (ste) mutants (1, 94, 166, 167, 270, 271), biochemical analyses to determine the activity corresponding to the mutant gene (7, 18, 33, 108, 112–114, 136, 186, 233, 237, 255), pulse-chase metabolic labeling experiments to determine which step of a-factor biogenesis is blocked in mutant cells (1, 26, 56, 94, 116, 150, 231), and mutational analysis of MFA1 to define the residues of a-factor required for recognition by the biogenesis enzymes (43, 44, 56, 116, 172, 260). Most commonly, SDS-PAGE has been used to examine the 35S-labeled a-factor biosynthetic intermediates present in intracellular and extracellular fractions from wild-type and mutant cells (Fig. 4) (94, 116, 231). The a-factor biosynthetic intermediates that can be distinguished by differences in gel mobility by SDS-PAGE (P0, P1, P2, and M) are indicated in Fig. 3 and 4. Thin-layer chromatography (TLC) has also been employed to analyze a-factor intermediates, but it lacks optimal resolution properties for a-factor intermediates and is not currently in widespread use (44, 233).
Fig 4.
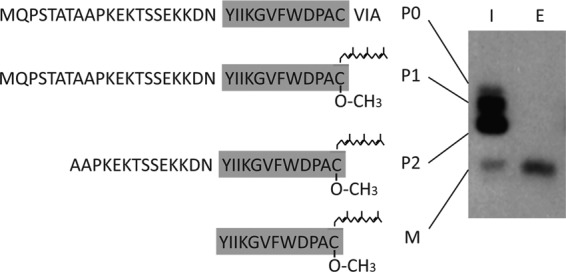
Gel analysis of a-factor biosynthetic intermediates. The precursor (P0, P1, and P2) and mature (M) forms of a-factor are resolved on a 16% SDS-polyacrylamide gel, as discussed in the text (see also Fig. 3). Cells were pulse-labeled for 5 min with Tran35S-label, intracellular (I) and extracellular (E) fractions prepared, and proteins immunoprecipitated with a-factor antibodies. (Reprinted from reference 56.)
Properties of a-factor biosynthetic intermediates.
Three major intracellular a-factor biosynthetic intermediates (P1, P2, and M) can be detected by metabolic labeling of wild-type cells with [35S]cysteine, immunoprecipitation with a-factor antibodies, and SDS-PAGE (Fig. 4 [note that Fig. 4 shows the a-factor species present after a 5-min pulse-labeling period]). The identity of each species has been ascertained by amino acid sequence analysis, mass spectrometry, and fractionation of cell lysates (farnesylated species are in the 100,000 × g pellet, and nonfarnesylated species are soluble) (56). The full-length unmodified a-factor precursor (P0) is so quickly converted to the fully CAAX-modified intermediate P1 that very little P0 is present at any given moment in a wild-type strain (thus, only a minor P0 band is seen in Fig. 4, and this is not always detectable) (56, 116). However, this unmodified P0 precursor species is the sole species present in a ram1 mutant or when analyzing a mutant version of a-factor in which the CAAX motif cysteine is altered (C33S) (56, 108).
A typical feature of most biosynthetic pathways is the complete conversion of a precursor species to its mature final form, as occurs for α-factor biogenesis. The biogenesis of a-factor is unusual in this regard, in that considerable amounts of the P1, P2, and M intracellular species accumulate in pulse-chase experiments that do not chase to mature extracellular a-factor (56, 116). The persistence of such stable unconvertible intracellular intermediates may reflect the fact that these a-factor species are in the incorrect location to be further processed or are in an improper configuration to be converted to mature a-factor. Attempts to “mobilize” these species for more efficient maturation, for instance, by promoting mating conditions (by addition of MATα cells or of synthetic α-factor) do not improve their conversion efficiency (56).
Discovery of the P2 intermediate was unanticipated and foreshadowed the discovery of the Ste24 protease.
The P2 species of a-factor (Fig. 3 and 4) was not originally predicted from a comparison of the precursor and mature forms of a-factor. Instead, P2 appeared unexpectedly in pulse-chase experiments (as illustrated in Fig. 4) and was shown by Edman degradation to correspond to a form of the a-factor precursor lacking the N-terminal 7 amino acids (56). The existence of this novel intermediate provided the first indication that N-terminal endoproteolytic cleavage of a-factor involves an obligatory two-step process (Fig. 3, steps 4 and 5).
We now know that Ste24 mediates the first of these steps to yield P2 (94, 237). Thus, surprisingly, Ste24 plays dual roles in a-factor biogenesis, first mediating C-terminal CAAX processing redundantly with Rce1 (Fig. 3 step 2) and then carrying out N-terminal proteolysis, where it alone acts (Fig. 3, step 4) (33, 34, 237, 254, 255). The ability of Ste24 to recognize two distinct cleavage sites in the a-factor precursor is a notable and not well understood feature, since proteases generally have specific recognition sites for cleavage.
Yeast a-Factor Biogenesis Enzymes Are Highly Conserved
In the sections below, we discuss each of the enzymes involved in a-factor biogenesis. We review the historical context of their discovery, as well as what is currently known about their biochemical activities, topologies, and cellular localizations. Many, but not all, of these enzymes are membrane proteins, and their topologies are indicated in Fig. 5 and Fig. 6. For all of the enzymes discussed below, mammalian homologs are known. For many cases, including those of Ram1, Rce1, Ste24, and Ste14, the yeast protein was the founding member of their respective enzyme family, and research on these yeast enzymes provided the groundwork for further studies in mammalian cells. Special emphasis is placed on Ste24 and its metazoan homolog ZMPSTE24, which have led to insights into several arenas, including mammalian lamin A biogenesis, the premature-aging disorder Hutchinson-Gilford progeria syndrome (HGPS), and the related progeroid disorders mandibuloacral dysplasia (MAD) and restrictive dermopathy (RD) (19, 279).
Fig 5.
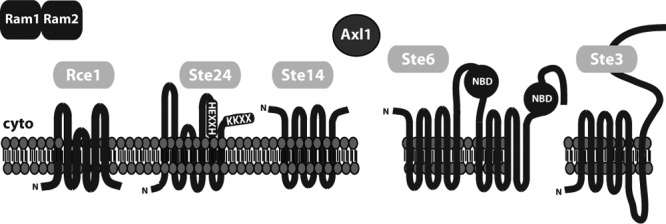
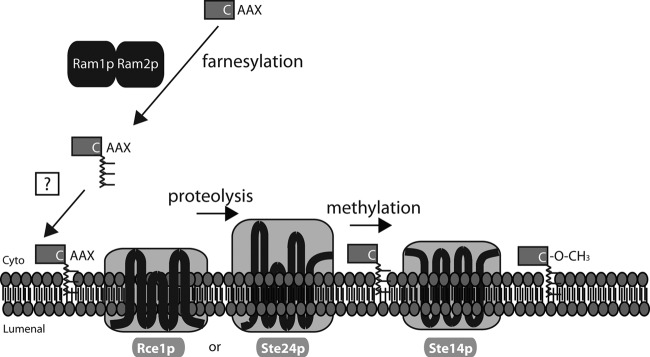
Topology of a-factor biogenesis components. The predicted or demonstrated membrane topology of each integral membrane biogenesis component is shown and is discussed in the text. Rce1, Ste24, and Ste14 are in the ER membrane of MATa and MATα haploid cells and MATa/MATα diploid cells, Ste6 is in the plasma membrane of MATa cells only, and Ste3 is in the plasma membrane of MATα cells only. The Ram1/Ram2 prenyltransferase and the Axl1 zinc metalloprotease do not contain predicted transmembrane spans. In Ste24, the HEXXH zinc metalloprotease motif and the KKXX ER retrieval motif are indicated.
Fig 6.
Is there an escort/ER delivery protein involved in CAAX processing? The components and cell biological features of CAAX processing are discussed in the text. Prenylation is carried out by Ram1/2 in the cytosol. The potential existence of an escort or chaperone protein (question mark) that shields the lipophilic prenyl group of a newly prenylated protein and delivers it to the postprenylation processing enzymes in the ER membrane is discussed in the text.
C-TERMINAL CAAX MODIFICATION OF a-FACTOR IS MEDIATED BY Ram1/2, Rce1 OR Ste24, AND Ste14
Farnesylation by Ram1/2, the Heterodimeric Farnesyltransferase
Genetic screens yielded the RAM1 and RAM2 genes.
The heterodimeric yeast farnesyltransferase (FTase) enzyme consists of the β and α subunits Ram1 and Ram2, respectively (108, 185, 198, 207, 232, 233) (Fig. 5 and 6). The Ram1/Ram2 FTase covalently joins the 15-carbon-long isoprenoid moiety farnesyl to the cysteine sulfhydryl in CAAX proteins, via a thioether bond. Ram2 can alternatively heterodimerize with Cdc43 to form the GGTase I enzyme, which joins the 20-carbon isoprenoid geranylgeranyl to proteins in which the last residue of the CAAX motif is either Leu or Phe (23, 50, 91, 198).
(i) Ram1, the β subunit of FTase.
The identification of the gene encoding Ram1, the FTase β subunit in yeast, contributed an important clue to the early views of a-factor processing. RAM1 was identified in two unrelated and independent yeast mutant hunts, one for sterile (ste) mutants defective in a-factor activity (yielding a mutation called ste16), and another for a suppressor of a hyperactive allele of RAS2 (yielding a suppressor called supH that was temperature-sensitive [Ts−] for viability). The ste16 mutant had an unanticipated growth rate defect that was not expected for a mutant defective solely in a-factor production, while the supH mutant had a defect in a-factor production resulting in MATa-specific sterility, which was not expected for a Ts− mutant defective solely in Ras production (185, 207, 271). Thus, the two mutations, ste16 and supH, caused similar phenotypes (compromised viability and a lack of a-factor) and were shown by functional and positional tests to represent alleles of a single gene, renamed RAM1 (for Ras and a-factor maturation) (207). A third independent screen yielded a mutation leading to mutant defective in both Ras processing and a-factor production (dpr1), which also represented an allele of the RAM1 gene (96). In ram1 mutants, Ras and a-factor both fail to undergo a characteristic gel mobility shift, which was eventually shown to be due to a complete lack of CAAX processing (96, 207, 233). The striking genetic connection between the otherwise unrelated proteins Ras and a-factor, and the observation that both contained a CAAX motif (which was then of unknown function), was a significant clue to understanding this motif.
However, it took a number of years to determine that Ram1 mediates prenylation. At the time that the ram1 mutant was identified, in 1986, prenylation had been observed in only a few obscure fungal species and was not widely appreciated (101). The posttranslational modifications of a-factor had not yet been shown. Furthermore, the Ras proteins H-Ras and N-Ras were known to be palmitoylated (palmitate is a 16-carbon-long fatty acid, which is very different in structure from farnesyl). Thus, Ram1 was originally suggested to mediate palmitoylation of both Ras and the lipophilic a-factor (207). Indeed, in a yeast ram1 mutant, palmitoylation of Ras did not occur (96, 207). However, subsequent biochemical and mass spectrometry studies revealed that both a-factor and Ras are indeed farnesylated and that farnesylation is a prerequisite for palmitoylation of Ras (5, 106), thus leading to the revised hypothesis that Ram1 is involved in farnesylation. The purification of the mammalian farnesyltransferase as a heterodimeric enzyme (57, 212) containing a subunit homologous to Ram1 and the demonstration that prenylation was defective in a ram1 mutant (108, 233) provided definitive proof that Ram1 is a subunit of the yeast FTase.
(ii) Ram2, the α subunit shared by the FTase and GGTase I.
Ram2 was identified as a temperature-sensitive mutant in a directed two-part genetic screen for strains defective in both Ras and a-factor activity (108). First, the Ras-based screen that had yielded the supH allele of RAM1 was repeated, and additional suppressors of hyperactive Ras2 that mapped to complementation groups different from RAM1 were identified. Subsequently, these mutants were screened for a MATa-specific mating defect, leading to identification of the RAM2 gene. FTase enzymatic activity was detected in extracts prepared from Escherichia coli coexpressing RAM1 and RAM2 (or by mixing E. coli extracts expressing these individually), demonstrating conclusively that Ram1 and Ram2 together comprise the yeast FTase (108).
(iii) Historical interplay between yeast and mammalian studies.
Soon after yeast RAM1 provided the initial link between a-factor and Ras, yeast FTase studies were eclipsed by dramatic advances from the Brown and Goldstein groups (57, 212), who purified the mammalian FTase α and β subunits. Ultimately, the purification, biochemical characterization, crystallization, and structural analysis of the mammalian FTase advanced the field significantly and prompted the development of farnesyltransferase inhibitors (FTIs) that initially held great promise as potential chemotherapeutic agents for Ras-based cancers (21, 23, 29, 99, 156). FTIs have undergone clinical trials for cancer chemotherapy, but for Ras-based cancers they have been less effective than hoped, most likely due to the alternative geranylgeranylation of Ras by GGTase in FTI-treated tumor cells (156). Clinical trials to determine the efficacy of FTIs for treating progeria are now under way and are discussed below.
Farnesylation is required for a-factor biogenesis: fate of a-factor in ram1 and ram2 mutants.
Prenylation is the first step in a-factor biogenesis (Fig. 3, step 1). Analysis of a-factor biogenesis in ram1 and ram2 mutants permitted the assessment of the fate of the nonprenylated a-factor precursor, P0. In both mutants, P0 is soluble, undergoes no subsequent a-factor processing steps, and is not stable (56). Similarly, a Cys-to-Ser substitution mutation in the a-factor CAAX motif (C33S) leads to the same fate (56). Thus, prenylation is obligatory for stability and for all subsequent steps in a-factor biogenesis. One possibility to explain the need for prenylation in a-factor biogenesis is that without its prenyl group, a-factor cannot reach the membrane, where most other processing enzymes reside (Fig. 3). In addition, the prenyl group appears to be directly required for the recognition of a-factor by its processing enzymes (41, 44, 112, 172, 231). Interestingly, when a-factor is forced to undergo geranylgeranylation instead of farnesylation (by mutation of its CAAX motif from CVIA to CVIL), there is no impact on a-factor processing, export, or mating, indicating that either form of prenylation is sufficient for maintaining the biological properties of a-factor (43).
Viability of ram1 and ram2 mutants.
A ram1 deletion mutant is viable at 30°C, albeit significantly sick, whereas a ram2 deletion mutant is inviable (108, 207). Since numerous farnesyltransferase substrates, including Ras proteins, are essential for viability in yeast, the viability of the ram1 mutant seems surprising at first glance. Most likely, the cross-prenylation of essential proteins such as Ras and others by the Cdc43/Ram2 GGTase I in the absence of FTase can account for the viability of the ram1 mutant (261). Indeed, overexpression of Cdc43 partially suppresses growth defects of the ram1 mutant, and the capability to cross-prenylate has been demonstrated for both the Cdc43/Ram2 GGTase I and the Ram1/Ram2 FTase (261).
Notably, the slow-growing ram1 mutant throws off suppressors that permit enhanced growth but are still unable to produce bioactive prenylated a-factor (108; S. Michaelis, unpublished data). The gene that is altered in these suppressor strains is not known. Although they are unable to promote prenylation of a-factor, it remains possible that these suppressors can directly or indirectly enhance cross-prenylation of certain CAAX substrates, such as Ras1 and Ras2 by the yeast GGTase I, but not other substrates, such as a-factor. Not surprisingly, given the critical role of Ram2 as a common subunit of both the GGTase I and FTase enzymes, yeast ram2 deletion mutants are fully inviable, and suppressors do not arise (108).
Rce1 and Ste24 Are the Founding Members of Two Distinct Families of CAAX Endoproteases, both Localized to the ER Membrane
All of the postprenylation CAAX-processing enzymes discussed below (Rce1, Ste24, and Ste14) were first identified through genetic and biochemical studies of yeast a-factor. Because the yeast and mammalian genes encoding them are so highly conserved, studies of the yeast enzymes have paved the way for understanding how their mammalian counterparts function. There is currently considerable interest in identifying small-molecule inhibitors of these enzymes, as they represent potentially new chemotherapeutic agents (29, 99).
Ste24 and Rce1 were identified through directed genetic studies of a-factor.
Genes encoding the CAAX endoprotease enzymes (also called AAXing enzymes) were not identified in standard yeast screens for fully sterile mutants. In hindsight, this is understandable, as we now know that there are two functionally redundant enzymes, Rce1 and Ste24, that can mediate processing of the a-factor CAAX motif CVIA (8, 33, 34, 94, 254). Thus, a single mutant would not have had a completely AAXing-defective phenotype. The existence of two functionally related AAXing activities, each with distinct enzymatic properties, also confounded biochemical attempts to identify a single CAAX endoprotease in yeast and mammalian cells (8, 10, 11, 278).
The discovery of the two yeast CAAX proteases, Ste24 and Rce1, relied on a set of targeted screens performed by Rine and coworkers (33). To identify STE24 (also called AFC1, for a-factor-converting enzyme), these investigators made use of an mfa1 mfa2 deletion strain transformed with a plasmid expressing a mutant form of a-factor with an altered CAAX motif (CAMQ). We now know that the CAMQ motif, from rabbit muscle glycogen phosphorylase kinase, is recognized well by Ste24 and poorly by Rce1 (33, 109). Using this strain and an autocrine arrest selection scheme, a loss-of-function mutation in STE24 was identified. The second gene, RCE1 (for Ras-converting enzyme), was found by two additional genetic screens in the same study by Rine and coworkers. In one case, a mutation that blocked the residual a-factor activity in a ste24 mutant was found. In parallel, high-copy-number plasmids that could partially restore a-factor production in a ste24 mutant expressing solely the CAMQ form of a-factor were sought (33). Both screens identified the same gene, RCE1.
While Rce1 and Ste24 can function redundantly to produce bioactive a-factor, Rce1 is solely responsible in vivo for the AAXing of yeast Ras1 and Ras2 (whose CAAX motifs are CIIC and CIIS). Disruption of the RCE1 gene, but not of STE24, leads to a defect in Ras localization and signaling in yeast (33, 279). Other small GTPases also appear to be substrates for Rce1 and not Ste24 (8, 99, 137, 199). Interestingly, physiological substrates for Ste24, apart from a-factor, are not known in yeast, although several studies indirectly suggest that the HSP40 CAAX protein, Ydj1, may be a substrate of Ste24 (188, 260; Michaelis, unpublished data).
Ste24 and Rce1 have nonidentical but partially overlapping substrate specificities for differing CAAX motifs.
The substrate specificity of Ste24 versus Rce1 for distinct CAAX motifs has been examined using a-factor constructs with altered CAAX motifs (260). Such constructs are expressed from a plasmid transformed into a series of yeast strains (wild type, ste24Δ mutant, rce1Δ mutant, and ste24Δ rce1Δ double mutant) in which both, one, or neither of the AAXing enzymes is present (note that these strains are also deleted for the chromosomal a-factor genes). Thus, the amounts of a-factor produced by these strains, assayed by the halo or mating assays discussed below, reflect the efficacy of AAXing. In this setting, certain CAAX motifs are found to be strictly Rce1 specific (i.e., CTLM from Ste18, the γ subunit of a yeast G protein), some are Ste24 specific (i.e., CAMQ from rabbit muscle phosphorylase kinase), and some can be AAXed by either Rce1 or Ste24 (i.e., CVIA from a-factor) and others by neither (147, 204, 260, 279). Definitive predictive rules concerning Ste24 versus Rce1 dependency have not yet emerged, as all possible CAAX motifs have not yet been tested in this assay (260).
Determinants other than the CAAX motif may also be able to influence AAXing specificity, at least for certain proteins. Notably, we have observed that the CAAX motif of Ras2 (CIIS), when placed at the C terminus of a-factor, can be processed by either Rce1 or Ste24 in vivo (Michaelis, unpublished data). However, in the context of Ras2 itself, Rce1 appears to be solely responsible for AAXing, as Ras2 localization in yeast is completely disrupted in the rce1Δ mutant and is unaffected in the ste24Δ mutant (33, 279). Thus, the commonly employed a-factor swap assay discussed above in which the a-factor CAAX motif is replaced by a “test” CAAX motif may not always provide a completely accurate assessment of inherent Rce1 or Ste24 specificity for a particular CAAX protein (188). It should be noted that a few CAAX motifs that can be cleaved independently of Rce1 or Ste24 have also been identified (147), for which the protease remains unknown.
Commonalities and differences between Ste24 and Rce1: Ste24 is a bona fide zinc metalloprotease, but whether Rce1 is itself a protease is not completely clear.
Ste24 and Rce1 both contribute to a-factor CAAX processing in yeast and are multispanning membrane proteins localized in the ER membrane (236) (Fig. 3, 5, and 6). Ste24 has also been shown to localize to the inner nuclear membrane (INM), a location contiguous with the ER membrane (15), and this is also likely the case for Rce1. Such a dual location in the ER membrane and the INM would ensure that Ste24 and Rce1 can proteolyze both cytosolic and nuclear CAAX substrates. Ste14, the CAAX methyltransferase, also exhibits dual ER and INM localization (15).
Endoproteolysis of the a-factor CAAX motif by either Rce1 or Ste24 is strictly dependent on prior prenylation of the CAAX motif cysteine (33). Surprisingly, Rce1 and Ste24 are not homologous, share no features in common (aside from multiple transmembrane [TM] spans), and may have very different mechanisms of action from one another. Purified Ste24 has been demonstrated to be a zinc metalloprotease (255), as discussed in detail below. Rce1, on the other hand, has been refractory to purification and lacks a recognizable protease motif (33, 199, 204, 206). Thus, it remains possible that Rce1 may be necessary, but not sufficient, for AAXing, functioning to activate an as-yet-unidentified protease. Resolving this issue will be important given the current interest in Rce1 as a potential anticancer drug target for Ras-based tumors (99, 272). The individual biochemical and cell biological features of the Ste24 and Rce1 proteins are discussed in more detail in the following sections.
Cell biological features of Ste24.
Ste24 is a 52-kDa (453-amino-acid) integral membrane protein (17, 19, 33, 94, 236, 255). Ste24 is predicted by hydropathy analysis to possess 7 TM spans (Fig. 5). The N terminus of Ste24 appears to be luminal and the C terminus cytosolic, based on protease protection studies using N- and C-terminally hemagglutinin (HA)-tagged versions of Ste24 (255) (Fig. 5). The C terminus of Ste24 contains a canonical dilysine ER retrieval motif (KKXX). Mutational analysis indicates that this motif is not required for ER localization of Ste24 in yeast (94), although it could play an important role to retrieve a low percentage of Ste24 molecules that may escape to the Golgi apparatus. As noted above, Ste24 is also found in the inner nuclear membrane, where it could act on nuclear CAAX proteins (15).
Ste24 contains a conserved zinc metalloprotease consensus motif (HEXXH), placing it in the M48A family of Zn metalloproteases (18). The HEXXH motif lies within a large cytosolic loop between the predicted TM spans 6 and 7 (Fig. 5) (255). Mutations in the conserved His and Glu residues in this motif (H335A and E336A) abolish Ste24 activity (94). The predicted location of this active site in the cytosol, but close to the membrane, is appropriate for the endoproteolytic cleavage of a membrane-bound prenylated substrate such as a-factor. As discussed in more detail below, Ste24 is notable in that it is responsible for two distinct activities in the biogenesis of a-factor (Fig. 3, steps 2 and 4) (17, 19, 34, 254): (i) C-terminal AAXing of a-factor, in which it is functionally redundant with Rce1 (Fig. 3, step 2), and (ii) the first step in N-terminal cleavage of a-factor (Fig. 3, step 4). The ability of Ste24 to recognize two completely different sites for processing makes it unusual among proteases.
Purification of Ste24 and demonstration of its protease activity.
The purification of Ste24 was a particularly important achievement, as it led to the unambiguous demonstration that Ste24 possesses proteolytic activity in and of itself, rather than acting indirectly to activate another protease(s) (255). The purification of a multispanning membrane protein such as Ste24 presents challenges, requiring the identification of a detergent that can both extract the protein from the membrane and also maintain its enzymatic activity. The strategy involved one-step purification of a His-tagged version of Ste24p from detergent-solubilized yeast membranes (115, 255). An optimal detergent was found to be dodecyl-maltoside.
Purified Ste24 was shown to efficiently mediate the endoproteolytic cleavage of a synthetic farnesylated version of a-factor containing an intact CAAX motif. The identity of the VIA peptide resulting from Ste24 cleavage was confirmed by mass spectrometry (255). Ste24 was shown to act in a zinc-dependent manner. This original study demonstrated that Ste24, on its own, possesses CAAX proteolytic cleavage activity (255). Purified Ste24 was also shown to directly mediate the first N-terminal processing step in a-factor biogenesis (Fig. 3, step 4) (237, 255).
In addition to its use in the direct mass spectrometry assay described above, synthetic a-factor with an intact farnesylated CAAX motif can also be used in two convenient, albeit indirect, assays for AAXing activity. In one of these, C-terminal AAXing by Ste24 can be measured in a 2-step coupled AAXing and carboxylmethylation assay (10, 11, 114, 255). In this assay, the extent of carboxylmethylation is determined after the AAXing reaction is completed, using radiolabeled S-adenosylmethionine (SAM) as a methyl donor and membranes as the source of the Ste14 carboxylmethyltransferase. Radiolabeled carboxylmethyl groups are quantitated by a base hydrolysis/vapor diffusion assay and reflect the prior level of AAXing by Ste24. Alternatively, because AAXing and subsequent carboxylmethylation of the farnesylated synthetic a-factor yield biologically active a-factor, a quantitative halo dilution assay (see below) can be used to determine the relative amount of AAXing activity present in the original test sample (255).
When Ste24 was purified, it represented the first eukaryotic multispanning membrane protein known at that time to possess intrinsic protease activity (255). Since then, a class of integral membrane proteases, called the intramembrane cleaving proteases (I-CLIPs), has been well-characterized biochemically (155). I-CLIPs include the S2P metalloprotease (required to cleave the membrane-anchored transcription factor SREBP, which is involved in cholesterol biosynthesis), the Rhomboid family of serine proteases (which mediate epidermal growth factor release), and two aspartyl proteases, the signal peptide peptidase (SPP) (which is involved in clearance of cleaved signal sequences) and γ-secretase (which generates the peptides implicated in causing Alzheimer's disease) (155). The I-CLIPs contain their catalytic subunits within transmembrane spans and likewise hydrolyze peptide bonds within their substrates' transmembrane domains. Like the I-CLIPs, Ste24 is a multispanning membrane protease. However, in contrast to I-CLIPS, Ste24's site of cleavage on prenylated substrates is proximal to the prenyl anchor, rather than within a transmembrane domain.
Ste24 is present in all eukaryotes.
Homologs of STE24 from a number of eukaryotic organisms, including mammals, plants, and parasites, encode enzymes that are functionally similar to Ste24, in that when heterologously expressed in yeast they can mediate AAXing of the a-factor CAAX motif (35, 36, 39, 40, 93, 100, 188, 254). Mammalian ZMPSTE24 (for zinc metalloprotease Ste24) is the best-characterized homolog (19). Quite strikingly, like yeast Ste24, human ZMPSTE24 (also called HsSte24 or FACE-1) can mediate both C- and N-terminal processing of a-factor in yeast, as evidenced by mating and halo assays (254). The characterization of the zmpste24−/− knockout mouse led to the discovery that ZMPSTE24 is the lamin A endoprotease (24, 203). Mammalian ZMPSTE24 and the role of mutations in this gene in human progeroid disorders are discussed further below.
Rce1 remains refractory to purification, precluding the demonstration that it is in fact a protease.
Rce1 (Ras-converting enzyme) is a 36-kDa (315-amino-acid) integral membrane protein, predicted to contain 6 transmembrane spans (Fig. 5). However, the topology of Rce1 has not been experimentally investigated. CAAX processing of Ras proteins appears to be solely dependent on Rce1 in vivo, while that of a-factor can be mediated by either Rce1 or Ste24 (33).
Whether Rce1, like Ste24, possesses protease activity or whether Rce1 simply activates another protease remains an open question, since the purification of Rce1 in an active form has not yet been achieved. Rce1 lacks a defined protease motif for any of the four standard categories of proteases: aspartyl, cysteine, serine/threonine, or metal dependent. At least one report has suggested that Rce1 is a cysteine protease (82), but the sole Cys residue in Rce1 is not required for enzyme function (204). Other reports have suggested that Rce1 may be a noncanonical zinc metalloprotease (202). Several residues important for activity have been identified by mutational analysis but do not provide further clarification of the protease category to which Rce1 belongs (204), nor do standard protease inhibitor studies (206). Until Rce1 can be purified, it will not be possible to know whether it is solely responsible for endoproteolysis or instead whether it requires a binding partner or stimulates another protease.
Rce1 is specifically required for the maturation and activity of Ras proteins. Because the oncoprotein Ras is mutated in many cancers, small-molecule inhibitors of Rce1 are of clinical and therapeutic interest (99, 272). Several of these have been identified, including those from a compound library screening effort (77, 171, 206). Of the latter inhibitors, some are specific for Rce1, while others inhibit both Rce1 and Ste24. It remains to be determined if such compounds could be useful for cancer treatment.
Ste14, the Founding Member of the Isoprenylcysteine Carboxylmethyltransferase Family, Carries out the Final Step of CAAX Processing
Ste14 is the founding member of the isoprenylcysteine carboxylmethyltransferase (ICMT) family of enzymes (9, 112–114, 219, 220, 231). Ste14 mediates carboxylmethylation of endoproteolyzed CAAX proteins by transferring a methyl group from S-adenosylmethionine (SAM) to the free carboxyl of the prenylated cysteine residue (7). The STE14 gene was identified in a mutant hunt for MATa-specific sterile mutant (271). Membranes from wild-type yeast were shown to possess ICMT biochemical activity, and this activity was lacking in membranes from a ste14 mutant (112, 113, 174).
Ste14 is 239 amino acids in length, with a molecular mass of 28 kDa. Topology studies suggest that Ste14 contains six transmembrane spans (Fig. 5), with the last two spans comprising a hairpin turn with no extracellular loop. A substrate binding domain has not yet been identified; however, a conserved tripartite consensus motif is located in the C-terminal region of Ste14 and includes transmembrane domains 5 and 6 and the hydrophilic regions just N and C terminal to these spans (219).
Production of active ICMT activity in E. coli heterologously expressing the STE14 gene provided the first evidence that Ste14 is the sole component required for ICMT activity (113, 114). Subsequently, purification of detergent-solubilized Ste14 from yeast and its reconstitution into artificial lipid vesicles led to unambiguous proof that Ste14 is both necessary and sufficient for ICMT activity. Ste14 was shown to be a zinc-dependent enzyme (7).
Ste14 carboxylmethylation activity can occur independently of any peptide context, as reflected by the fact that a commonly used substrate for Ste14 is N-acetyl farnesylcysteine (N-AFC) (7, 112, 113). Recent evidence suggests that Ste14 forms a dimer or other higher-order oligomer through a GXXXG motif in its first transmembrane span (104, 218). Heterologous expression of either the Schizosaccharomyces pombe STE14 homolog, Mam4, or the mammalian ICMT can functionally complement a yeast ste14Δ mutant, indicating that distant homologs have conserved function (68, 220).
Ste14 is located in the ER membrane, although neither ER retention signals nor ER retrieval motifs are evident (220, 236). Interestingly, adding an HA epitope tag to Ste14, at either the N or C terminus or internally, disrupts ER localization and results in transport of Ste14 to the Golgi membrane (220). Ste14 is also in the inner nuclear membrane, as is the case for Ste24, where it can mediate the carboxylmethylation of nuclear CAAX proteins (15).
Ras and a-factor are the two best-characterized cellular substrates of Ste14 in yeast. In a ste14Δ mutant, the lack of Ste14 activity results in biologically inactive a-factor (hence, a sterile phenotype), and the localization of Ras is altered from the plasma membrane to puncta in the cytosol (113, 231, 279). In a ste14Δ mutant, nonmethylated a-factor is processed at its N terminus by Ste24 and Axl1 (116, 231). However, this nonmethylated, but otherwise fully processed, a-factor cannot be exported by the Ste6 transporter, either because it cannot be recognized by Ste6 or because Ste6 cannot support the transport of a-factor in its nonmethylated form (231). The nonmethylated form of a-factor that is present internally in a ste14Δ strain is highly unstable (231). Even if nonmethylated a-factor were exported, it would be essentially biologically inactive, based on the finding that a synthetic version of a-factor that is farnesylated but lacks the carboxylmethyl group cannot stimulate the Ste3 receptor on MATα cells (41, 44). Thus, carboxylmethylation plays multiple important roles for a-factor and is required for its intracellular stability, export, and receptor activation (41, 231). Notably, a ste14Δ mutant is viable, with no observable growth defects, suggesting that lack of carboxylmethylation does not affect the activity of Ras or other essential CAAX proteins, at least under standard growth conditions (231).
Ste24 AND Axl1 MEDIATE SEQUENTIAL STEPS IN THE N-TERMINAL CLEAVAGE OF a-FACTOR
The First Step in N-Terminal Cleavage of a-Factor Is Mediated by Ste24
Following C-terminal CAAX processing, a-factor undergoes N-terminal cleavage, involving two sequential steps (Fig. 3, steps 4 and 5). The first cleavage is between residues T7 and A8, and the second is between residues N21 and Y22. The latter processing event was predicted from comparison of the precursor and mature forms of a-factor (Fig. 2). However, the first step was unexpected and was revealed by the presence of an unanticipated biosynthetic intermediate, P2, discovered in metabolic labeling experiments to identify a-factor biosynthetic intermediates, as discussed above (Fig. 4) (56). P2 was shown to contain A8 as its N-terminal amino acid by Edman degradation (56). Mutants with mutations at or near the P1 to P2 cleavage site (A8G, A8T, and A9P) are defective for this first N-terminal cleavage step and block production of mature a-factor (94, 116).
Ste24 mediates this first N-terminal cleavage of the a-factor precursor and was first identified in our laboratory in a mutant hunt for “leaky” sterile mutants (94). Shortly thereafter, Ste24 was independently identified as a CAAX endoprotease that functions redundantly with Rce1, as discussed above (33). While Ste24's apparent role in two completely different steps of a-factor biogenesis appeared at first to be contradictory, the dual role of Ste24 was confirmed by genetic and biochemical studies (34, 254, 255). Because of the overlapping roles of Rce1 and Ste24 in CAAX proteolysis, only the N-terminal cleavage step of a-factor biogenesis is blocked in a ste24Δ mutant (94, 116). It is notable that the sterile phenotype of a ste24Δ mutant is only partial, resulting in residual mating. This is because a small percentage of a-factor (2 to 5%) is N-terminally cleaved to mature a-factor, even in the absence of Ste24, likely due to the ability of Axl1 or its homolog Ste23 (see below) to either bypass or substitute for Ste24 (116).
The fact that Ste24 promotes cleavage at two sites in a-factor (C-terminal CAAX processing and N-terminal cleavage) has been amply demonstrated (34, 237, 254, 255). Such a dual role is unprecedented among proteases, particularly because the cleavage sites in a-factor bear little resemblance to one another. The C-terminal CAAX cleavage occurs between a prenylated cysteine (C33) and valine (V34), and the N-terminal Ste24 cleavage site is between threonine (T8) and alanine (A9) (Fig. 3 and 4). While it has been suggested that there may be multiple active sites within Ste24 that allow it to mediate cleavage of a-factor at two very different sites (66), this seems unlikely, since a mutation in the HEXXH zinc metalloprotease domain of Ste24 disrupts both C-terminal and N-terminal processing of a-factor (33, 237). It is possible that although there is a single catalytic site, there could be two substrate recognition regions within Ste24.
It is notable that the dual roles for Ste24 appear to be conserved throughout evolution, as mammalian ZMPSTE24 expressed in a ste24Δ yeast mutant can complement for both activities (254). In addition, ZMPSTE24 also mediates two processing steps for another protein, the mammalian lamin A precursor. These two steps are C-terminal AAXing and endoproteolytic cleavage of the lamin A tail, 15 residues away from the prenylated C-terminal cysteine (note that in a-factor, the site of Ste24 N-terminal cleavage is 26 residues away from the prenylated cysteine) (19, 24, 160, 203; S. Michaelis and C. Hrycyna, unpublished data). Processing of lamin A by ZMPSTE24 is discussed in more detail below.
Axl1 Is a Predicted Zinc Metalloprotease That Generates Mature a-Factor
Axl1 possesses an inverted zinc metalloprotease motif.
The final step in N-terminal processing of a-factor (Fig. 4, step 5) is carried out by the zinc metalloprotease Axl1 (1, 94, 186). Axl1 cleaves between residues N21 and Y22 of the a-factor precursor, but only after Ste24 has removed the first seven residues of the N-terminal extension. AXL1 was identified in a screen for partially sterile mutants and was originally called ste22 (1). The final step of a-factor N-terminal cleavage was shown to be defective in the axl1 mutant, resulting in accumulation of the P2 a-factor intermediate (Fig. 3 and 4) (1, 94). Axl1 possesses an “inverted” zinc metalloprotease motif (HXXEH), strongly suggesting it directly mediates a-factor proteolysis, although definitive proof that Axl1 acts alone awaits its purification and the demonstration of proteolytic activity in vitro. The HXXEH motif is critical for Axl1 function, since mutations in this motif block the production of mature a-factor (1). The reason that an axl1Δ mutant is not fully sterile and has residual a-factor activity is that yeast encodes an Axl1 homolog, Ste23, that can partially compensate for a-factor processing when Axl1 activity is absent (1). Ste23 also possesses an HXXEH motif. Both Axl1 and Ste23 are members of the M16A subfamily of zinc metalloproteases (140, 186).
Recognition determinants for Axl1 may be complex. A number of mutations that alter residues flanking the Axl1 cleavage site in the a-factor precursor are defective in processing (116). Point mutations and deletions elsewhere in the N-terminal extension can also block Axl1-mediated cleavage of a-factor (116). In an axl1Δ mutant, a-factor remains incompletely processed and cannot be exported by Ste6 (1, 94, 116). This could be due to the failure of Ste6 to recognize forms of a-factor such as P2 that are too long, or alternatively, it could be that Axl1 is required for the delivery of a-factor to the Ste6 transporter.
Additional cellular roles and cell biological properties of Axl1.
Interestingly, Axl1 has a cellular role in haploid MATa and MATα cells that appears to be completely unrelated to a-factor biogenesis. Axl1 was first discovered and named based on its role in promoting an axial budding pattern in haploid yeast, and it was shown to be expressed exclusively in haploid cells and not in diploid cells (95). It is notable that Axl1 mutants defective in the HXXEH zinc metalloprotease motif are not defective in axial budding, suggesting that the role of Axl1 in haploid bud site selection, in contrast to its role in a-factor biogenesis, does not involve proteolysis (1).
Axl1 is a large protein of 1,208 amino acids (138 kDa). The cellular site where the Axl1 processing of a-factor occurs is not known. The Ste24-mediated N-terminal cleavage step that occurs prior to Axl1 cleavage takes place on the cytosolic face of the ER membrane (Fig. 3 and 5). However, Axl1 is not predicted to contain any transmembrane spans and thus could be a soluble protein (Fig. 5). One report shows that Axl1 can localize to the mother-bud neck junction which could occur through protein-protein interactions (164) and would be consistent with Axl1's role in budding but not necessarily in a-factor processing. Whether Axl1 is present at sites in the cell other than the mother-bud neck junction is not known. The steady-state level of Axl1 is diminished in a yeast mutant deleted for the YDJ1 gene, which encodes a cytosolic Hsp40 chaperone in yeast. Interestingly, Ydj1 is required both for Axl1 mRNA accumulation and for Axl1 protein stabilization. However, the biological significance of these two forms of regulation of Axl1 by Ydj1 is not understood (180).
Axl1 is homologous to the mammalian insulin-degrading enzyme (IDE), also called insulysin (1). IDE has broad substrate specificity and can cleave numerous physiologically significant small molecules, including insulin and the Aβ peptide, which is implicated in Alzheimer's disease (90). Interest in this enzyme has grown, as IDE deficiency correlates with an increased risk for type 2 diabetes and Alzheimer's disease, although the physiological importance of IDE1 for human health and disease remains to be firmly established (159). Mammalian IDE heterologously expressed in yeast can substitute for Axl1 to mediate a-factor biogenesis (140). Thus, yeast may provide a tractable model system for studying the functional properties of human IDE1.
Ste6, AN ABC TRANSPORTER, MEDIATES THE NONCLASSICAL EXPORT OF a-FACTOR
Ste6 Is a Dedicated Pump for a-Factor and a Model Protein for ABC Transporter Function
The export of a-factor out of the cell by the ABC transporter protein Ste6 represents an unconventional mode of export, distinct from the classical secretory pathway (150, 179, 181). The STE6 gene was identified in a search for MATa-specific sterile mutants. DNA sequence analysis revealed that Ste6 is 1,290 residues long and belongs to the ATP binding cassette (ABC) transporter superfamily (182, 201, 253). Ste6 was one of the earliest members of this important class of transporters to be identified (26, 150, 179, 181). The STE6 gene and the two a-factor genes, MFA1 and MFA2, are transcriptionally coregulated. All three genes are expressed in MATa cells but not in MATα or diploid cells (270). This coregulated expression of Ste6 and a-factor, along with the fact that the sole phenotype of a ste6Δ mutant is a lack of extracellular a-factor, suggests that Ste6 serves as a dedicated, cell-type-specific pump for a-factor (149, 150, 181).
Ste6 is a prototypical ABC transporter.
Members of the ABC superfamily share a conserved overall architecture, consisting of two homologous halves, each with multiple (usually 6) transmembrane domains and an ATP nucleotide binding domain (NBD) that couples nucleotide hydrolysis to substrate transport (201, 253). Ste6 also exhibits this canonical domain organization (Fig. 5) and has been useful as an experimental model for structure-function studies of ABC transporters. In early studies, specific residues within the NBD consensus sequences of Ste6 were probed by mutational analysis, providing evidence for the functional importance of many of them (26). Coexpression of Ste6 partial molecules (half and quarter molecules) yielded insights into the flexible domain structure of this class of transporters (26, 27). Another ABC family member is the mammalian cystic fibrosis transmembrane conductance regulator (CFTR), an ion channel that is defective in individuals with cystic fibrosis. Mutational studies of STE6-CFTR chimeras expressed in yeast have been immensely valuable for providing insights into CFTR structure (75, 200, 256, 257). The detection of translational read-through of premature termination codons (PTC) in STE6 by Bedwell and colleagues (89) has had implications for the development of drugs to treat genetic diseases caused by PTC (134).
In most cases, the only known substrates for ABC transporters are xenobiotic compounds and not physiological native ones. The closest mammalian homolog of yeast Ste6 is the P-glycoprotein multidrug resistance (MDR) protein, encoded by the mammalian MDR1 gene (4, 182, 201, 253). The MDR1 transporter can mediate the efflux of numerous chemically distinct hydrophobic drugs that diffuse into mammalian cells, thereby promoting multidrug resistance in those cells (4). In contrast to mammalian MDR1, Ste6 represents one of the few examples of an ABC transporter for which a specific physiological substrate, namely, a-factor, is known.
The mechanism of recognition between ABC transporters and their substrates is an intriguing area of study but remains unclear. It is notable that functional complementation of a yeast ste6Δ strain for mating by expression of mouse MDR3 demonstrated that this mammalian transporter could promote a-factor export in yeast, albeit at a significantly lower level than STE6 itself (210, 211). Interestingly human MDR1 was found to be unable to complement a yeast ste6Δ mutant (151). Several reports seemed to provide evidence that a Plasmodium falciparum MDR3 homolog and the mammalian multidrug resistance-related protein MRP1 could also substitute for Ste6 to mediate the export of a-factor when heterologously expressed in yeast (222, 224, 226, 264). However, these reports could not be substantiated, and several of them were ultimately retracted (221, 223, 225). The reason why some, but not all, eukaryotic MDR proteins can complement a ste6Δ mutant for mating is not understood at this point, but ultimately these findings could provide clues to the mechanism of ABC transporter substrate recognition.
Role of a-factor CAAX modifications in Ste6 recognition.
Defects in the prenylation, AAXing, or carboxylmethylation of a-factor all result in a lack of a-factor export. For instance, as noted above, in a ste14 mutant, in which mature but unmethylated a-factor is generated, the export of a-factor by Ste6 is blocked (231). Thus, carboxylmethylation may be crucial for recognition of a-factor by Ste6. Not surprisingly, export of a-factor is also lacking in an rce1Δ ste24Δ double mutant, since AAXing is required for subsequent methylation (116).
Prenylation is also required for a-factor export by Ste6, but since prenylation is also required for all steps of a-factor localization and processing, including methylation, it is not possible to conclude that the prenyl group per se is a direct recognition determinant for Ste6 (56). The length of a-factor could also be a recognition determinant for Ste6. In mutants blocked for the Axl1-mediated N-terminal cleavage of a-factor, the resulting P2 form of a-factor (which retains the 14-amino-acid-long extension that is N terminal to the mature 12-mer) accumulates within the cell due to a lack of export (1, 94, 116). Thus, the existing evidence suggests that the farnesyl, carboxylmethyl group, and length of a-factor are all critical for export. However, whether these features reflect specific recognition determinants for the binding of a-factor to Ste6 or, alternatively, whether they reflect the intracellular accessibility or deliverability of a-factor to Ste6 is not clear.
Role of a-factor amino acids in Ste6 recognition.
It is likely that the recognition of a-factor by Ste6 does not rely on a highly sequence-specific amino acid recognition code. Several pieces of evidence support this view. First, when Ste6 is heterologously expressed in Schizosaccharomyces pombe, it can export the 9-amino-acid-long M-factor pheromone, which is prenylated and carboxylmethylated but otherwise shares little homology with a-factor (59). Second, and conversely, when the Candida albicans STE6 homolog HST6 is expressed in S. cerevisiae, it can transport a-factor, even though its native C. albicans pheromone substrate is different from a-factor (209). Third, several prenylated pheromones from the Basidiomycete fungus Schizophyllum commune can be exported by Ste6 when expressed in S. cerevisiae, even though these pheromones share no homology with a-factor (92). Interestingly, the genomes of Basidiomycetes can encode up to hundreds of different pheromones and receptors, but multiple transporters have not been reported. Finally, even the mouse STE6 homolog mdr3 can complement a yeast ste6 mutant for a-factor export and mating, as noted above (210, 211), further suggesting a low level of specificity between a-factor and its transporter.
There are, however, a few amino acid substitutions in MFA1 that are not allowable for a-factor export. These cause either a complete (G26V) or partial (G26C, P31Q, and A32K) block in export (116). These nonallowable amino acid changes may ultimately help to provide insight into any “rules” of recognition that may exist between Ste6 and its transport substrate a-factor.
Ste6 as a Model Protein for Studies of Endocytosis and ER Quality Control
In addition to serving as a model protein for ABC transporters, Ste6 has also been an important model protein for cell biological studies, in particular for dissecting the processes of endocytosis and ER quality control. Ste6 is localized to the plasma membrane in yeast, and from this site it undergoes endocytosis into endosomes, followed by either recycling to the plasma membrane or delivery to the vacuole, where it is degraded (25, 135, 142, 148, 162). Targeting to the vacuole involves an intermediate step, in which Ste6 is incorporated into intraluminal vesicles within the multivesicular body, mediated by the ESCRT pathway (25, 135, 141–143, 162). Ste6 endocytosis studies were among the first to establish the role for monoubiquitination as a key endocytic sorting signal for plasma membrane proteins and also as a key signal for recruitment to the ESCRT pathway (25, 135, 141–143, 148, 162, 165). Ste6 studies were also important for defining several components of the ESCRT pathway (146).
In terms of ER quality control, Ste6 mutants were specifically sought that were rapidly degraded by ER-associated degradation (ERAD) and could serve as defined substrates for this process (163). One such mutant protein is designated Ste6* (also called the ste6-166 mutant). Ste6* fails to reach the plasma membrane (PM) (163). Instead, it is retained in the ER membrane and undergoes ubiquitin-proteasome-mediated proteolysis by ERAD. Accordingly, Ste6* has been useful as a model in defining the ERAD machinery and demonstrating that there are several ERAD pathways (117, 118, 194, 263).
Ste6* and additional ste6 mutants have provided evidence for another aspect of ER quality control, namely, the existence of a novel ER quality control compartment called the ER-associated compartment (ERAC) (117). The ERAC is adjacent to the ER and is directly connected to it. Misfolded forms of Ste6 are concentrated in the ERAC. It remains to be determined whether the ERAC is identical to other recently defined ER-associated structures, i.e., the juxta-nuclear quality control compartment (JUNQ) (130) or the compartment called the ER-containing autophagosomes (ERA) (28). Preliminary data from our laboratory indicate that ERACs and JUNQs may represent the same compartment (280).
Ste3, THE RECEPTOR FOR EXTRACELLULAR a-FACTOR
Ste3 is the receptor for extracellular a-factor on the surface of MATα cells (105) (Fig. 3). It is a classical G-protein coupled receptor with seven transmembrane spans (Fig. 5). Upon interaction with a-factor (Fig. 3, step 7), Ste3 stimulates an intracellular signal transduction pathway that promotes cell-cell fusion between MATα and MATa cells, followed by nuclear fusion and diploid formation (Fig. 1) (157, 246). MATα cells lacking Ste3 are sterile.
Both the primary sequence of a-factor and its CAAX modifications contribute to optimal activation of Ste3. Numerous mutational alterations of residues within the mature portion of a-factor prevent the activation of Ste3, without affecting the biogenesis or export of a-factor (44, 116). Synthetic versions of a-factor lacking the methyl group, the farnesyl group, or both are significantly defective in the mating and halo assays discussed below, possessing 1%, 0.1%, and 0.0025%, respectively, of the activity level of fully CAAX-modified a-factor (41, 172). Small alterations of the farnesyl moiety can also influence a-factor activity (74). Notably, a synthetic a-factor analog containing a d-Ala5 instead of l-Ala5 acts in a hyperactive fashion, presumably due to its improved interaction with Ste3 (42). Simply exchanging the farnesyl group on a-factor with a geranylgeranyl group does not have a dramatic impact on activation of Ste3 (43).
The interaction interface between Ste3 and a-factor remains undefined, in part because a-factor is so hydrophobic that it adheres to membranes nonspecifically, complicating binding studies. In contrast, the hydrophilic α-factor exhibits specific binding to its receptor Ste2 (193), and residue-to-residue contact sites between a new cross-linkable version of α-factor and Ste2 have recently been reported (262). By analogy, recent studies involving improved methods for preparing photoaffinity-labeled a-factor could potentially lead to advances in the ability to probe the interactions of a-factor with its receptor Ste3 and its transporter Ste6 (192).
UNANSWERED QUESTIONS RAISED BY THE UNUSUAL BIOGENESIS PATHWAY AND PROPERTIES OF a-FACTOR
As evident in Fig. 3 and from the sections above, we now have a relatively comprehensive overview of the a-factor biogenesis pathway, and we understand many individual steps in detail. Even so, a number of important and interesting questions remain to be answered. For instance, whether a-factor requires certain escort proteins to travel through the cell and to diffuse in the external environment is not known. Also, it is not known whether other factors are required to optimize the a-factor pheromone gradient. These issues are discussed below. The answers to these questions may ultimately help us to understand why such an elaborate biogenesis pathway has evolved to produce a secreted molecule as small and as lipophilic as a-factor.
Intracellular Trafficking of a-Factor: Is There a CAAX-Processing Chaperone?
FTase is a soluble enzyme and is thought to carry out prenylation in the cytosol. How, then, are prenylated CAAX proteins such as a-factor efficiently delivered to the surface of the ER membrane, where the postprenylation processing enzymes are located (236)? One possibility is that a chaperone or escort protein that has yet to be identified may aid in this process (Fig. 6). Such a postulated chaperone for farnesylated a-factor might be similar to the RabGDI or REP proteins, which regulate the activity and intracellular trafficking of geranylgeranylated Rab proteins (161). It is possible that analogous factors could be identified in comprehensive screens for MATa-specific sterile mutants.
As mentioned above, where in the cell a-factor is processed by Axl1 remains unclear. Furthermore, we do not know how fully processed mature a-factor reaches its transporter Ste6 on the plasma membrane. Mature a-factor may travel on the surface of intracellular vesicles, as can occur for Ras proteins (274), or alternatively, a protein chaperone may escort a-factor to Ste6 at the plasma membrane. The Ste6 transporter itself reaches the plasma membrane by transiting through the secretory pathway, and it also exists on endosomes (25, 135, 148). Thus, whether a-factor is transported by Ste6 across the plasma membrane, or possibly across an intracellular membrane, has not been firmly established.
Is There an Extracellular a-Factor Protease?
An optimal gradient of pheromones must be detected by MATa and MATα partner cells to maximize the efficiency of mating (122, 123, 126). To ensure that the concentration of α-factor is not in excess, MATa cells secrete an aspartyl protease called barrier factor, encoded by the BAR1 gene, that cleaves and inactivates α-factor, thereby influencing the α-factor pheromone gradient (54, 126, 168). Evidence for an analogous protease, called “a-factorase,” that cleaves a-factor in the culture medium and/or the cell surface of MATα cells has been reported. However, the gene encoding this putative protease has not been identified, nor has the activity been purified (41, 173, 247). The confirmation of such an a-factor-degrading enzyme activity would be of interest, as it may help us to understand how signaling between mating partners is optimized.
Is Extracellular a-Factor Always in a High-Molecular-Mass Complex?
Gel filtration of a-factor isolated from a yeast culture supernatant reveals that extracellular a-factor (1.6 kDa) appears to be part of a high-molecular-mass complex (>600 kDa) (30, 196). It has been suggested that this may reflect an association of a-factor with carbohydrates (30). Alternatively, a-factor may be in micelles, membrane vesicles, or complexed with an extracellular carrier protein(s). It is not known whether the high-molecular-mass a-factor complex is generated intracellularly prior to export by Ste6, whether the complex forms only outside cells, or whether it is simply an experimental artifact that is generated only in specific media or under particular purification conditions.
It is noteworthy that chemically synthesized a-factor, which is not part of a complex, is nevertheless capable of efficiently stimulating G1 cell cycle arrest and the mating response in MATα cells and of producing an a-factor halo (5, 44, 172). This finding suggests that the components in the high-molecular-mass complex are not necessary for a-factor activity and rather may aid in diffusion or solubility.
It would be of interest to gain a better understanding of the precise physical form of extracellular a-factor. It is notable that extracellular a-factor is surprisingly diffusible, as indicated by the sizeable a-factor “halo” seen in agar petri plate assays for a-factor (see Fig. 7 and 8 and the next section). Future studies of the physical nature and/or associations of extracellular a-factor could provide insights relevant to lipid-modified signaling molecules in mammalian cells, such as Hedgehog and Wnt, that mediate long-range signaling in various tissues (248). Interestingly, exogenous a-factor has been variably reported to restore or not to restore mating to an a-factor-less (mfa1Δ mfa2Δ) strain (172, 184). What accounts for these differences is not well understood.
Fig 7.
Qualitative mating and halo assays to measure a-factor activity. Mating and halo assays are shown for a wild-type (WT) strain and the indicated mutants. For the mating assay, MATa cells are patched onto a lawn of MATa mating tester cells spread onto SD medium, on which only diploids can grow. For halo assays, the MATa cells are patched onto a lawn of a MATa sst2 halo tester cells on rich medium. The extent of growth inhibition of the underlying lawn reflects the amount of a-factor present and is called an a-factor halo. The assays are discussed in the text.
Fig 8.
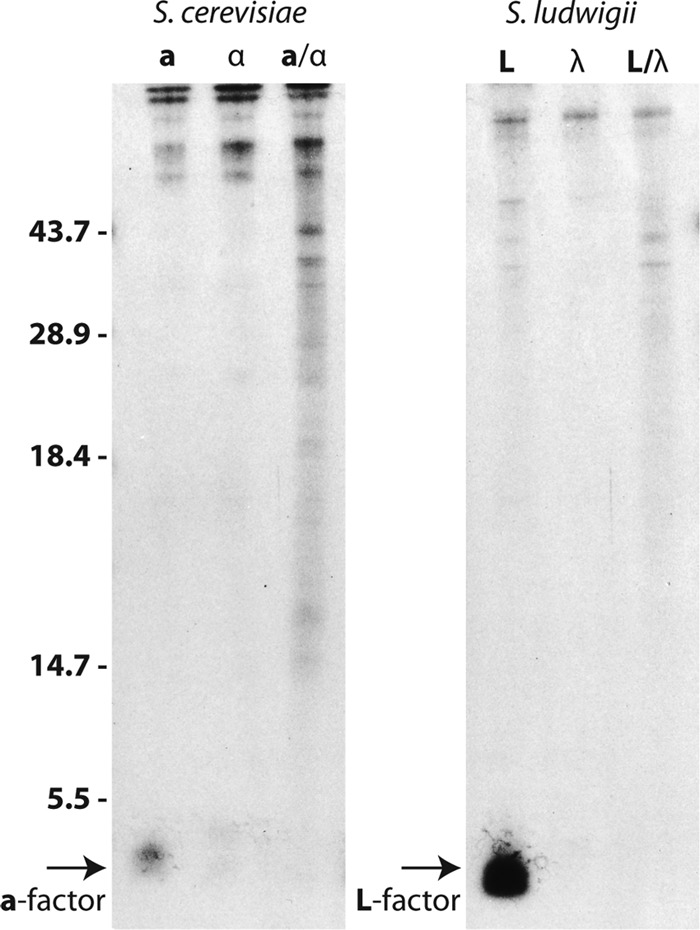
Identification of a secreted S. ludwigii mating pheromone similar to a-factor. Log-phase cultures of the indicated species of haploid and diploid cells were radiolabeled with [35S]cysteine for 30 min. Proteins in the culture media were concentrated by TCA precipitation, directly run on a 16% SDS-polyacrylamide gel, and analyzed by autoradiography.
BIOASSAYS FOR a-FACTOR ACTIVITY
Two sensitive bioassays, the “mating” and “halo” assays, can be used to measure a-factor activity. These are based on the ability of a-factor to stimulate mating responses in MATα cells and can be performed in a quantitative or qualitative way (Fig. 7) (196). In the mating assay shown here (Fig. 7, left panel), wild-type and mutant MATa strains are patched onto a lawn of MATα cells spread on synthetic depleted (SD) medium that lacks added amino acids or nucleotides. The MATa and MATα mating partners contain complementary auxotrophies, so that diploids that result from mating of cells within the patch can grow on the SD medium, while the unmated haploids in the patch and lawn cannot grow. In Fig. 7, mating is seen for the wild-type strain but not for the ste6, ste14, or ram1 a-factor-defective mutant. This mating assay can also be performed in a quantitative manner by mixing MATa and MATα cells, allowing a 4-hour mating period, and plating selectively for diploids at dilutions that allow quantitation of mating efficiency. Remarkably, the quantitative mating assay is accurate over at least six orders of magnitude, and thus it has been extensively used to test mutations in the a-factor biogenesis machinery and the impact of mutations that alter residues in a-factor itself (26, 116, 196).
In the halo assay (Fig. 7, right panel), patches of wild-type or mutant MATa cells are replica plated onto a lawn of MATα sst2 cells. An early step in the mating response pathway is G1 cell cycle arrest, which is followed by cell cycle recovery. In response to the a-factor secreted by the cells in the patch, the MATα cells containing the hypersensitizing sst2 mutation become permanently G1 arrested and fail to grow (53, 54). The zone of growth inhibition in the lawn surrounding these patches is referred to as an a-factor “halo.” The width of the halo roughly reflects the amount of active a-factor that is secreted. Inclusion of a low concentration of Triton X-100 in the lawn helps to promote a-factor diffusion and results in optimal halos (116). The a-factor halo assay is extremely sensitive, such that even strains producing 1 to 5% of the wild-type level of a-factor show a significant halo in this assay (26, 196). In Fig. 7 (right), the wild-type patch is surrounded by a significant a-factor halo, whereas the ram1 and ste14 mutants show no halo whatsoever, as expected, due to a lack of CAAX-processed a-factor. The small residual halo surrounding the ste6 mutant is due to lysis of a small percentage of cells in the patch and liberation of the intracellular a-factor that is properly processed but not exported (196). The cells that lyse to produce this residual halo are no longer alive and thus cannot mate. The phenomenon of cell lysis occurs on petri plates, but not during liquid growth, so no extracellular a-factor is detected in liquid cultures of ste6 mutants (196).
Halo tests for a-factor can also be done in a quantitative manner. In this case, cell-free culture supernatant from MATa cells or synthetic a-factor is serially diluted and spotted onto the sst2 lawn. The a-factor titer is deduced from the highest dilution that produces a spot of growth inhibition (26, 172, 255). Quantitative halo tests have been useful to test the severity of mutant forms of a-factor or mutant biogenesis components and to deduce the titers of synthetic versions of a-factor. It should be noted that synthetic a-factor can stimulate Ste3 in the halo assay, but it does not efficiently support mating to wild-type MATα cells, possibly because a key aspect of normal mating involves a pheromone gradient generated between the a-factor-producing cells and the responding cells (172, 184). Current evidence suggest that there are two mating modes, “chemotropic” and “default,” and that these two types of mating involve distinct but overlapping pathways (83, 84, 122, 123).
The a-factor mating pheromone was initially identified based on its ability to cause MATα cells to undergo a morphological change called shmooing, which can be microscopically observed (85). Shmoos are cellular projections formed when a pheromone from one cell type binds to receptors on the cell surface of the opposite mating type (Fig. 1). This shmoo assay is not easily quantifiable and is thus not in standard use. In contrast, halo and mating assays can be easily performed and are more commonly used (26, 172, 184, 196, 255).
BIOCHEMICAL ANALYSIS OF a-FACTOR
The extremely small size of both the mature and precursor forms of a-factor can present challenges for the experimentalist working with a-factor. These challenges are amplified by a-factor's hydrophobic posttranslational modifications. Alternatively, these same properties of small size and hydrophobicity can be used to great advantage, for instance, in facilitating a-factor purification. In the sections below, we discuss various biochemical procedures used to analyze and purify a-factor and their utility for purifying molecules that resemble a-factor.
Technical Aspects Regarding Detection of a-Factor Biosynthetic Intermediates
The hydrophobic properties of a-factor make it “sticky.”
Its farnesyl and carboxylmethyl modifications render a-factor extremely “sticky,” promoting its efficient adherence to the walls of standard plastic or glass tubes and pipette tips, thereby resulting in variable recovery. If it is important to maintain a-factor activity for quantitation purposes, methanol washes can be performed to remove a-factor from these surfaces. The a-factor can subsequently be dried down and reconstituted in dimethyl sulfoxide (DMSO) without loss of activity (55, 56, 196). Alternatively, an excess of blocking protein, generally bovine serum albumin (BSA), can be added to the culture medium as a competitor to prevent a-factor sticking to plastic surfaces (196). If activity is not an issue for the researcher but quantitative recovery is nevertheless important (for instance, for SDS-PAGE analysis), a detergent such as SDS can be used to efficiently solubilize a-factor from plastic surfaces (56).
SDS-PAGE conditions for the optimal separation of a-factor species.
Critical for understanding the a-factor biogenesis pathway has been the biochemical analysis of a-factor biosynthetic intermediates present in mutants defective in particular steps of biogenesis compared to those in wild-type cells. The detection of a-factor biosynthetic intermediates is generally carried out in the following way: cellular proteins are metabolically labeled by adding 35[S]cysteine and/or 35[S]methionine to growing cells (either by pulse-labeling or by steady-state labeling), extracellular and cellular fractions are collected separately, cells are lysed, proteins in both fractions are concentrated by trichloroacetic acid (TCA) precipitation, the radiolabeled a-factor species are immunoprecipitated with a-factor antibodies, and the a-factor species are analyzed by SDS-PAGE and phosphorimager analysis. In this way, a-factor biogenesis intermediates, as well as the intracellular and extracellular forms of mature a-factor, can be visualized in wild-type or mutant strains (Fig. 4) (1, 26, 56, 94, 108, 116, 231).
However, achieving ideal resolution of a-factor biosynthetic intermediates is complicated, due to the small size of a-factor (1.6 kDa and 4.3 kDa for the mature and precursor species, respectively) and its lipophilic nature. These properties render separation by SDS-PAGE challenging (56, 58, 116). We have found, counterintuitively, that running gels rapidly at high voltage minimizes diffusion of these small species and results in tight gel bands, even though the gel becomes quite hot (56, 58). For reasons that are unclear, despite the expectation that heat would promote diffusion of small species and cause bands to be less sharp, this is apparently not the case for a-factor. We have also found that 16% acrylamide gels are optimal for visualizing a-factor intermediates, with gel bands becoming less sharp when either higher or lower acrylamide percentages are used. Furthermore, we have observed that that pH of the bottom buffer in an SDS-polyacrylamide gel can make an enormous difference in being able to detect discrete a-factor bands, such that differences as small as a few tenths of a pH unit from the standard Laemmli resolving gel buffer can cause smearing of bands. These issues are discussed in more detail elsewhere (56, 58).
Finally, it is worth noting that some intermediates in CAAX processing are difficult to reliably distinguish from one another, even under optimized conditions. For instance, the prenylated but non-AAXed and nonmethylated precursor is difficult to distinguish by gel migration from the fully C-terminally modified P1 species (Fig. 4) (44, 56, 116, 231). Like most other prenylated proteins, the farnesylated form of a-factor migrates more rapidly on SDS-PAGE than the nonfarnesylated form, presumably due to alteration in SDS binding properties (56). For intermediates that differ in AAXing or carboxylmethylation status, gel migration differences can occasionally be detected between fully CAAX-processed a-factor (P1) and either the nonmethylated species (produced in a ste14Δ mutant) or non-AAXed and nonmethylated a-factor (produced in an rce1Δ ste24Δ double mutant); however, these differences are not reproducibly seen (94, 231).
Challenges of performing a-factor immunoblotting.
The small size of a-factor intermediates renders them difficult to capture on either nitrocellulose or polyvinylidene difluoride (PVDF) membranes. Thus, transfer times must be kept brief (≤10 min) to prevent migration of these species through the membrane. Interestingly, a-factor is able to migrate through even the smallest pore sizes of nitrocellulose membranes. However, PVDF membranes are capable of capturing a-factor efficiently (56). As an alternative method, thin-layer chromatography can sometimes be helpful for distinguishing certain a-factor intermediates (44, 233).
The small size of a-factor distinguishes it from other secreted molecules and facilitates its direct visualization in the culture medium.
Conveniently, the small size of a-factor is a feature that distinguishes it from most other secreted proteins. Thus, radiolabeled a-factor from the culture supernatant can be detected on its own, running near the dye front, without the use of antibodies and immunoprecipitation (Fig. 8) (55). As can be seen in Fig. 8 (left panel), a-factor is in the culture fluid of MATa cells and is absent from the culture fluid of haploid MATα or diploid MATa/α cells. The combined features of a-factor's unique small size and ease of metabolic labeling of the CAAX motif cysteine with 35S has allowed the direct identification not only of a-factor but also of other small extracellular lipidated molecules, such as L-factor and an a-factor-related peptide (AFRP) (55, 195) (discussed below).
Purification Methods for a-Factor
The fully processed and secreted a-factor pheromone exists in a high-molecular-mass complex, as discussed above (30). Conveniently, this property allows a-factor to be highly concentrated by the use of high-molecular-mass ultrafiltration cutoff filters such as Centricon filters. Thus, despite the fact that the molecular mass of a-factor on its own is 1.6 kDa, when isolated from the culture fluid it is completely retained by filters with a molecular mass cutoff of 30 kDa, permitting a significant degree of concentration (26, 196).
Another way to concentrate a-factor is to use Amberlite XAD2 resin (55, 196, 251), which consists of polymeric beads that are commonly used to extract nonpolar, hydrophobic compounds from aqueous or polar solutions containing organic compounds. XAD2 resin can be added to “conditioned medium” obtained by the removal of cells from a yeast culture grown to saturation (55, 196, 251). The beads are incubated with cell-free culture medium for several hours, during which any hydrophobic molecules in the medium, including a-factor, stick quantitatively to the beads. After the beads are collected and washed with water, the a-factor can be rinsed off with a small amount of n-propanol, permitting many orders of magnitude of concentration. Concentrated material can then be fractionated by high-pressure liquid chromatography (HPLC) and analyzed by mass spectrometry. This methodology was used to determine the structure of a-factor and an a-factor-related peptide (AFRP) (5, 55). Importantly, this technique can be used to identify small secreted prenylated proteins from any eukaryote and does not require that such a molecule be part of a high-molecular-mass complex.
Purification of a Novel Fungal Pheromone, Saccharomycodes ludwigii L-Factor: Application of Lessons Learned from a-Factor Studies
Our laboratory has used the methods described above (XAD2 resin, HPLC, and mass spectrometry) to purify a novel lipophilic pheromone that we have called L-factor (195). L-factor is secreted by the yeast Saccharomycodes ludwigii, a fungal species that divides by an unusual mode of bipolar budding and is not closely related to S. cerevisiae (148). S. ludwigii has two haploid mating types (275), which we have designated MAT-L and MAT-λ, by analogy with S. cerevisiae MATa and MATα. L-factor, secreted by S. ludwigii MAT-L cells, elicits a mating-type-specific zone of growth inhibition, or “halo,” on a lawn of S. ludwigii MAT-λ cells, as shown in Fig. 9D, but not on a lawn of MAT-L cells (data not shown). Interestingly, L-factor can also elicit a mating-type-specific response in the unrelated yeast S. cerevisiae, producing a halo on a lawn of MATα cells (Fig. 9B), similar to an a-factor halo (Fig. 9A). This finding suggested the composition of L-factor could be similar to that of a-factor (148). Note that unlike L-factor, S. cerevisiae a-factor acts solely on S. cerevisiae cells and not on S. ludwigii cells (Fig. 9C), indicating that a-factor and L-factor must not be identical. Just as extracellular a-factor can be detected directly without the need for antibodies (Fig. 8, left panel), we can detect L-factor as a discrete low-molecular-mass protein species secreted from S. ludwigii MAT-L simply by direct SDS-PAGE comparison of the culture supernatants from haploid MAT-L and MAT-λ and diploid MAT-L/λ cells after metabolic labeling of cells with [35S]cysteine and methionine (Fig. 8, right panel).
Fig 9.
S. ludwigii produces a pheromone with an activity similar to that of S. cerevisiae a-factor. Halo assays were performed by patching the indicated strains onto a lawn of MATα S. cerevisiae or MATλ S. ludwigii cells. The secreted L-factor from S. ludwigii is recognized by the receptor on S. cerevisiae, but not vice versa, as discussed in the text.
The gene that encodes L-factor has not been identified, and therefore the L-factor primary amino acid sequence is unknown. We purified L-factor based on its activity, using Amberlite XAD2 resin and HPLC, as discussed above (195). Mass spectrometry revealed that L-factor is a farnesylated and carboxylmethylated 12-mer with considerable homology to a-factor (6 of 12 positionally conserved residues are identical, and 3 of 12 are similar) (148; S. Michaelis and S. Carr, unpublished data). Apparently, this level of similarity is sufficient for L-factor to interact with S. cerevisiae Ste3 and to elicit a mating-type-specific response (Fig. 9). Interestingly, S. cerevisiae a-factor does not effectively interact with the S. ludwigii L-factor receptor, since no halo is observed (Fig. 9).
Purification of a-Factor-Related Peptide
We have also used the methods discussed above to purify and determine the composition of a novel secreted molecule in S. cerevisiae, which we discovered during our a-factor studies, called a-factor-related peptide (AFRP) (55). AFRP is a prenylated and carboxylmethylated species present in the culture medium of MATa cells, in equal abundance to a-factor itself (note that the gel resolution in Fig. 8 is not sufficient to resolve a-factor and AFRP). Certain a-factor antibodies recognize AFRP, but others do not (55). We have shown that AFRP is derived from the a-factor precursor and undergoes CAAX processing but, surprisingly, does not require Ste6 for its export (55). Mass spectrometry analyses indicate that AFRP corresponds to the C-terminal seven residues of a-factor, including the prenylated and carboxylmethylated Cys residue, but AFRP neither stimulates nor inhibits a-factor activity (55). Thus, the function of AFRP and its mode of export remain intriguing and unsolved questions.
The same methodology that we used here for AFRP and L-factor studies is likely to have general utility for the analysis of secreted prenylated signaling molecules from other fungi and possibly from metazoan species, where such secreted a-factor-like signaling molecules could exist.
LIPOPHILIC PHEROMONES ARE COMMON AMONG FUNGI
Prenylated secreted pheromones are a common feature of the two best-studied fungal classes, Ascomycetes and Basidiomycetes (32, 38, 51, 52). These classes differ based on their mode of spore generation, either inside (Ascomycetes) or outside (Basidiomycetes) of the mother cell. Recent studies indicate they also differ in the types of pheromone precursors they secrete, with Ascomycetes producing both hydrophobic and hydrophilic pheromones and Basidiomycetes producing solely hydrophobic ones such as a-factor (32, 38, 41, 51, 79, 269). This issue is discussed in detail below.
The Ascomycetes Secrete Both Hydrophobic (Prenylated) and Hydrophilic (Unmodified) Pheromones, Analogous to S. cerevisiae a-Factor and α-Factor
Similar to the case for S. cerevisiae, most Ascomycetes examined to date encode both hydrophobic (a-factor-like) and hydrophilic (α-factor-like) pheromones, expressed by two opposite mating types (41, 79, 138, 269). In general, in cases where the DNA sequence is known, the precursors for the lipophilic pheromones are relatively short, ranging in length from ∼23 to 45 amino acids (138). The single shared sequence feature among the lipophilic pheromone precursor genes is a C-terminal CAAX motif. It is notable that when the coding sequence for these fungal precursors are compared to one another, generally neither the N-terminal extension, cleavage sites, nor mature coding portions of their coding sequences exhibit obvious similarities (138). Thus, due to lack of homology and a very small gene size (∼150 bp), it is difficult to scan open reading frames (ORFs) of fungal genomes and pick out those that could encode a-factor-like precursors.
Species of the Ascomycetes for which putative prenylated precursors have been reported in the literature include the following: the fission yeast Schizosaccharomyces pombe (69–71, 266), the human pathogen Candida albicans (79), the filamentous fungi Neurospora crassa (31, 138) and Sordaria macrospora (176, 205), the chestnut blight fungus Cryphonectria parasitica (282), the rice blast pathogen Magnathorpe grisea (242), the white blight pathogen Gibberella zeae (139, 158), and the cellulase producer Hypocrea jecorina (238). For H. jecorina, the precursor is unusual, sharing commonalities with S. cerevisiae a-factor and α-factor precursors. The H. jecorina precursor contains three adjacent CAAX peptides separated by Kex2-like protease sites, implying a requirement for an initial processing step to generate C-terminal CAAX motifs (238). Pheromones from several additional filamentous Ascomycetes have been compared (31, 138). As discussed above and illustrated in Fig. 8 and 9, we have shown that the ascomycete S. ludwigii also produces a prenylated pheromone, L-factor, which bears substantial similarity to S. cerevisiae a-factor (195). In at least one case for the Ascomycetes, that of S. pombe M-factor, a synthetic prenylated and carboxylmethylated 9-mer peptide has been demonstrated to have bioactivity (266).
The issue of why Ascomycetes of opposite mating types produce two distinct types of pheromones, one lipid soluble like a-factor and the other water soluble like α-factor, is not understood (38, 51, 103, 269). This comes at some cost, as cells must express unique and complex processing machinery for the production of each pheromone. Differences in the solubility and diffusibility of the two pheromone types are likely to be biologically important. It is possible that the existence of two distinct chemical types of pheromones ensures that mating partners of the ascomycete fungi will be able to communicate, no matter whether they find themselves on the waxy lipophilic surface of a fruit or washed off that surface by the rain into a water-based (hydrophilic) milieu. In either case, at least one of the mating partners will secrete a pheromone that can efficiently reach the other partner to elicit mating responses. Once the mating response has been initiated in the mating partner responding to the more environmentally favored pheromone, this would in turn lead to the upregulation and increased secretion of the more environmentally challenged pheromone in the partner of the opposite mating type (86, 157, 250). In this way, the initiation of a mating response could help to compensate for, and overcome, conditions that clearly favor one pheromone over another. This issue has been discussed by Whiteway and colleagues (79, 269).
It is of interest that in a recent study, S. cerevisiae was “reprogrammed” to generate exclusively lipid-modified pheromones or hydrophilic pheromones and their cognate receptors, rather than one of each (103). In either case, when both partners secreted a-factor-like or α-factor-like pheromones, mating was observed. Although mating occurred with significantly reduced efficiency, this study suggests that the expression of two physically distinct pheromone types is not an absolute requirement for mating in Ascomycetes (103, 269).
The Basidiomycetes Secrete Exclusively Hydrophobic (Prenylated) Pheromones
In Basidiomycetes, all known pheromones appear to be of the lipophilic prenylated (a-factor-like) type (38, 51, 269). Basidiomycete species can exist as multiple mating types, ranging from two to thousands. In all cases, the pheromones of Basidiomycetes are encoded as precursors with a C-terminal CAAX motif. Examples of species for which such pheromones are known include Tremella mesenterica (229), Tremella brasiliensis (120), and Rhodosporidium toruloides (132, 133) (as discussed above, farnesylation was discovered as a posttranslational modification in pheromones secreted by these species). For R. toruloides, the precursor gene encodes multiple copies of the pheromone, and an initial processing step is required to reveal C-terminal CAAX motifs for each (3, 63). Other Basidiomycetes for which pheromones are known are Usitlago maydis (32, 144, 244, 252), Usitlago hordei (6, 145), Cryptococcus neoformans (72, 177, 189, 243), Schizophyllum commune (92, 267), Coprinus cinereus (38, 52, 197), and Sporisorium reilianium (235). For S. reilianium, at least 8 pheromone precursor genes have been identified, ranging in size from 10 to 74 residues, all encoding CAAX motifs. Multiple pheromone precursors are also known for C. cinereus and S. commune (38, 52, 92, 215). Notably, S. commune pheromone-receptor gene pairs have been expressed in S. cerevisiae and can substitute for the endogenous S. cerevisiae receptors and pheromones (92). Genetic and biochemical evidence supports the importance of prenylation for several Basidiomycete pheromones (145), and a few of these pheromones have been purified and/or synthesized to demonstrate their activity (6, 144, 197).
The reason why Basidiomycetes produce exclusively lipophilic, water-insoluble pheromones, in contrast to Ascomycetes, which produce hydrophilic and hydrophobic mating factors, is not known. However, having a single type, versus two pheromone types, may reflect differences between the two classes of fungi in terms of their distinct sexual life cycles. The pheromones of Basidiomycetes may be constrained to being lipophilic because of the specific roles they play in cell fusion, nuclear fusion, and/or nuclear migration in this fungal class (52).
How Prevalent Are Lipophilic Signaling Molecules?
A particularly intriguing question is whether eukaryotes other than fungi can also secrete prenylated signaling molecules. For instance, do secreted prenylated hormones exist in higher eukaryotes such as mammals? This question remains unanswered, as such signaling molecules could be challenging to detect directly. Hormones are present in the bloodstream in vanishingly small amounts. Furthermore, a hypothetical prenylated hormone might be so hydrophobic that it is readily adsorbed to circulating polypeptides or to the surfaces of the tubes or pipette tips used by researchers to manipulate samples for the purpose of detecting such novel components.
Searching for potential a-factor-like signaling molecules by genomic methods also has drawbacks. The small size of the MFA1 and MFA2 genes encoding a-factor precursors in S. cerevisiae (108 or 114 nucleotides), and likewise those of other fungal species, often precludes them from being annotated as ORFs (37, 79, 184). While it should theoretically be possible to identify short ORFs terminating with a CAAX motif in any organism, the overabundance of candidates makes this type of analysis challenging, even in species with small genomes, as most candidate short ORFs would likely not be translated. Ultimately, comparative genomics and the development of methods such as ribosome profiling (119) could provide a means of identifying short translated ORFs that encode novel prenylated signaling molecules, even within the larger genomes of mammalian cells.
KNOWLEDGE OF a-FACTOR BIOGENESIS PROVIDES INSIGHTS INTO SEEMINGLY UNRELATED MOLECULES: MAMMALIAN LAMIN A AND A DROSOPHILA GERM CELL CHEMOATTRACTANT
The a-factor biogenesis pathway comprises three modular steps (C-terminal CAAX modification, N-terminal processing, and nonclassical export). Two fascinating examples suggest that some or all of these modules may be used to generate other kinds of biologically important prenylated molecules, namely, the mammalian nuclear scaffold component lamin A, defects in which result in the premature aging disorder Hutchinson-Gilford progeria syndrome (HGPS) (19, 279), and a secreted chemoattractant involved in germ cell migration in Drosophila (214). These proteins provide powerful examples of the how the knowledge of a-factor biogenesis has suggested specific candidate genes required for the activity of other prenylated proteins, thereby significantly accelerating the pace of discovery in two very different areas of research.
Role of Defective Zmpste24-Mediated Processing of Lamin A in Progeria and Related Diseases
The mammalian nuclear scaffold proteins lamin A and lamin B were among the first proteins determined to undergo prenylation, in addition to fungal pheromones and Ras (22, 88, 111, 265). Nuclear lamins are intermediate filament proteins that polymerize to form the meshwork that underlies the nuclear envelope, presumably providing membrane support and a platform for the organization of heterochromatin, transcription factors, and nuclear pore complexes. While lacking in yeast, lamins are present in all metazoans (80, 273).
There are two main types of lamins, B type and A type. The B-type lamins (encoded by two different genes) undergo CAAX modification and are not further processed, retaining their hydrophobic farnesyl and carboxylmethyl modifications that may serve to anchor them to the nuclear envelope (76, 80, 273). The A-type lamins consist of two splice isoforms, lamin A and lamin C (encoded by a single gene, LMNA). Lamin C is a short isoform that does not terminate in a CAAX motif. Lamin A, however, is synthesized as a precursor, prelamin A, that undergoes CAAX processing, followed by an additional endoproteolytic cleavage event that occurs 15 residues upstream of the prenylated Cys residue, resulting in the cleavage of the modified tail from the protein (Fig. 10) (16, 22). This cleavage event is reminiscent of the N-terminal processing of a-factor by Ste24 (26 residues from the prenylated Cys residue) (Fig. 10) and provided the impetus for experiments to examine lamin A processing in zmpste24−/− mouse embryonic fibroblasts. These studies showed that the product of the mouse Zmpste24 gene, (called ZMPSTE24 in humans) mediates the final step of lamin A processing (Fig. 10) (24, 203). The reason why cells modify the lamin A tail only to subsequently cleave it off is not clear. However, this step in lamin A biogenesis is important for human health and longevity, since mutational alterations of either lamin A or ZMPSTE24 that diminish lamin A cleavage by ZMPSTE24 cause a spectrum of progeroid disorders (45, 73, 279). Apparently, the persistently prenylated form of lamin A causes problems in a variety of tissues by a mechanism that is not yet understood.
Fig 10.
Comparison of yeast a-factor processing and mammalian nuclear lamin A processing in healthy cells and disease cells defective in the ZMPSTE24 processing step. The processing pathway of the nuclear protein lamin A (middle) is analogous to that of yeast a-factor (left), as shown. Following CAAX processing, the modified lamin A tail is removed by ZMPSTE24, the mammalian homolog of yeast Ste24. Defective processing of pre-lamin A that leads to progeroid diseases is also shown (right). As discussed in the text, the failure to remove the tail due to the absence of the ZMPSTE24 cleavage site within pre-lamin A causes Hutchinson-Gilford progeria syndrome (HGPS). Failure to remove the tail due to mutations in ZMPSTE24 lead to the related progeroid disorders mandibuloacral dysplasia type B (MAD-B) and restrictive dermopathy (RD).
Among progeroid disorders, the best-studied is Hutchinson-Gilford progeria syndrome (HGPS), a rare and devastating disease that causes children to age prematurely and rapidly (45, 73). Children are asymptomatic at birth but begin to manifest features of premature aging at about 1 year of age. Symptoms include failure to thrive, hair loss, thin skin, lipodystrophy, and cardiovascular disease. These children typically die in their mid-teens from complications of atherosclerosis. HGPS is caused by a point mutation in LMNA that alters splicing and leads to production a form of lamin A that contains a 50-amino-acid in-frame deletion (78, 87). This mutant form of lamin A, called progerin, lacks the Zmpste24 cleavage site, resulting in its persistent prenylation, which in turn causes disease (Fig. 10). While it remains unclear how the persistently prenylated lamin A molecule elicits disease, farnesyltransferase inhibitors (FTIs), which were originally tested for treatment of Ras-based cancers, have shown promising results in cell culture and mouse models of progeria (46, 47, 102, 169, 259, 276, 277). The results of the first clinical trial testing the efficacy of FTIs in children with HGPS will soon be reported.
Mutations in ZMPSTE24 also cause progeroid disorders, either lesser or greater in severity than HGPS. Restrictive dermopathy (RD) is more severe than HGPS. RD is fatal in utero or shortly after birth and results from a complete ZMPSTE24 null phenotype, due to truncation or frameshift mutations in both alleles of ZMPSTE24 (191). All of the lamin A produced in the cells of individuals with RD is predicted to exist in the permanently prenylated toxic form. In contrast, the disease mandibuloacral dysplasia type B (MAD-B) is milder than HGPS but shares with it generalized lipodystrophy and skeletal abnormalities (2). The majority of MAD-B patients have one null and one partially active ZMPSTE24 allele, thus impeding but not totally preventing ZMPSTE24 cleavage of the lamin A tail. Taken together, the current data suggest that MAD, HGPS, and RD represent a spectrum of similar diseases of increasing severity that correlates with increasingly greater amounts of the persistently prenylated form of lamin A (19, 20, 45, 73). Mammalian ZMPSTE24 can complement a yeast ste24Δ rce1Δ mutant for a-factor activity. Thus, yeast mating and halo assays can be used to assess the severity of human ZMPSTE24 disease alleles (2, 20).
Drugs that inhibit ZMPSTE24 through off-target effects have now also been suggested to be associated with progeroid symptoms (49, 64, 65). Drug cocktails used to treat HIV patients include rationally designed protease inhibitors (PIs) that target the HIV retroviral aspartyl protease (62). However, it has been demonstrated in cell culture systems that some of these drugs can also reduce ZMPSTE24 zinc metalloprotease activity, leading to an accumulation of the prenylated uncleaved form of lamin A (49, 64, 65). These HIV PIs also inhibit yeast Ste24 in vitro (115). Whether or not the toxic lamin A is the direct cause of the progeroid and lipodystrophy symptoms experienced by some HIV patients taking PI cocktails is currently unclear, but this remains a potential explanation.
Finally, there is evidence that Zmpste24 cleavage of lamin A may be important during normal aging. Zmpste24 activity is diminished in blood vessel smooth muscle cells from aged individuals, possibly due to downregulation of Zmpste24 (208). In addition, the progerin form of lamin A, which cannot be cleaved by Zmpste24, is present in increased amounts in skin biopsy specimens from old versus young individuals and in in vitro aged tissue culture cells, suggesting that Zmpste24 cleavage of lamin A may play a role in physiological aging (178, 217). The discovery of Ste24 based on its a-factor-processing activity in turn led to its demonstration as the lamin A endoprotease and has provided a striking example of the unexpected implications of a-factor biogenesis research.
Drosophila Germ Cell Migration: Biogenesis of a Germ Cell Attractant Involves a Pathway Similar to That for a-Factor
Germ cell migration is a process that may involve a signaling molecule analogous to a-factor. Primordial germ cells in the embryos of metazoans migrate from their site of origin to a specific mesodermal location in the gonad, where they develop into mature egg and sperm (214). While chemoattractants are commonly hydrophilic secreted molecules, evidence from two model organisms, zebrafish and Drosophila, suggests that in both cases germ cell migration is guided in part by a secreted attractant that is geranylgeranylated. This conclusion is based on the findings that mutations or pharmacological treatments that diminish Drosophila or zebrafish GGTase I prenylation activity cause a defect in germ cell migration (213, 230, 258).
Recently, Ricardo and Lehmann have provided genetic evidence that a pathway strikingly similar to that of a-factor biogenesis is implicated in the production of the proposed prenylated germ cell migration factor in Drosophila (213). Mutations in Drosophila homologs for genes in each of the three a-factor biogenesis modules cause germ cell migration defects. These include Drosophila genes likely to mediate CAAX processing (βggt1 and Dme1/ste14), N-terminal cleavage (Dme1/ste24), and export (mdr49). Strikingly, mdr49 is the closest homolog in Drosophila to the yeast ABC transporter gene STE6, and expression of yeast STE6 in the Drosophila mdr49 mutant restores proper germ cell migration (213).
An ex vivo germ cell migration assay established by the Lehmann group has been utilized to probe the genetic requirements for the secretion of this prenylated chemoattractant (213). In this assay, germ cells are scored for their ability to migrate to an adjacent chamber containing conditioned medium from wild-type or mutant Drosophila tissue culture (KC) cells. Wild-type KC cells secrete the chemoattractant and prompt the migration of germ cells, but the mutant KC cells that are defective in the CAAX-processing enzymes or the ABC transporter mdr49 do not. Secretion of the chemoattractant is not inhibited in secretory mutants, suggesting that like for a-factor, an unconventional export route may be utilized. The identity of the germ cell migration chemoattractant has not yet been determined. However, the fractionation of conditioned culture medium should allow the purification of the intriguing secreted and prenylated chemoattractant. The use of biochemical methods, including capture on the XAD2 resin to which a-factor and other small hydrophobic molecules readily bind (55, 251), should facilitate purification of the germ cell attractant.
Clearly, the thorough understanding of the a-factor biogenesis pathway provided important groundwork for the characterization of the Drosophila germ cell attractant. It is of interest to note that a common pathway appears to be important in both yeast and flies for processes that are essential for reproduction in both organisms. Whether a prenylated ABC transporter-dependent chemoattractant also plays a role in mammalian germ cell migration is now a fascinating possibility to explore.
ACKNOWLEDGMENTS
We thank our many colleagues in the a-factor field and former laboratory members who have contributed so very much to our understanding of a-factor biogenesis and function. In particular, we thank Peng Chen and Stephanie Sapperstein, who initiated the a-factor work in this laboratory, Konomi Fujimura Kamada, who identified STE24, and Greg Huyer, who helped to lay the groundwork for this review.
This work was funded by grants from the NIH (GM41223 and GM51508) to S.M.
REFERENCES
- 1. Adames N, Blundell K, Ashby MN, Boone C. 1995. Role of yeast insulin-degrading enzyme homologs in propheromone processing and bud site selection. Science 270:464–467 [DOI] [PubMed] [Google Scholar]
- 2. Agarwal AK, Fryns JP, Auchus RJ, Garg A. 2003. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum. Mol. Genet. 12:1995–2001 [DOI] [PubMed] [Google Scholar]
- 3. Akada R, et al. 1989. Multiple genes coding for precursors of rhodotorucine A, a farnesyl peptide mating pheromone of the basidiomycetous yeast Rhodosporidium toruloides. Mol. Cell. Biol. 9:3491–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. 2003. P-glycoprotein: from genomics to mechanism. Oncogene 22:7468–7485 [DOI] [PubMed] [Google Scholar]
- 5. Anderegg RJ, Betz R, Carr SA, Crabb JW, Duntze W. 1988. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J. Biol. Chem. 263:18236–18240 [PubMed] [Google Scholar]
- 6. Anderson CM, et al. 1999. Molecular analysis of the pheromone and pheromone receptor genes of Ustilago hordei. Gene 240:89–97 [DOI] [PubMed] [Google Scholar]
- 7. Anderson JL, Frase H, Michaelis S, Hrycyna CA. 2005. Purification, functional reconstitution, and characterization of the Saccharomyces cerevisiae isoprenylcysteine carboxylmethyltransferase Ste14p. J. Biol. Chem. 280:7336–7345 [DOI] [PubMed] [Google Scholar]
- 8. Ashby MN. 1998. CaaX converting enzymes. Curr. Opin. Lipidol. 9:99–102 [DOI] [PubMed] [Google Scholar]
- 9. Ashby MN, Errada PR, Boyartchuk VL, Rine J. 1993. Isolation and DNA sequence of the STE14 gene encoding farnesyl cysteine: carboxyl methyltransferase. Yeast 9:907–913 [DOI] [PubMed] [Google Scholar]
- 10. Ashby MN, King DS, Rine J. 1992. Endoproteolytic processing of a farnesylated peptide in vitro. Proc. Natl. Acad. Sci. U. S. A. 89:4613–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashby MN, Rine J. 1995. Ras and a-factor converting enzyme. Methods Enzymol. 250:235–251 [DOI] [PubMed] [Google Scholar]
- 12. Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. 1988. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell 54:335–344 [DOI] [PubMed] [Google Scholar]
- 13. Bardwell L. 2005. A walk-through of the yeast mating pheromone response pathway. Peptides 26:339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bardwell L, Cook JG, Inouye CJ, Thorner J. 1994. Signal propagation and regulation in the mating pheromone response pathway of the yeast Saccharomyces cerevisiae. Dev. Biol. 166:363–379 [DOI] [PubMed] [Google Scholar]
- 15. Barrowman J, Hamblet C, George CM, Michaelis S. 2008. Analysis of prelamin A biogenesis reveals the nucleus to be a CaaX processing compartment. Mol. Biol. Cell 19:5398–5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barrowman J, Hamblet C, Kane MS, Michaelis S. 2012. Requirements for efficient proteolytic cleavage of prelamin A by ZMPSTE24. PLoS One 7:e32120 doi:10.1371/journal.pone.0032120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barrowman J, Michaelis S. 2011. CAAX processing and yeast a-factor biogenesis, p 13–41 In Hrycyna CA, Bergo MO, Tamanoi F. (ed), The enzymes, vol 30 Academic Press, Burlington, VT [Google Scholar]
- 18. Barrowman J, Michaelis S. The Ste24p protease. In Barrett AJ, Rawlings N, Woessner JF. (ed), Handbook of proteolytic enzmyes, 3rd ed, in press Academic Press, London, United Kingdom. [Google Scholar]
- 19. Barrowman J, Michaelis S. 2009. ZMPSTE24, an integral membrane zinc metalloprotease with a connection to progeroid disorders. Biol. Chem. 390:761–773 [DOI] [PubMed] [Google Scholar]
- 20. Barrowman J, Wiley PA, Hudon-Miller SE, Hrycyna CA, Michaelis S. 2012. Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity. Hum. Mol. Gen. [Epub ahead of print.] doi:10.1093/hmg/dds233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basso AD, Kirschmeier P, Bishop WR. 2006. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J. Lipid Res. 47:15–31 [DOI] [PubMed] [Google Scholar]
- 22. Beck LA, Hosick TJ, Sinensky M. 1990. Isoprenylation is required for the processing of the lamin A precursor. J. Cell Biol. 110:1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benetka W, Koranda M, Eisenhaber F. 2006. Protein prenylation: an (almost) comprehensive overview on discovery history, enzymology, and significance in physiology and disease. Monatshefte Chemie. 137:1241–1281 [Google Scholar]
- 24. Bergo MO, et al. 2002. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl. Acad. Sci. U. S. A. 99:13049–13054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berkower C, Loayza D, Michaelis S. 1994. Metabolic instability and constitutive endocytosis of STE6, the a-factor transporter of Saccharomyces cerevisiae. Mol. Biol. Cell 5:1185–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berkower C, Michaelis S. 1991. Mutational analysis of the yeast a-factor transporter STE6, a member of the ATP binding cassette (ABC) protein superfamily. EMBO J. 10:3777–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berkower C, Taglicht D, Michaelis S. 1996. Functional and physical interactions between partial molecules of STE6, a yeast ATP-binding cassette protein. J. Biol. Chem. 271:22983–22989 [DOI] [PubMed] [Google Scholar]
- 28. Bernales S, McDonald KL, Walter P. 2006. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 4:e423 doi:10.1371/journal.pbio.0040423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berndt N, Hamilton AD, Sebti SM. 2011. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 11:775–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Betz R, MacKay VL, Duntze W. 1977. a-factor from Saccharomyces cerevisiae: partial characterization of a mating hormone produced by cells of mating type a. J. Bacteriol. 132:462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bobrowicz P, Pawlak R, Correa A, Bell-Pedersen D, Ebbole DJ. 2002. The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and the circadian clock. Mol. Microbiol. 45:795–804 [DOI] [PubMed] [Google Scholar]
- 32. Bolker M, Urban M, Kahmann R. 1992. The a mating type locus of U. maydis specifies cell signaling components. Cell 68:441–450 [DOI] [PubMed] [Google Scholar]
- 33. Boyartchuk VL, Ashby MN, Rine J. 1997. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275:1796–1800 [DOI] [PubMed] [Google Scholar]
- 34. Boyartchuk VL, Rine J. 1998. Roles of prenyl protein proteases in maturation of Saccharomyces cerevisiae a-factor. Genetics 150:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bracha K, Lavy M, Yalovsky S. 2002. The Arabidopsis AtSTE24 is a CAAX protease with broad substrate specificity. J. Biol. Chem. 277:29856–29864 [DOI] [PubMed] [Google Scholar]
- 36. Bracha-Drori K, Shichrur K, Lubetzky TC, Yalovsky S. 2008. Functional analysis of Arabidopsis postprenylation CaaX processing enzymes and their function in subcellular protein targeting. Plant Physiol. 148:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brake AJ, Brenner C, Najarian R, Laybourn P, Merryweather J. 1985. Structure of genes encoding precursors of the yeast peptide mating pheromone a-factor, p 103–108 In Gething MJ. (ed), Protein transport and secretion. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Brown AJ, Casselton LA. 2001. Mating in mushrooms: increasing the chances but prolonging the affair. Trends Genet. 17:393–400 [DOI] [PubMed] [Google Scholar]
- 39. Cadinanos J, et al. 2003. AtFACE-2, a functional prenylated protein protease from Arabidopsis thaliana related to mammalian Ras-converting enzymes. J. Biol. Chem. 278:42091–42097 [DOI] [PubMed] [Google Scholar]
- 40. Cai GB, et al. 2006. A membrane-associated metalloprotease of Taenia solium metacestode structurally related to the FACE-1/Ste24p protease family. Int. J. Parasitol. 36:925–935 [DOI] [PubMed] [Google Scholar]
- 41. Caldwell GA, Naider F, Becker JM. 1995. Fungal lipopeptide mating pheromones: a model system for the study of protein prenylation. Microbiol. Rev. 59:406–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caldwell GA, Wang SH, Dawe AL, Naider F, Becker JM. 1993. Identification of a hyperactive mating pheromone of Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 197:1173–1178 [DOI] [PubMed] [Google Scholar]
- 43. Caldwell GA, Wang SH, Naider F, Becker JM. 1994. Consequences of altered isoprenylation targets on a-factor export and bioactivity. Proc. Natl. Acad. Sci. U. S. A. 91:1275–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Caldwell GA, et al. 1994. Molecular determinants of bioactivity of the Saccharomyces cerevisiae lipopeptide mating pheromone. J. Biol. Chem. 269:19817–19825 [PubMed] [Google Scholar]
- 45. Capell BC, Collins FS. 2006. Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet. 7:940–952 [DOI] [PubMed] [Google Scholar]
- 46. Capell BC, et al. 2005. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 102:12879–12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Capell BC, et al. 2008. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc. Natl. Acad. Sci. U. S. A. 105:15902–15907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caplan S, Green R, Rocco J, Kurjan J. 1991. Glycosylation and structure of the yeast MF alpha 1 alpha-factor precursor is important for efficient transport through the secretory pathway. J. Bacteriol. 173:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caron M, Auclair M, Sterlingot H, Kornprobst M, Capeau J. 2003. Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. AIDS. 17:2437–2444 [DOI] [PubMed] [Google Scholar]
- 50. Casey PJ, Seabra MC. 1996. Protein prenyltransferases. J. Biol. Chem. 271:5289–5292 [DOI] [PubMed] [Google Scholar]
- 51. Casselton LA. 2002. Mate recognition in fungi. Heredity 88:142–147 [DOI] [PubMed] [Google Scholar]
- 52. Casselton LA, Olesnicky NS. 1998. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 62:55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chan RK, Otte CA. 1982. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol. Cell. Biol. 2:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chan RK, Otte CA. 1982. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol. Cell. Biol. 2:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen P, Choi JD, Wang R, Cotter RJ, Michaelis S. 1997. A novel a-factor-related peptide of Saccharomyces cerevisiae that exits the cell by a Ste6p-independent mechanism. Mol. Biol. Cell 8:1273–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen P, Sapperstein SK, Choi JD, Michaelis S. 1997. Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor. J. Cell Biol. 136:251–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen WJ, Andres DA, Goldstein JL, Russell DW, Brown MS. 1991. cDNA cloning and expression of the peptide-binding beta subunit of rat p21ras farnesyltransferase, the counterpart of yeast DPR1/RAM1. Cell 66:327–334 [DOI] [PubMed] [Google Scholar]
- 58. Choi JD. 1999. Analysis of processing, processing efficiency, and non-classical export of the Saccharomyces cerevisiae mating pheromone a-factor. The Johns Hopkins University, Baltimore, MD [Google Scholar]
- 59. Christensen PU, Davey J, Nielsen O. 1997. The Schizosaccharomyces pombe mam1 gene encodes an ABC transporter mediating secretion of M-factor. Mol. Gen. Genet. 255:226–236 [DOI] [PubMed] [Google Scholar]
- 60. Clarke S. 1992. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem. 61:355–386 [DOI] [PubMed] [Google Scholar]
- 61. Clarke S, Vogel JP, Deschenes RJ, Stock J. 1988. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc. Natl. Acad. Sci. U. S. A. 85:4643–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Clarke SG. 2007. HIV protease inhibitors and nuclear lamin processing: getting the right bells and whistles. Proc. Natl. Acad. Sci. U. S. A. 104:13857–13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coelho MA, Rosa A, Rodrigues N, Fonseca A, Goncalves P. 2008. Identification of mating type genes in the bipolar basidiomycetous yeast Rhodosporidium toruloides: first insight into the MAT locus structure of the Sporidiobolales. Eukaryot. Cell 7:1053–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Coffinier C, et al. 2007. HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells. Proc. Natl. Acad. Sci. U. S. A. 104:13432–13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coffinier C, et al. 2008. A potent HIV protease inhibitor, darunavir, does not inhibit ZMPSTE24 or lead to an accumulation of farnesyl-prelamin A in cells. J. Biol. Chem. 283:9797–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Corrigan DP, et al. 2005. Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem. J. 387:129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cross FR. 1988. DAF1, a mutant gene affecting size control, pheromone arrest, and cell cycle kinetics of Saccharomyces cerevisiae. Mol. Cell. Biol. 8:4675–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dai Q, et al. 1998. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J. Biol. Chem. 273:15030–15034 [DOI] [PubMed] [Google Scholar]
- 69. Davey J. 1996. M-factor, a farnesylated mating factor from the fission yeast Schizosaccharomyces pombe. Biochem. Soc. Trans. 24:718–723 [DOI] [PubMed] [Google Scholar]
- 70. Davey J. 1992. Mating pheromones of the fission yeast Schizosaccharomyces pombe: purification and structural characterization of M-factor and isolation and analysis of two genes encoding the pheromone. EMBO J. 11:951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Davey J, Davis K, Hughes M, Ladds G, Powner D. 1998. The processing of yeast pheromones. Semin. Cell Dev. Biol. 9:19–30 [DOI] [PubMed] [Google Scholar]
- 72. Davidson RC, Moore TD, Odom AR, Heitman J. 2000. Characterization of the MFalpha pheromone of the human fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 38:1017–1026 [DOI] [PubMed] [Google Scholar]
- 73. Davies BS, Fong LG, Yang SH, Coffinier C, Young SG. 2009. The posttranslational processing of prelamin A and disease. Annu. Rev. Genomics Hum. Genet. 10:153–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dawe AL, et al. 1997. Novel modifications to the farnesyl moiety of the a-factor lipopeptide pheromone from Saccharomyces cerevisiae: a role for isoprene modifications in ligand presentation. Biochemistry 36:12036–12044 [DOI] [PubMed] [Google Scholar]
- 75. DeCarvalho AC, Gansheroff LJ, Teem JL. 2002. Mutations in the nucleotide binding domain 1 signature motif region rescue processing and functional defects of cystic fibrosis transmembrane conductance regulator delta f508. J. Biol. Chem. 277:35896–35905 [DOI] [PubMed] [Google Scholar]
- 76. Dechat T, Gesson K, Foisner R. 2010. Lamina-independent lamins in the nuclear interior serve important functions. Cold Spring Harb Symp. Quant Biol. 75:533–543 [DOI] [PubMed] [Google Scholar]
- 77. Dechert AM, et al. 2010. Modulation of the inhibitor properties of dipeptidyl (acyloxy)methyl ketones toward the CaaX proteases. Bioorg. Med. Chem. 18:6230–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. De Sandre-Giovannoli A, et al. 2003. Lamin a truncation in Hutchinson-Gilford progeria. Science 300:2055. [DOI] [PubMed] [Google Scholar]
- 79. Dignard D, El-Naggar AL, Logue ME, Butler G, Whiteway M. 2007. Identification and characterization of MFA1, the gene encoding Candida albicans a-factor pheromone. Eukaryot. Cell 6:487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dittmer TA, Misteli T. 2011. The lamin protein family. Genome Biol. 12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dmochowska A, Dignard D, Henning D, Thomas DY, Bussey H. 1987. Yeast KEX1 gene encodes a putative protease with a carboxypeptidase B-like function involved in killer toxin and alpha-factor precursor processing. Cell 50:573–584 [DOI] [PubMed] [Google Scholar]
- 82. Dolence JM, Steward LE, Dolence EK, Wong DH, Poulter CD. 2000. Studies with recombinant Saccharomyces cerevisiae CaaX prenyl protease Rce1p. Biochemistry 39:4096–4104 [DOI] [PubMed] [Google Scholar]
- 83. Dorer R, Boone C, Kimbrough T, Kim J, Hartwell LH. 1997. Genetic analysis of default mating behavior in Saccharomyces cerevisiae. Genetics 146:39–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dorer R, Pryciak PM, Hartwell LH. 1995. Saccharomyces cerevisiae cells execute a default pathway to select a mate in the absence of pheromone gradients. J. Cell Biol. 131:845–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Duntze W, MacKay V, Manney TR. 1970. Saccharomyces cerevisiae: a diffusible sex factor. Science 168:1472–1473 [DOI] [PubMed] [Google Scholar]
- 86. Elion EA. 2000. Pheromone response, mating and cell biology. Curr. Opin. Microbiol. 3:573–581 [DOI] [PubMed] [Google Scholar]
- 87. Eriksson M, et al. 2003. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423:293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Farnsworth CC, Wolda SL, Gelb MH, Glomset JA. 1989. Human lamin B contains a farnesylated cysteine residue. J. Biol. Chem. 264:20422–20429 [PMC free article] [PubMed] [Google Scholar]
- 89. Fearon K, McClendon V, Bonetti B, Bedwell DM. 1994. Premature translation termination mutations are efficiently suppressed in a highly conserved region of yeast Ste6p, a member of the ATP-binding cassette (ABC) transporter family. J. Biol. Chem. 269:17802–17808 [PubMed] [Google Scholar]
- 90. Fernandez-Gamba A, Leal MC, Morelli L, Castano EM. 2009. Insulin-degrading enzyme: structure-function relationship and its possible roles in health and disease. Curr. Pharm. Des. 15:3644–3655 [DOI] [PubMed] [Google Scholar]
- 91. Finegold AA, et al. 1991. Protein geranylgeranyltransferase of Saccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires the CDC43 gene product but not the DPR1 gene product. Proc. Natl. Acad. Sci. U. S. A. 88:4448–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fowler TJ, DeSimone SM, Mitton MF, Kurjan J, Raper CA. 1999. Multiple sex pheromones and receptors of a mushroom-producing fungus elicit mating in yeast. Mol. Biol. Cell 10:2559–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Freije JM, et al. 1999. Identification and chromosomal location of two human genes encoding enzymes potentially involved in proteolytic maturation of farnesylated proteins. Genomics 58:270–280 [DOI] [PubMed] [Google Scholar]
- 94. Fujimura-Kamada K, Nouvet FJ, Michaelis S. 1997. A novel membrane-associated metalloprotease, Ste24p, is required for the first step of NH2-terminal processing of the yeast a-factor precursor. J. Cell Biol. 136:271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fujita A, et al. 1994. A yeast gene necessary for bud-site selection encodes a protein similar to insulin-degrading enzymes. Nature 372:567–570 [DOI] [PubMed] [Google Scholar]
- 96. Fujiyama A, Matsumoto K, Tamanoi F. 1987. A novel yeast mutant defective in the processing of ras proteins: assessment of the effect of the mutation on processing steps. EMBO J. 6:223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fuller RS, Sterne RE, Thorner J. 1988. Enzymes required for yeast prohormone processing. Annu. Rev. Physiol. 50:345–362 [DOI] [PubMed] [Google Scholar]
- 98. Gammie AE, Rose MD. 2002. Assays of cell and nuclear fusion. Methods Enzymol. 351:477–498 [DOI] [PubMed] [Google Scholar]
- 99. Gelb MH, et al. 2006. Therapeutic intervention based on protein prenylation and associated modifications. Nat. Chem. Biol. 2:518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gillespie JR, et al. 2007. C-terminal proteolysis of prenylated proteins in trypanosomatids and RNA interference of enzymes required for the posttranslational processing pathway of farnesylated proteins. Mol. Biochem. Parasitol. 153:115–124 [DOI] [PubMed] [Google Scholar]
- 101. Glomset JA, Gelb MH, Farnsworth CC. 1990. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem. Sci. 15:139–142 [DOI] [PubMed] [Google Scholar]
- 102. Glynn MW, Glover TW. 2005. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 14:2959–2969 [DOI] [PubMed] [Google Scholar]
- 103. Goncalves-Sa J, Murray A. 2011. Asymmetry in sexual pheromones is not required for ascomycete mating. Curr. Biol. 21:1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Griggs AM, Hahne K, Hrycyna CA. 2010. Functional oligomerization of the Saccharomyces cerevisiae isoprenylcysteine carboxyl methyltransferase, Ste14p. J. Biol. Chem. 285:13380–13387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hagen DC, McCaffrey G, Sprague GF., Jr 1986. Evidence the yeast STE3 gene encodes a receptor for the peptide pheromone a factor: gene sequence and implications for the structure of the presumed receptor. Proc. Natl. Acad. Sci. U. S. A. 83:1418–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hancock JF, Magee AI, Childs JE, Marshall CJ. 1989. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57:1167–1177 [DOI] [PubMed] [Google Scholar]
- 107. Hansen W, Garcia PD, Walter P. 1986. In vitro protein translocation across the yeast endoplasmic reticulum: ATP-dependent posttranslational translocation of the prepro-alpha-factor. Cell 45:397–406 [DOI] [PubMed] [Google Scholar]
- 108. He B, et al. 1991. RAM2, an essential gene of yeast, and RAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc. Natl. Acad. Sci. U. S. A. 88:11373–11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Heilmeyer LM, Jr, et al. 1992. Farnesylcysteine, a constituent of the alpha and beta subunits of rabbit skeletal muscle phosphorylase kinase: localization by conversion to S-ethylcysteine and by tandem mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 89:9554–9558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Herskowitz I. 1989. A regulatory hierarchy for cell specialization in yeast. Nature 342:749–757 [DOI] [PubMed] [Google Scholar]
- 111. Holtz D, Tanaka RA, Hartwig J, McKeon F. 1989. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell 59:969–977 [DOI] [PubMed] [Google Scholar]
- 112. Hrycyna CA, Clarke S. 1990. Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:5071–5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hrycyna CA, Sapperstein SK, Clarke S, Michaelis S. 1991. The Saccharomyces cerevisiae STE14 gene encodes a methyltransferase that mediates C-terminal methylation of a-factor and RAS proteins. EMBO J. 10:1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hrycyna CA, Wait SJ, Backlund PS, Jr, Michaelis S. 1995. Yeast STE14 methyltransferase, expressed as TrpE-STE14 fusion protein in Escherichia coli, for in vitro carboxylmethylation of prenylated polypeptides. Methods Enzymol. 250:251–266 [DOI] [PubMed] [Google Scholar]
- 115. Hudon SE, et al. 2008. HIV-protease inhibitors block the enzymatic activity of purified Ste24p. Biochem. Biophys. Res. Commun. 374:365–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Huyer G, et al. 2006. Saccharomyces cerevisiae a-factor mutants reveal residues critical for processing, activity, and export. Eukaryot. Cell 5:1560–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huyer G, et al. 2004. A striking quality control subcompartment in Saccharomyces cerevisiae: the endoplasmic reticulum-associated compartment. Mol. Biol. Cell 15:908–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Huyer G, et al. 2004. Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble luminal protein. J. Biol. Chem. 279:38369–38378 [DOI] [PubMed] [Google Scholar]
- 119. Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. 2009. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324:218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ishibashi Y, Sakagami Y, Isogai A, Suzuki A. 1984. Structures of tremerogen-a-9291-i and tremerogen-a-9291-vii—peptidyl sex-hormones of Tremella brasiliensis. Biochemistry 23:1399–1404 [DOI] [PubMed] [Google Scholar]
- 121. Ishibashi Y, Sakagami Y, Isogai A, Suzuki A, Bandoni RJ. 1983. Isolation of tremerogens A-9291-I and A-9291-II, novel sex hormones of Tremella brasiliensis. Can. J. Biochem. Cell Biol. 61:796–801 [DOI] [PubMed] [Google Scholar]
- 122. Jackson CL, Hartwell LH. 1990. Courtship in S. cerevisiae: both cell types choose mating partners by responding to the strongest pheromone signal. Cell 63:1039–1051 [DOI] [PubMed] [Google Scholar]
- 123. Jackson CL, Hartwell LH. 1990. Courtship in Saccharomyces cerevisiae: an early cell-cell interaction during mating. Mol. Cell. Biol. 10:2202–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jensen D, Schekman R. 2011. COPII-mediated vesicle formation at a glance. J. Cell Sci. 124:1–4 [DOI] [PubMed] [Google Scholar]
- 125. Jiang Y, Rossi G, Ferro-Novick S. 1993. Bet2p and Mad2p are components of a prenyltransferase that adds geranylgeranyl onto Ypt1p and Sec4p. Nature 366:84–86 [DOI] [PubMed] [Google Scholar]
- 126. Jin M, et al. 2011. Yeast dynamically modify their environment to achieve better mating efficiency. Sci. Signal. 4:ra54 doi:10.1126/scisignal.2001763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Julius D, Blair L, Brake A, Sprague G, Thorner J. 1983. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell 32:839–852 [DOI] [PubMed] [Google Scholar]
- 128. Julius D, Brake A, Blair L, Kunisawa R, Thorner J. 1984. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell 37:1075–1089 [DOI] [PubMed] [Google Scholar]
- 129. Julius D, Schekman R, Thorner J. 1984. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell 36:309–318 [DOI] [PubMed] [Google Scholar]
- 130. Kaganovich D, Kopito R, Frydman J. 2008. Misfolded proteins partition between two distinct quality control compartments. Nature 454:1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kamiya Y, Sakurai A, Tamura S, Takahashi N. 1979. Structure confirmation of s-trans-trans-farnesylcysteine in rhodotorucine-a by application of sulfonium salt cleavage reaction. Agric. Biol. Chem. 43:1049–1053 [Google Scholar]
- 132. Kamiya Y, Sakurai A, Tamura S, Takahashi N. 1978. Structure of rhodotorucine A, a novel lipopeptide, inducing mating tube formation in Rhodosporidium toruloides. Biochem. Biophys. Res. Commun. 83:1077–1083 [DOI] [PubMed] [Google Scholar]
- 133. Kamiya Y, et al. 1979. Structure of rhodotorucine-a, a peptidyl factor, inducing mating tube formation in Rhodosporidium toruloides. Agric. Biol. Chem. 43:363–369 [DOI] [PubMed] [Google Scholar]
- 134. Keeling KM, Bedwell DM. 2011. Suppression of nonsense mutations as a therapeutic approach to treat genetic diseases. Wiley Interdiscip. Rev. RNA 2:837–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kelm KB, Huyer G, Huang JC, Michaelis S. 2004. The internalization of yeast Ste6p follows an ordered series of events involving phosphorylation, ubiquitination, recognition and endocytosis. Traffic 5:165–180 [DOI] [PubMed] [Google Scholar]
- 136. Ketchum CJ, Schmidt WK, Rajendrakumar GV, Michaelis S, Maloney PC. 2001. The yeast a-factor transporter Ste6p, a member of the ABC superfamily, couples ATP hydrolysis to pheromone export. J. Biol. Chem. 276:29007–29011 [DOI] [PubMed] [Google Scholar]
- 137. Kim E, et al. 1999. Disruption of the mouse Rce1 gene results in defective Ras processing and mislocalization of Ras within cells. J. Biol. Chem. 274:8383–8390 [DOI] [PubMed] [Google Scholar]
- 138. Kim H, Metzenberg RL, Nelson MA. 2002. Multiple functions of mfa-1, a putative pheromone precursor gene of Neurospora crassa. Eukaryot. Cell 1:987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kim HK, Lee T, Yun SH. 2008. A putative pheromone signaling pathway is dispensable for self-fertility in the homothallic ascomycete Gibberella zeae. Fungal Genet. Biol. 45:1188–1196 [DOI] [PubMed] [Google Scholar]
- 140. Kim S, Lapham AN, Freedman CG, Reed TL, Schmidt WK. 2005. Yeast as a tractable genetic system for functional studies of the insulin-degrading enzyme. J. Biol. Chem. 280:27481–27490 [DOI] [PubMed] [Google Scholar]
- 141. Kolling R. 2002. Mutations affecting phosphorylation, ubiquitination and turnover of the ABC-transporter Ste6. FEBS Lett. 531:548–552 [DOI] [PubMed] [Google Scholar]
- 142. Kolling R, Hollenberg CP. 1994. The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. EMBO J. 13:3261–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kolling R, Losko S. 1997. The linker region of the ABC-transporter Ste6 mediates ubiquitination and fast turnover of the protein. EMBO J. 16:2251–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Koppitz M, Spellig T, Kahmann R, Kessler H. 1996. Lipoconjugates: structure-activity studies for pheromone analogues of Ustilago maydis with varied lipophilicity. Int. J. Pept. Protein Res. 48:377–390 [DOI] [PubMed] [Google Scholar]
- 145. Kosted PJ, Gerhardt SA, Anderson CM, Stierle A, Sherwood JE. 2000. Structural requirements for activity of the pheromones of Ustilago hordei. Fungal Genet. Biol. 29:107–117 [DOI] [PubMed] [Google Scholar]
- 146. Kranz A, Kinner A, Kolling R. 2001. A family of small coiled-coil-forming proteins functioning at the late endosome in yeast. Mol. Biol. Cell 12:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Krishnankutty RK, Kukday SS, Castleberry AJ, Breevoort SR, Schmidt WK. 2009. Proteolytic processing of certain CaaX motifs can occur in the absence of the Rce1p and Ste24p CaaX proteases. Yeast 26:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Krsmanovic T, Pawelec A, Sydor T, Kolling R. 2005. Control of Ste6 recycling by ubiquitination in the early endocytic pathway in yeast. Mol. Biol. Cell 16:2809–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kuchler K, Goransson HM, Viswanathan MN, Thorner J. 1992. Dedicated transporters for peptide export and intercompartmental traffic in the yeast Saccharomyces cerevisiae. Cold Spring Harbor Symp. Quant. Biol. 57:579–592 [DOI] [PubMed] [Google Scholar]
- 150. Kuchler K, Sterne RE, Thorner J. 1989. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 8:3973–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kuchler K, Thorner J. 1992. Functional expression of human mdr1 in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 89:2302–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kurjan J. 1985. Alpha-factor structural gene mutations in Saccharomyces cerevisiae: effects on alpha-factor production and mating. Mol. Cell. Biol. 5:787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kurjan J. 1992. Pheromone response in yeast. Annu. Rev. Biochem. 61:1097–1129 [DOI] [PubMed] [Google Scholar]
- 154. Kurjan J, Herskowitz I. 1982. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell 30:933–943 [DOI] [PubMed] [Google Scholar]
- 155. Lal M, Caplan M. 2011. Regulated intramembrane proteolysis: signaling pathways and biological functions. Physiology (Bethesda) 26:34–44 [DOI] [PubMed] [Google Scholar]
- 156. Lane KT, Beese LS. 2006. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J. Lipid Res. 47:681–699 [DOI] [PubMed] [Google Scholar]
- 157. Leberer E, Thomas DY, Whiteway M. 1997. Pheromone signalling and polarized morphogenesis in yeast. Curr. Opin. Genet. Dev. 7:59–66 [DOI] [PubMed] [Google Scholar]
- 158. Lee J, Leslie JF, Bowden RL. 2008. Expression and function of sex pheromones and receptors in the homothallic ascomycete Gibberella zeae. Eukaryot. Cell 7:1211–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Leissring MA, et al. 2003. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40:1087–1093 [DOI] [PubMed] [Google Scholar]
- 160. Leung GK, et al. 2001. Biochemical studies of Zmpste24-deficient mice. J. Biol. Chem. 276:29051–29058 [DOI] [PubMed] [Google Scholar]
- 161. Leung KF, Baron R, Seabra MC. 2006. Thematic review series: lipid posttranslational modifications. Geranylgeranylation of Rab GTPases. J. Lipid Res. 47:467–475 [DOI] [PubMed] [Google Scholar]
- 162. Loayza D, Michaelis S. 1998. Role for the ubiquitin-proteasome system in the vacuolar degradation of Ste6p, the a-factor transporter in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Loayza D, Tam A, Schmidt WK, Michaelis S. 1998. Ste6p mutants defective in exit from the endoplasmic reticulum (ER) reveal aspects of an ER quality control pathway in Saccharomyces cerevisiae. Mol. Biol. Cell 9:2767–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lord M, et al. 2002. Subcellular localization of Axl1, the cell type-specific regulator of polarity. Curr. Biol. 12:1347–1352 [DOI] [PubMed] [Google Scholar]
- 165. Losko S, Kopp F, Kranz A, Kolling R. 2001. Uptake of the ATP-binding cassette (ABC) transporter Ste6 into the yeast vacuole is blocked in the doa4 Mutant. Mol. Biol. Cell 12:1047–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mackay V, Manney TR. 1974. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. I. Isolation and phenotypic characterization of nonmating mutants. Genetics 76:255–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Mackay V, Manney TR. 1974. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. II. Genetic analysis of nonmating mutants. Genetics 76:273–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. MacKay VL, et al. 1988. The Saccharomyces cerevisiae BAR1 gene encodes an exported protein with homology to pepsin. Proc. Natl. Acad. Sci. U. S. A. 85:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Mallampalli MP, Huyer G, Bendale P, Gelb MH, Michaelis S. 2005. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 102:14416–14421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Maltese WA, Sheridan KM. 1987. Isoprenylated proteins in cultured cells: subcellular distribution and changes related to altered morphology and growth arrest induced by mevalonate deprivation. J. Cell Physiol. 133:471–481 [DOI] [PubMed] [Google Scholar]
- 171. Manandhar SP, Hildebrandt ER, Schmidt WK. 2007. Small-molecule inhibitors of the Rce1p CaaX protease. J. Biomol. Screen. 12:983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Marcus S, et al. 1991. Significance of C-terminal cysteine modifications to the biological activity of the Saccharomyces cerevisiae a-factor mating pheromone. Mol. Cell. Biol. 11:3603–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Marcus S, Xue CB, Naider F, Becker JM. 1991. Degradation of a-factor by a Saccharomyces cerevisiae alpha-mating-type-specific endopeptidase: evidence for a role in recovery of cells from G1 arrest. Mol. Cell. Biol. 11:1030–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Marr RS, Blair LC, Thorner J. 1990. Saccharomyces cerevisiae STE14 gene is required for COOH-terminal methylation of a-factor mating pheromone. J. Biol. Chem. 265:20057–20060 [PubMed] [Google Scholar]
- 175. Marsh L, Neiman AM, Herskowitz I. 1991. Signal transduction during pheromone response in yeast. Annu. Rev. Cell Biol. 7:699–728 [DOI] [PubMed] [Google Scholar]
- 176. Mayrhofer S, Weber JM, Poggeler S. 2006. Pheromones and pheromone receptors are required for proper sexual development in the homothallic ascomycete Sordaria macrospora. Genetics 172:1521–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. McClelland CM, Fu J, Woodlee GL, Seymour TS, Wickes BL. 2002. Isolation and characterization of the Cryptococcus neoformans MATa pheromone gene. Genetics 160:935–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. McClintock D, et al. 2007. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One 2:e1269 doi:10.1371/journal.pone.0001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. McGrath JP, Varshavsky A. 1989. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature 340:400–404 [DOI] [PubMed] [Google Scholar]
- 180. Meacham GC, et al. 1999. Mutations in the yeast Hsp40 chaperone protein Ydj1 cause defects in Axl1 biogenesis and pro-a-factor processing. J. Biol. Chem. 274:34396–34402 [DOI] [PubMed] [Google Scholar]
- 181. Michaelis S. 1993. STE6, the yeast a-factor transporter. Semin. Cell Biol. 4:17–27 [DOI] [PubMed] [Google Scholar]
- 182. Michaelis S, Berkower C. 1995. Sequence comparison of yeast ATP-binding cassette proteins. Cold Spring Harbor Symp. Quant. Biol. 60:291–307 [DOI] [PubMed] [Google Scholar]
- 183. Michaelis S, Chen P, Berkower C, Sapperstein S, Kistler A. 1992. Biogenesis of yeast a-factor involves prenylation, methylation and a novel export mechanism. Antonie Van Leeuwenhoek 61:115–117 [DOI] [PubMed] [Google Scholar]
- 184. Michaelis S, Herskowitz I. 1988. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol. Cell. Biol. 8:1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Michaelis S, Powers S. 1988. Biogenesis of yeast mating pheromone a-factor and ras proteins. UCLA Symp. Mol. Cell. Biol. New Ser. 76:193–202 [Google Scholar]
- 186. Michaelis S, Schmidt WK. 2004. The Axl1p protease, p 879–882 In Barrett AJ, Rawlings N, Woessner JF. (ed), The handbook of proteolytic enzymes, 2nd ed Academic Press, London, United Kingdom [Google Scholar]
- 187. Miyakawa T, Tabata M, Tsuchiya E, Fukui S. 1985. Biosynthesis and secretion of tremerogen a-10, a polyisoprenyl peptide mating pheromone of tremella-mesenterica. Eur. J. Biochem. 147:489–493 [DOI] [PubMed] [Google Scholar]
- 188. Mokry DZ, Manandhar SP, Chicola KA, Santangelo GM, Schmidt WK. 2009. Heterologous expression studies of Saccharomyces cerevisiae reveal two distinct trypanosomatid CaaX protease activities and identify their potential targets. Eukaryot. Cell 8:1891–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Moore TD, Edman JC. 1993. The alpha-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol. Cell. Biol. 13:1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Moulson CL, et al. 2007. Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Hum. Mutat. 28:882–889 [DOI] [PubMed] [Google Scholar]
- 191. Moulson CL, et al. 2005. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J. Investig. Dermatol. 125:913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Mullen DG, et al. 2011. Synthesis of a-factor peptide from Saccharomyces cerevisiae and photoactive analogues via Fmoc solid phase methodology. Bioorg. Med. Chem. 19:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Naider F, Becker JM. 2004. The alpha-factor mating pheromone of Saccharomyces cerevisiae: a model for studying the interaction of peptide hormones and G protein-coupled receptors. Peptides 25:1441–1463 [DOI] [PubMed] [Google Scholar]
- 194. Nakatsukasa K, Huyer G, Michaelis S, Brodsky JL. 2008. Dissecting the ER-associated degradation of a misfolded polytopic membrane protein. Cell 132:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Nijbroek GL. 1998. Distinct classes of yeast ste6 mutants provide insight into yeast ER quality control. The Johns Hopkins University, Baltimore, MD [Google Scholar]
- 196. Nijbroek GL, Michaelis S. 1998. Functional assays for analysis of yeast ste6 mutants. Methods Enzymol. 292:193–212 [DOI] [PubMed] [Google Scholar]
- 197. Olesnicky NS, Brown AJ, Dowell SJ, Casselton LA. 1999. A constitutively active G-protein-coupled receptor causes mating self-compatibility in the mushroom Coprinus. EMBO J. 18:2756–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Omer CA, Gibbs JB. 1994. Protein prenylation in eukaryotic microorganisms: genetics, biology and biochemistry. Mol. Microbiol. 11:219–225 [DOI] [PubMed] [Google Scholar]
- 199. Otto JC, Kim E, Young SG, Casey PJ. 1999. Cloning and characterization of a mammalian prenyl protein-specific protease. J. Biol. Chem. 274:8379–8382 [DOI] [PubMed] [Google Scholar]
- 200. Paddon C, Loayza D, Vangelista L, Solari R, Michaelis S. 1996. Analysis of the localization of STE6/CFTR chimeras in a Saccharomyces cerevisiae model for the cystic fibrosis defect CFTR delta F508. Mol. Microbiol. 19:1007–1017 [DOI] [PubMed] [Google Scholar]
- 201. Paumi CM, Chuk M, Snider J, Stagljar I, Michaelis S. 2009. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol. Mol. Biol. Rev. 73:577–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Pei J, Grishin NV. 2001. Type II CAAX prenyl endopeptidases belong to a novel superfamily of putative membrane-bound metalloproteases. Trends Biochem. Sci. 26:275–277 [DOI] [PubMed] [Google Scholar]
- 203. Pendas AM, et al. 2002. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31:94–99 [DOI] [PubMed] [Google Scholar]
- 204. Plummer LJ, et al. 2006. Mutational analysis of the ras converting enzyme reveals a requirement for glutamate and histidine residues. J. Biol. Chem. 281:4596–4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Poggeler S. 2000. Two pheromone precursor genes are transcriptionally expressed in the homothallic ascomycete Sordaria macrospora. Curr. Genet. 37:403–411 [DOI] [PubMed] [Google Scholar]
- 206. Porter SB, et al. 2007. Inhibition of the CaaX proteases Rce1p and Ste24p by peptidyl (acyloxy)methyl ketones. Biochim. Biophys. Acta 1773:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Powers S, et al. 1986. RAM, a gene of yeast required for a functional modification of RAS proteins and for production of mating pheromone a-factor. Cell 47:413–422 [DOI] [PubMed] [Google Scholar]
- 208. Ragnauth CD, et al. 2010. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation 121:2200–2210 [DOI] [PubMed] [Google Scholar]
- 209. Raymond M, et al. 1998. A Ste6p/P-glycoprotein homologue from the asexual yeast Candida albicans transports the a-factor mating pheromone in Saccharomyces cerevisiae. Mol. Microbiol. 27:587–598 [DOI] [PubMed] [Google Scholar]
- 210. Raymond M, Gros P, Whiteway M, Thomas DY. 1992. Functional complementation of yeast ste6 by a mammalian multidrug resistance mdr gene. Science 256:232–234 [DOI] [PubMed] [Google Scholar]
- 211. Raymond M, Ruetz S, Thomas DY, Gros P. 1994. Functional expression of P-glycoprotein in Saccharomyces cerevisiae confers cellular resistance to the immunosuppressive and antifungal agent FK520. Mol. Cell. Biol. 14:277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Reiss Y, Goldstein JL, Seabra MC, Casey PJ, Brown MS. 1990. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell 62:81–88 [DOI] [PubMed] [Google Scholar]
- 213. Ricardo S, Lehmann R. 2009. An ABC transporter controls export of a Drosophila germ cell attractant. Science 323:943–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Richardson BE, Lehmann R. 2010. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat. Rev. Mol. Cell Biol. 11:37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Riquelme M, Challen MP, Casselton LA, Brown AJ. 2005. The origin of multiple B mating specificities in Coprinus cinereus. Genetics 170:1105–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. 2002. Precursor processing by kex2/furin proteases. Chem. Rev. 102:4525–4548 [DOI] [PubMed] [Google Scholar]
- 217. Rodriguez S, Coppede F, Sagelius H, Eriksson M. 2009. Increased expression of the Hutchinson-Gilford progeria syndrome truncated lamin A transcript during cell aging. Eur. J. Hum. Genet. 17:928–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Romano JD. 2000. Localization and topological analysis of Ste14p, the Saccharomyces cerevisiae prenylcysteine carboxyl methyltransferase. The Johns Hopkins University, Baltimore, MD [Google Scholar]
- 219. Romano JD, Michaelis S. 2001. Topological and mutational analysis of Saccharomyces cerevisiae Ste14p, founding member of the isoprenylcysteine carboxyl methyltransferase family. Mol. Biol. Cell 12:1957–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Romano JD, Schmidt WK, Michaelis S. 1998. The Saccharomyces cerevisiae prenylcysteine carboxyl methyltransferase Ste14p is in the endoplasmic reticulum membrane. Mol. Biol. Cell 9:2231–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Ruetz S, Brault M, Dalton W, Gros P. 1999. Functional interactions between synthetic alkyl phospholipids and the ABC transporters P-glycoprotein, Ste-6, MRP, and Pgh 1. Biochemistry 38:2860. [PubMed] [Google Scholar]
- 222. Ruetz S, Brault M, Dalton W, Gros P. 1997. Functional interactions between synthetic alkyl phospholipids and the ABC transporters P-glycoprotein, Ste-6, MRP, and Pgh 1. Biochemistry 36:8180–8188 [DOI] [PubMed] [Google Scholar]
- 223. Ruetz S, et al. 1999. Functional expression of the multidrug resistance-associated protein in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274:2592. [PubMed] [Google Scholar]
- 224. Ruetz S, et al. 1996. Functional expression of the multidrug resistance-associated protein in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 271:4154–4160 [DOI] [PubMed] [Google Scholar]
- 225. Ruetz S, Delling U, Brault M, Schurr E, Gros P. 1999. The pfmdr1 gene of Plasmodium falciparum confers cellular resistance to antimalarial drugs in yeast cells. Proc. Natl. Acad. Sci. U. S. A. 96:1810. [PMC free article] [PubMed] [Google Scholar]
- 226. Ruetz S, Delling U, Brault M, Schurr E, Gros P. 1996. The pfmdr1 gene of Plasmodium falciparum confers cellular resistance to antimalarial drugs in yeast cells. Proc. Natl. Acad. Sci. U. S. A. 93:9942–9947 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 227. Ruohola H, Kabcenell AK, Ferro-Novick S. 1988. Reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex in yeast: the acceptor Golgi compartment is defective in the sec23 mutant. J. Cell Biol. 107:1465–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Sakagami Y, et al. 1979. Structure of tremerogen-a-10, a peptidal hormone inducing conjugation tube formation in Tremella mesenterica. Agric. Biol. Chem. 43:2643–2645 [Google Scholar]
- 229. Sakagami Y, Yoshida M, Isogai A, Suzuki A. 1981. Peptidal sex hormones inducing conjugation tube formation in compatible mating-type cells of Tremella mesenterica. Science 212:1525–1527 [DOI] [PubMed] [Google Scholar]
- 230. Santos AC, Lehmann R. 2004. Isoprenoids control germ cell migration downstream of HMGCoA reductase. Dev. Cell 6:283–293 [DOI] [PubMed] [Google Scholar]
- 231. Sapperstein S, Berkower C, Michaelis S. 1994. Nucleotide sequence of the yeast STE14 gene, which encodes farnesylcysteine carboxyl methyltransferase, and demonstration of its essential role in a-factor export. Mol. Cell. Biol. 14:1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Schafer WR, Rine J. 1992. Protein prenylation: genes, enzymes, targets, and functions. Annu. Rev. Genet. 26:209–237 [DOI] [PubMed] [Google Scholar]
- 233. Schafer WR, et al. 1990. Enzymatic coupling of cholesterol intermediates to a mating pheromone precursor and to the ras protein. Science 249:1133–1139 [DOI] [PubMed] [Google Scholar]
- 234. Schekman R, et al. 1995. Coat proteins and selective protein packaging into transport vesicles. Cold Spring Harbor Symp. Quant. Biol. 60:11–21 [DOI] [PubMed] [Google Scholar]
- 235. Schirawski J, Heinze B, Wagenknecht M, Kahmann R. 2005. Mating type loci of Sporisorium reilianum: novel pattern with three a and multiple b specificities. Eukaryot. Cell 4:1317–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235a. Schmidt RA, Schneider CJ, Glomset JA. 1984. Evidence for posttranslational incorporation of a product of mevalonic acid into Swiss 3T3 cell proteins. J. Biol. Chem. 259:10175–10180 [PubMed] [Google Scholar]
- 236. Schmidt WK, Tam A, Fujimura-Kamada K, Michaelis S. 1998. Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc. Natl. Acad. Sci. U. S. A. 95:11175–11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Schmidt WK, Tam A, Michaelis S. 2000. Reconstitution of the Ste24p-dependent N-terminal proteolytic step in yeast a-factor biogenesis. J. Biol. Chem. 275:6227–6233 [DOI] [PubMed] [Google Scholar]
- 238. Schmoll M, Seibel C, Tisch D, Dorrer M, Kubicek CP. 2010. A novel class of peptide pheromone precursors in ascomycetous fungi. Mol. Microbiol. 77:1483–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Schwartz D, Decker CJ, Parker R. 2003. The enhancer of decapping proteins, Edc1p and Edc2p, bind RNA and stimulate the activity of the decapping enzyme. RNA. 9:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Seidah NG. 2011. What lies ahead for the proprotein convertases? Ann. N. Y. Acad. Sci. 1220:149–161 [DOI] [PubMed] [Google Scholar]
- 241. Seidah NG, Chretien M. 1997. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr. Opin. Biotechnol. 8:602–607 [DOI] [PubMed] [Google Scholar]
- 242. Shen WC, Bobrowicz P, Ebbole DJ. 1999. Isolation of pheromone precursor genes of Magnaporthe grisea. Fungal Genet. Biol. 27:253–263 [DOI] [PubMed] [Google Scholar]
- 243. Shen WC, Davidson RC, Cox GM, Heitman J. 2002. Pheromones stimulate mating and differentiation via paracrine and autocrine signaling in Cryptococcus neoformans. Eukaryot. Cell 1:366–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Spellig T, Bolker M, Lottspeich F, Frank RW, Kahmann R. 1994. Pheromones trigger filamentous growth in Ustilago maydis. EMBO J. 13:1620–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Sprague GF, Jr, Blair LC, Thorner J. 1983. Cell interactions and regulation of cell type in the yeast Saccharomyces cerevisiae. Annu. Rev. Microbiol. 37:623–660 [DOI] [PubMed] [Google Scholar]
- 246. Sprague GF, Thorner JW. 1992. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae, p 657–744 In Jones EW, Pringle JR, Broach JR. (ed), The molecular and cellular biology of the yeast Saccharomyces: gene expression. Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 247. Steden M, Betz R, Duntze W. 1989. Isolation and characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by the mating hormone a-factor. Mol. Gen. Genet. 219:439–444 [DOI] [PubMed] [Google Scholar]
- 248. Steinhauer J, Treisman JE. 2009. Lipid-modified morphogens: functions of fats. Curr. Opin. Genet. Dev. 19:308–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Stimmel JB, Deschenes RJ, Volker C, Stock J, Clarke S. 1990. Evidence for an S-farnesylcysteine methyl ester at the carboxyl terminus of the Saccharomyces cerevisiae RAS2 protein. Biochemistry 29:9651–9659 [DOI] [PubMed] [Google Scholar]
- 250. Strazdis JR, MacKay VL. 1983. Induction of yeast mating pheromone a-factor by alpha cells. Nature 305:543–545 [DOI] [PubMed] [Google Scholar]
- 251. Strazdis JR, MacKay VL. 1982. Reproducible and rapid methods for the isolation and assay of a-factor, a yeast mating hormone. J. Bacteriol. 151:1153–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Szabo Z, Tonnis M, Kessler H, Feldbrugge M. 2002. Structure-function analysis of lipopeptide pheromones from the plant pathogen Ustilago maydis. Mol. Genet. Genomics 268:362–370 [DOI] [PubMed] [Google Scholar]
- 253. Taglicht D, Michaelis S. 1998. Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol. 292:130–162 [DOI] [PubMed] [Google Scholar]
- 254. Tam A, et al. 1998. Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CAAX processing. J. Cell Biol. 142:635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Tam A, Schmidt WK, Michaelis S. 2001. The multispanning membrane protein Ste24p catalyzes CAAX proteolysis and NH2-terminal processing of the yeast a-factor precursor. J. Biol. Chem. 276:46798–46806 [DOI] [PubMed] [Google Scholar]
- 256. Teem JL, et al. 1993. Identification of revertants for the cystic fibrosis delta F508 mutation using STE6-CFTR chimeras in yeast. Cell 73:335–346 [DOI] [PubMed] [Google Scholar]
- 257. Teem JL, Carson MR, Welsh MJ. 1996. Mutation of R555 in CFTR-delta F508 enhances function and partially corrects defective processing. Receptors Channels 4:63–72 [PubMed] [Google Scholar]
- 258. Thorpe JL, Doitsidou M, Ho SY, Raz E, Farber SA. 2004. Germ cell migration in zebrafish is dependent on HMGCoA reductase activity and prenylation. Dev. Cell 6:295–302 [DOI] [PubMed] [Google Scholar]
- 259. Toth JI, et al. 2005. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. U. S. A. 102:12873–12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Trueblood CE, et al. 2000. The CaaX proteases, Afc1p and Rce1p, have overlapping but distinct substrate specificities. Mol. Cell. Biol. 20:4381–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Trueblood CE, Ohya Y, Rine J. 1993. Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:4260–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Umanah GK, et al. 2010. Identification of residue-to-residue contact between a peptide ligand and its G protein-coupled receptor using periodate-mediated dihydroxyphenylalanine cross-linking and mass spectrometry. J. Biol. Chem. 285:39425–39436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Vashist S, Ng DT. 2004. Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J. Cell Biol. 165:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Volkman SK, Cowman AF, Wirth DF. 1995. Functional complementation of the ste6 gene of Saccharomyces cerevisiae with the pfmdr1 gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 92:8921–8925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Vorburger K, Kitten GT, Nigg EA. 1989. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J. 8:4007–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Wang SH, Xue CB, Nielsen O, Davey J, Naider F. 1994. Chemical synthesis of the M-factor mating pheromone from Schizosaccharomyces pombe. Yeast 10:595–601 [DOI] [PubMed] [Google Scholar]
- 267. Wendland J, et al. 1995. The mating-type locus B alpha 1 of Schizophyllum commune contains a pheromone receptor gene and putative pheromone genes. EMBO J. 14:5271–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. White JM, Rose MD. 2001. Yeast mating: getting close to membrane merger. Curr. Biol. 11:R16–R20 [DOI] [PubMed] [Google Scholar]
- 269. Whiteway M. 2011. Yeast mating: trying out new pickup lines. Curr. Biol. 21:R626–R628 [DOI] [PubMed] [Google Scholar]
- 270. Wilson KL, Herskowitz I. 1984. Negative regulation of STE6 gene expression by the alpha 2 product of Saccharomyces cerevisiae. Mol. Cell. Biol. 4:2420–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Wilson KL, Herskowitz I. 1987. STE16, a new gene required for pheromone production by a cells of Saccharomyces cerevisiae. Genetics 115:441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Winter-Vann AM, et al. 2005. A small-molecule inhibitor of isoprenylcysteine carboxyl methyltransferase with antitumor activity in cancer cells. Proc. Natl. Acad. Sci. U. S. A. 102:4336–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Worman HJ, Fong LG, Muchir A, Young SG. 2009. Laminopathies and the long strange trip from basic cell biology to therapy. J. Clin. Invest. 119:1825–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Wright LP, Philips MR. 2006. Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J. Lipid Res. 47:883–891 [DOI] [PubMed] [Google Scholar]
- 275. Yamazaki T, Ohara Y, Oshima Y. 1976. Rare occurrence of the tetratype tetrads in Saccharomycodes ludwigii. J. Bacteriol. 125:461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Yang SH, et al. 2005. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc. Natl. Acad. Sci. U. S. A. 102:10291–10296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Yang SH, et al. 2006. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J. Clin. Invest. 116:2115–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Young SG, et al. 2001. The enzymes, vol 21, p 155–213 Academic Press, San Diego, CA [Google Scholar]
- 279. Young SG, Fong LG, Michaelis S. 2005. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria—new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J. Lipid Res. 46:2531–2558 [DOI] [PubMed] [Google Scholar]
- 280. Yu A. 2011. Identification of cytosolic protein quality control machinery in yeast. The Johns Hopkins University, Baltimore, MD [Google Scholar]
- 281. Zhang FL, Casey PJ. 1996. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65:241–269 [DOI] [PubMed] [Google Scholar]
- 282. Zhang L, Baasiri RA, Van Alfen NK. 1998. Viral repression of fungal pheromone precursor gene expression. Mol. Cell. Biol. 18:953–959 [DOI] [PMC free article] [PubMed] [Google Scholar]