Abstract
OBJECTIVE
Although there are established age-related differences in sweet preferences, it remains unknown whether children differ from mothers in their preference for and perception of fat (creaminess). We examined whether individual differences in sucrose and fat preferences and perception are related to age, genotype and lifestyle.
SUBJECTS
Children 5–10 years-old (n = 84) and their mothers (n = 67) chose the concentration of sucrose and fat most preferred in pudding and sucrose most preferred in water using identical, two-alternative, forced-choice procedures, and ranked pudding samples for intensity of sweetness and creaminess. Subjects were also weighed and measured for height, as well as genotyped for a sweet-receptor gene (TAS1R3).
RESULTS
Children preferred higher concentrations of sucrose in water (P = 0.03) and in pudding (P = 0.05) and lower concentrations of fat in pudding (P<0.01) than did mothers. Children and mothers were equally able= to rank the intensity of different concentrations of fat (P=0.12) but not sucrose in pudding (P = 0.01). Obese and lean children and mothers did not differ in preferences, but obese mothers were less able to correctly rank the concentration of fat in pudding than were lean mothers (P = 0.03). Mothers who smoked preferred a higher concentration of sucrose than did those who never smoked (P<0.01). Individual differences in sweet preference were associated with genetic variation within the TAS1R3 gene in mothers but not children (P = 0.04).
CONCLUSION
Irrespective of genotype, children prefer higher concentrations of sugar but lower concentrations of fat in puddings than do their mothers. Thus, reduced-fat foods may be better accepted by children than adults.
Keywords: fat, sweet, children, preferences, TAS1R3, psychophysics
INTRODUCTION
Although modernization and industrialization of the food supply have produced palatable foods, the negative consequences of eating diets rich in sugars and fats have become increasingly commonplace.1 Foods containing sugars, with or without fats, have strong hedonic appeal, especially for children,2 and overeating of these foods is not only prevalent in the diets of Americans but also often associated with obesity.3,4
Why are foods that contain sugars and fats so appealing? The sensations experienced when tasting something sweet are mediated by taste receptors in the periphery and by multiple brain substrates, which phylogenetically are remarkably well conserved5 and are associated with reward-related learning and behaviors.6,7 Although sweet is considered a basic taste, the sensations experienced when tasting foods rich in fats are more complex and likely an amalgam of taste, retronasal olfactory and somatosensory components.8–10 If there is a component in fats that is a chemical stimulus for the taste system, it is probably sensed by taste receptors as free fatty acids that are created in the mouth when lingual lipase, produced near taste receptor cells, hydrolyzes triglycerides.9,11 Here we use the term ‘fat preference’ to refer to the preferences for the complex perception that individuals experience when tasting fat, including its creaminess and mouth feel, as well as its taste.
Does children’s basic biology make them more vulnerable than adults to overeating foods that are rich in sugars and fats? Psychophysical research suggests that this may be the case for sweets: children not only prefer a more intense sweet sensation than do adults (see Mennella12 for a review), but sweets are also an analgesic for children, enabling them to tolerate painful stimulation longer when they are tasting a sweet-tasting liquid than when tasting water.13 Although there is evidence that fats can also act as an analgesic during development,14 few studies have focused on the amount of fat most preferred by children15,16 and even less is known about whether it differs from that of adults.
In the present study, we determined the concentration of fat most preferred in a food matrix (pudding) by children and their mothers, using identical procedures to allow for comparisons between the two age groups. Preferences for sweetness (in both liquid form and a food matrix, pudding) were also assessed because there are pronounced age-related differences in the intensity of sweetness most preferred.2 The primary goals of the study were to determine whether there are age-related differences in liking and in the ability to discriminate foods that differ in creaminess (fat content) and to determine whether fat preferences associate with sugar preferences. Exploring how factors, such as body weight and smoking (in adults only) relate to sweet and fat preferences and dietary habits were secondary goals of the study. Smoking and obesity were independently associated with specific food cravings in our previous studies of adult women17 and thus might contribute to preferences of mothers and in turn their children. In addition, to better understand individual differences, we assessed whether variation in a sweet taste gene (TAS1R3) with previously described effects on sweet perception18 was related to sweet preference in children and their mothers.
SUBJECTS AND METHODS
Subjects
Women with healthy children between 5 and 10 years of age were recruited from the Philadelphia area using an internal database of previous participants who had consented to be notified of future studies at the Monell Center. Eligibility for participation in the ‘Monell Fat/Sweet Preference’ study was determined by initial phone interview, during which mothers were given a description of the study procedures but were not told the goals of the study or hypotheses being tested. All the procedures were approved by the Office of Regulatory Affairs at the University of Pennsylvania. Informed consent was obtained from each mother and assent was obtained from each child ≥7 years of age. Mothers were paid for their participation.
Food stimuli
Two types of stimuli were used to determine the intensity of sweetness most preferred and one stimulus was used to determine the intensity of creaminess most preferred (Table 1); all stimuli were presented at room temperature. For sweetness, we assessed the liking of five solutions that differed in sucrose content (3, 6, 12, 24 and 36% wt/vol) and three vanilla pudding samples (Jello Pudding; General Foods, White Plains, NY, USA) that differed in sucrose content (13.4, 24.1 and 36.2% wt/wt). The base pudding, which had 13.4% wt/wt sugar content, was prepared by mixing 5.3 g vanilla pudding powder with 24.7 g milk (3.8% fat wt/wt) (Lehigh Valley Dairy Farms, Lansdale, PA, USA). The other two pudding samples with sugar contents of 24.1% and 36.2% wt/wt were prepared by adding 4.2 and 10.7 g sucrose, respectively, to 30 g base pudding.
Table 1.
Food stimuli
| Taste quality |
Matrix | Concentrations |
|---|---|---|
| Sweetness | Liquid (water) |
3, 6, 12, 24 and 36% sucrose (wt/vol) |
| Creaminess | Pudding Pudding |
13.4, 24.1 and 36.2% sucrose (wt/wt)a 3.1, 6.9 and 15.6% fat (wt/wt)b |
All sweet pudding samples were made with 3.8% milk (fat wt/wt). The standard pudding has 13.4% sugar wt/wt.
All samples had 13.4% sugar wt/wt; a drop of yellow food coloring was added to the 15.6% sample to mask color differences.
For creaminess, we used three vanilla pudding samples that differed in fat content (3.1, 6.9 and 15.6% fat wt/wt); the sugar content remained constant at 13.4% sucrose wt/wt. Preparation procedures followed from the methods of Mattes:19 samples were made by mixing 5.3 g vanilla pudding powder with 24.7 g milk with different fat content. Skim milk (0% fat) and heavy cream (33% fat) were mixed to prepare milks with fat contents of 3.8, 8.4 or 19% fat wt/wt. The 3.8% milk was prepared by mixing 84.6 g skim milk with 11.4 g heavy cream, the 8.4% milk was prepared by mixing 74.4 g skim milk with 25.6 g heavy cream and the 19% milk was prepared by mixing 42.5 g skim milk with 57.6 g heavy cream. One drop of yellow food coloring (McCormick & Co., Inc. Hunt Valley, MD, USA) was added to the 15.6% sample to mask color differences.
Procedures
Following abstinence from eating for at least 1 h, mothers and children were tested individually on two separate days in rooms specifically designed for sensory testing. If the mother was a smoker, she was also asked to refrain from smoking an hour before the testing session began. Carbon monoxide levels were measured using a Vitalograph-Breath CO monitor (Vitalograph Inc., Lenexa, KS, USA); CO levels were on average 11.5±1.0 parts per million, thus indicating compliance with this request.20 Each session lasted 20–30 min. In counterbalanced order, we determined their preferences and intensity ranking for the puddings, which differed in sugar concentration, during one test day and preferences and ranking for creaminess in pudding and the intensity of sucrose most preferred in water during the other. To mask visual differences among samples, the testing rooms were illuminated with red light.
Preference tests
Children tend to answer questions in the affirmative, have short attention spans, therefore, we used a forced-choice tracking technique that is sensitive to the cognitive limitations of children2,21 to determine the concentration of sugar or fat most preferred. Each type of taste stimulus was assessed individually, and to allow for comparisons, procedures were identical for children and adults. In brief, participants were presented with pairs of solutions (5 ml each) that differed in sucrose content (3, 6, 12, 24 and 36% wt/vol) or pairs of pudding samples (10 ml each) that differed in either sucrose (13.4, 24.1 and 36.2% wt/wt) or fat (3.1, 6.9 and 15.6% wt/wt) content. For the five sucrose solutions, the first pair presented was from the middle range (6 and 24% wt/vol), whereas for the three pudding samples, the first pair was the two extremes (13.4 and 36.2% for sucrose; 3.1 and 15.6% for fat).
Participants tasted each sample of the pair for 5 s without swallowing and then pointed to which of the pair they liked better, without instruction on how the stimuli differ. Each subsequent pair of solutions contained the participant’s preceding preferred concentration paired with an adjacent stimulus concentration. This pattern continued until the participant either chose two consecutive times the same concentration paired with both a higher and lower concentration or chose two consecutive times the highest or lowest concentration. Participants rinsed their mouth once with water after tasting each sample and twice between each pair of solutions; a timer was used to ensure a 1-min interval to separate each pair presentation. For the sucrose solutions, the entire task was repeated after a 3-min break, with stimulus pairs presented in reverse order (that is, weaker stimulus presented first in series 1 and stronger stimulus first in series 2), thus preventing children from reaching criterion response based on bias toward first or second position.
Intensity ranking procedures
To evaluate participants’ ability to detect differences in the amounts of fat and sugar in the puddings, a subset of participants were asked to sample without swallowing the three puddings that differed in sugar concentration during one session (n = 68 children, n = 55 the adults) and to sample the three puddings that differed in fat during another (n = 67 children, n = 56 adults). Subjects rinsed three times after tasting each pudding. After each sample was tasted, they were asked to rank the puddings from least to most sweet or from least to most creamy, respectively. Subject responses were scored from 0 to 2, following Stewart et al.22: a score of 2 indicated all correct (for example, 3.1, 6.9 and 15.6% wt/wt); a score of 1 indicated one was correct and the other two were adjacent to each other (for example, 3.1, 15.6 and 6.9% wt/wt); and a score of 0 indicated that all were incorrect (for example, 6.9, 15.6 and 3.1% wt/wt).
Anthropometry
Subjects were weighed and measured for height wearing light clothing and no shoes (model 439 physician scale; Detecto, Webb City, MO, USA). Body mass index (BMI) was computed by dividing the weight in kilograms by the squared height in meters, and subjects were then classified into BMI categories following the Center for Disease Control pediatric growth charts for children23 or standard BMI categories for adults.24 For children, age- and sex-specific BMI z-scores were calculated using software provided by the Center for Disease Control (EpiInfo 3.5). A second z-score was computed for all subjects by standardizing the residuals of a general linear regression using generation (mother and child) as a fixed factor and age in years as a continuous variable.
Demographics, food habits and preferences
Race/ethnicity for children was defined by the mother’s report of herself and the child’s father. We use the term ‘race/ethnicity’ because it describes both the genetic and cultural components of the groups in the sample.25 Individuals of non-Hispanic African-American and non-Hispanic European descent are hereafter referred to as Black and White, respectively. Mothers also completed the Harvard Service Food Frequency Questionnaire, a semi-quantitative dietary assessment tool that assesses dietary intake of 84 food items over the past month for herself26 and a modified version of this questionnaire for her child.27 From these data, we determined the average daily intake for selected food items related to fat and sweet tastes, such as dairy foods (for example, milk, hot chocolate, cheese, yogurt, ice cream and pudding), sweets (cookies, cake, pie, Jello, chocolate, other candy, donuts and sweet rolls) and sweetened beverages (soda, fruit juice and fruit drinks). Most distributions were skewed, so daily intake values were square-root transformed.
Sweet-receptor genotyping
Mothers and children provided DNA samples from cheek swabs, which were extracted following the recommendations of the manufacturer (BuccalAmp; Epicenter, Madison, WI, USA). DNA samples were diluted to a concentration of 5 ng μl−1, which was used as template in a Taqman assay for single-nucleotide polymorphisms (Applied Biosystems, Foster City, CA, USA).28 The genetic variant chosen for the study was in a regulatory region of the sweet-receptor TAS1R3 that was previously associated with sugar sensitivity in adults.18 There are three genotypes at this locus (rs35744813; CC, CT and TT), with the T genotype being associated with a poorer ability to detect low concentrations of sucrose compared with those with the C genotype.
Data analyses
Primary analyses were framed around understanding how age associated with the concentration of fat most preferred (hereafter referred to as fat preference) and the ability to discriminate pudding samples that differed in fat content (that is, intensity ranking), as well as whether there was concordance in the concentrations of fat most preferred with concentrations of sugars most preferred in a food matrix. To this end, separate one-way analyses of variance or Chi-Square analyses with generation (children and mothers) as a grouping factor were conducted, however, when cells had fewer than five subjects, Yates’ Chi-square was used. Follow-up partition analyses were then used to further examine these significant effects. To analyze the relationship between sweet preferences in water and pudding, as well as between sweet and fat preferences in pudding, separate correlational analyses were conducted for children and mothers. Secondary analyses examined how body weight (BMI z-scores) and the smoking habits of mothers associated with sweet and fat preferences, intensity ranking and daily food intakes of sweet and fatty foods.
Genotype–phenotype association analyses were conducted in two ways. When the dependent variable was a quantitative trait (sucrose preference in water), genotype and generation (child or mother) were fixed factors in a two-way analysis of variance with least significant difference post-hoc testing when main effects were found. When the dependent variable was categorical (concentration of sucrose or fat in the pudding), genotype and generation were used as fixed factors in an ordinal logistical regression. This analytical method was chosen because of the linear (additive) effect of the TAS1R3 genotypes.18 For all analyses, the threshold for statistical significance was set at P<0.05 and were computed using Statistica 8.0 or 10.0 (StatSoft, Tulsa, OK, USA).
RESULTS
Subject characteristics
The characteristics of the study population are presented in Table 2. The final sample of 67 mothers and 84 children (44 girls and 40 boys) included 10 sibling pairs and 3 sibling triads. The children were 8.0±1.9 years of age and their mothers were 34.7±7.3 years of age. Of the 67 mothers, 37% were current smokers; the remainder had never smoked in their lifetimes. One additional mother began testing but was excluded because she was on appetite enhancement medication, and a few children (n = 9) began testing but were excluded from some of the tasks because they did not understand or for noncompliance. The distribution of race/ethnicity, family income and educational levels of the mothers reflects the socioeconomic diversity of the city of Philadelphia.29 More than one-third of the children and two-thirds of the mothers were overweight or obese, a finding consistent with current trends.30,31
Table 2.
Demographic characteristics of participants by age group: Monell Fat/Sweet Preference Study, 2009–2010, Philadelphia, PA, USA
| Characteristic | Adults (n = 67) |
Children (n = 84) |
|---|---|---|
| Age (years): mean±s.d. (range) |
34.7±7.3 (21–52) |
8.0±1.9 (5.0–10.9) |
| Race/ethnicity: % of group, n | ||
| White | 32.8%, 22 | 29.8%, 25 |
| Black | 62.7%, 42 | 54.8%, 46 |
| Hispanic/Latino/Latina | 3%, 2 | 0%, 0 |
| Other/unknown/more than one race |
1.5%, 1 | 15.5%, 13 |
| BMI (kg m −2), mean±s.d. (range) |
29.5±6.7 (19.3–54.0) |
|
| Weight category by BMI: % of group, n | ||
| Underweight | 0%, 0 | 4.8%, 4 |
| Normal weight | 29.9%, 20 | 56.6%, 47 |
| Overweight | 29.9%, 20 | 19.3%, 16 |
| Obese | 40.2%, 27 | 19.3%, 16 |
| Socioeconomic data, mothers only | ||
| Highest education level: % of mothers, n | ||
| High school | 23.9%, 16 | |
| Some college/technical school |
35.8%, 24 | |
| College | 29.9%, 20 | |
| Graduated college or higher |
10.4%, 7 | |
| Income level: % of mothers, n | ||
| <$35 000 | 55.2%, 37 | |
| $35 000–$75 000 | 29.9%, 20 | |
| >$75 000 | 14.9%, 10 | |
Abbreviation: BMI (kg m −2), body mass index, a measure of obesity, where kg is weight in kilograms and m is height measured in meters. Family yearly income is reported in US dollars ($). Smoking status was determined through questionnaire and confirmed with empirical measures (breath carbon monoxide concentrations). School refers to formal education, high school, community or 4-year college. Categories to classify BMI are from the Center for Disease Control.
Age-related differences
As a group, children preferred a more intense concentration of sucrose in water (F(1, 141) = 4.70; P = 0.03) and pudding (Yates’ χ2(2) = 5.92; P = 0.05) but a lower concentration of fat in pudding (χ2(2) = 15.55; P = 0.0004) than did mothers (Table 3). There was a positive relationship between the concentration of sucrose most preferred in water and the concentration most preferred in pudding for children (r = 0.44, P<0.001; n = 70) and a tendency for this for mothers (r = 0.23, P = 0.06; n = 66). For mothers, daily intakes of sweetened beverages were positively related to the concentration of sugar most preferred in water (r = 0.25, P = 0.05; n = 65) and pudding (r = 0.28, P = 0.02; n = 64).
Table 3.
Age-related differences in sweet and fat preferences and intensity rankings
| Measure | Mothers | Children |
F or Yates’ Chi or Chia |
P-value |
|---|---|---|---|---|
| Preference tests | ||||
| Amount of sucrose in water most preferred (% sucrose wt/vol) b, mean±s.d., n | 16.1±9.9, 67 | 19.7±9.8, 76 | 4.7 | 0.03 |
| Amount of sucrose in pudding most preferred (% sucrose wt/wt) c, % of group, n | 5.9 | 0.05 | ||
| 13.4% | 18.2%, 12 | 4.0%, 3 | ||
| 24.1% | 57.6%, 38 | 65.3%, 49 | ||
| 36.2% | 24.2%, 16 | 30.7%, 23 | ||
| Amount of fat in pudding most preferred (% fat wt/wt)c, % of group, n | 15.5 | <0.01 | ||
| 3.1% | 15.6%, 10 | 6.6%, 5 | ||
| 6.9% | 45.3%, 29 | 77.6%, 59 | ||
| 15.6% | 39.1%, 25 | 15.8%, 12 | ||
| Intensity ranking tests, % of group, n | ||||
| Sweet puddings | 8.6 | 0.01 | ||
| 0 (all incorrect) | 14.5%, 8 | 35.3%, 24 | ||
| 1 | 25.5%, 14 | 27.9%, 19 | ||
| 2 (all correct) | 60.0%, 33 | 36.8%, 25 | ||
| Fat puddings | 4.3 | 0.12 | ||
| 0 (all incorrect) | 32.2%, 18 | 47.8%, 32 | ||
| 1 | 33.9%, 19 | 32.8%, 22 | ||
| 2 (all correct) | 33.9%, 19 | 19.4%, 13 |
Test statistics from analysis of variance (F; sucrose in water preference test) or Yates’ Chi-square (for sweet preference in pudding only) or Chi-square (χ2; for fat in pudding preference test and fat and sweet-ranking test).
Geometric mean±s.d. for the % sucrose (wt/vol) preferred in two trials of a forced-choice comparison preference test.
Percent of sucrose (wt/wt) or milk fat in pudding (wt/wt) chosen on the first trial of the preference test.
No significant relationships were found between the concentration of fat most preferred and concentration of sugar most preferred in pudding for children (r= −0.03, P = 0.78; n = 70) or their mothers (r = 0.13, P = 0.32; n = 64). However, there was a significant effect of generation (age group) on the ability of subjects to rank the puddings for sweetness (χ2(2)=8.58, P = 0.01) but not creaminess (χ2(2)=4.32, P = 0.12; Table 3). A greater proportion of children than mothers incorrectly ranked all of the sweet puddings (χ2(1)=6.80; P<0.01).
Individual differences
Body weight
There was no relationship between BMI z-scores and the concentration of sugar in water (r = 0.05, P = 0.55; n = 142) and the concentration of sugar (r = 0.09, P = 0.28; n = 140) or fat (r = 0.04, P = 0.67; n = 143) in pudding most preferred by mothers and children. For mothers, the ability to rank the creaminess (but not the ability to rank sweetness) was related to BMI z-scores (r = −0.30, P = 0.03; n = 56): the higher the BMI, the greater the inability to rank the puddings. Those women who incorrectly ranked the puddings ate more dairy (2.6±1.5 serving per day) than did the others (1.7±1.4; F(1, 52) 4.52; P = 0.04). The relationship between ranking ability and BMI was not evident in children.
Smoking status of mothers
Although the smoking status of mothers had no effect on their ability to rank the puddings that differed in sucrose or fat content, we found that those who were current smokers preferred a more intense sweetness in water (F(1, 65) = 8.96, P = 0.004; see Figure 1). No group differences were observed for sweet or fat preferences in pudding ((Yate’s χ2 (2) = 2.06, P = 0.36) and (Yate’s χ2 (2) 0.18, P = 0.92), respectively). Children’s preference for and ranking of sucrose in water or pudding and fat in pudding did not differ by their mother’s smoking status (Table 4).
Figure 1.
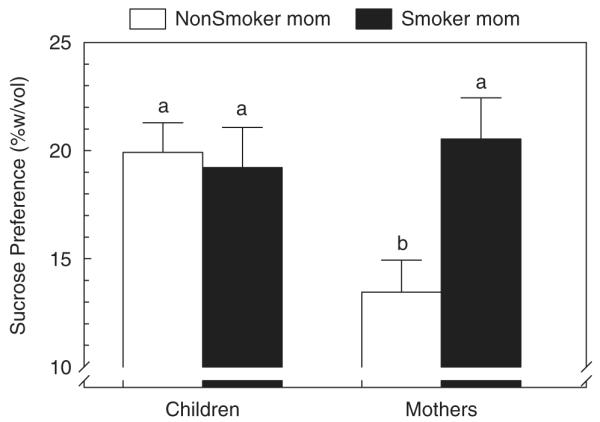
Concentration of sucrose most preferred in water (% wt/vol; mean±s.e.). Subjects were stratified by generation (mothers and children) and smoking status of the mother (smoker and nonsmoker). Groups that do not share a subscript differ by post-hoc testing.
Table 4.
Taste preferences and intensity ranking scores of mothers and children by mother’s smoking status
| Measure | Smoking status of mother |
P-value | ||
|---|---|---|---|---|
| Nonsmoker | Current smoker | F or Yates’ Chi or Chia | ||
| Mothers’ preference tests | (n = 42) | (n = 25) | ||
| Sucrose in water (% wt/vol sucrose)b | 13.5=±8.9 | 20.5=±10.1 | 9.0 | <0.01 |
| Sucrose in pudding (% wt/wt sugar)c | 2.1 | 0.36 | ||
| 13.4% | 22.0%, n = 9 | 12.0%, n = 3 | ||
| 24.1% | 61.0%, n = 25 | 52.0%, n = 13 | ||
| 36.2% | 17.1%, n = 7 | 36.0%, n = 9 | ||
| Fat in pudding (% wt/wt fat)c | 0.2 | 0.92 | ||
| 3.1% | 14.6%, n = 6 | 17.4%, n = 4 | ||
| 6.9% | 48.8%, n = 20 | 39.1%, n = 9 | ||
| 15.6% | 36.6%, n = 15 | 43.5%, n = 10 | ||
| Mothers’ ranking tests | ||||
| Sweet pudding | 1.4 | 0.50 | ||
| 0 (all incorrect) | 18.2%, n = 6 | 9.1%, n = 2 | ||
| 1 | 18.2%, n = 6 | 36.4%, n = 8 | ||
| 2 (all correct) | 63.6%, n = 21 | 54.5%, n = 12 | ||
| Fat pudding | 0.5 | 0.79 | ||
| 0 (all incorrect) | 32.4%, n = 11 | 31.8%, n = 7 | ||
| 1 | 38.2%, n = 13 | 27.3%, n = 6 | ||
| 2 (all correct) | 29.4%, n = 10 | 40.9%, n = 9 | ||
| Children’s preference | n = 55 | n = 29 | ||
| Sucrose in water (% wt/vol sucrose)b | 19.9=±9.2 | 19.2=±10.9 | 0.1 | 0.77 |
| Sucrose in pudding (% wt/wt sugar)c | 0.4 | 0.83 | ||
| 13.4% | 4.0%, n = 2 | 4.0%, n = 1 | ||
| 24.1% | 64.0%, n = 32 | 68.0%, n = 17 | ||
| 36.2% | 32.0%, n = 16 | 28.0%, n = 7 | ||
| Fat in pudding (% wt/wt fat) | 1.1 | 0.57 | ||
| 3.1% | 6.0%, n = 3 | 7.7%, n = 2 | ||
| 6.9% | 74.0%, n = 37 | 84.6%, n = 22 | ||
| 15.6% | 20.0%, n = 10 | 7.7%, n = 2 | ||
Test statistic from analysis of variance (F; sucrose in water preference test) or Yate’s χ2 (when n per cell <5) or χ2 (n per cell ≥5).
Geometric mean ± s.d. for the % sucrose (wt/vol) preferred in two trials of a forced-choice comparison preference test.
Percent of sucrose (wt/wt) or milk fat in pudding (wt/wt) chosen on the first trial of the preference test.
Sweet-receptor genotype
There was a relationship between the preference for sucrose in water, but not pudding, and alleles of the TAS1R3 sweet-receptor gene. For sucrose in water, the genotype and preference relationship differed between children and mothers. In mothers, there was an additive effect of the T genotype which increased sweet preference but there was no genotype–phenotype relationship in children (Table 5, Figure 2).
Table 5.
Sweet-receptor TAS1R3 genotype and sweet preference in children and their mothers (n = 135–144)
| Phenotype | Effect tested | F or Wald a | P-value |
|---|---|---|---|
| Sucrose in water, preference |
Generation | 2.6 | 0.11 |
| Genotype | 0.8 | 0.45 | |
| GXG | 3.3 | 0.04* | |
| Sucrose in pudding, preference |
Generation | 4.5 | 0.03* |
| Genotype | 2.1 | 0.34 | |
| GXG | 2.9 | 0.23 |
Abbreviation: GXG, TAS1R3 genotype by generation (that is, mother vs child). All tests had two df,
P<0.05.
Test statistics from analysis of variance (F; sucrose in water) or ordinal logistical regression (Wald; sucrose in pudding).
Figure 2.
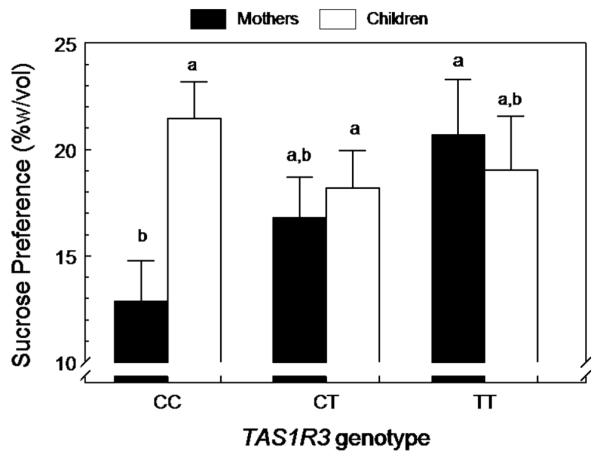
Concentration of sucrose most preferred in water (% wt/vol; mean±s.e.). Subjects stratified by generation (mother and children) and genotype (TAS1R3). The TAS1R3 protein is a component of the sweet receptor, and the DNA variant site studied here is in the promoter region and has three commonly observed genotypes, CC, CT or TT. Groups that do not share a subscript differ by post-hoc testing.
DISCUSSION
Children live in different sensory worlds than do adults. Children preferred a higher concentration of sugar but a lower concentration of fat than did their mothers, therefore, the age-related effects are not solely due to children’s preference for more intense stimulation. For both children and mothers, the higher the concentration of sucrose preferred in water, the higher the concentration of sucrose preferred in pudding. This intensity of sweetness most preferred, as measured in the laboratory, has been shown to be related to what they like to eat in the real world, such as the concentration of sugars in their most preferred beverages and breakfast cereals.28 Why do children prefer higher sweet but lower fat compared with their mothers? Several factors, not mutually exclusive, may explain these age-related differences.
Sweetness may be more salient than creaminess for children. The positive hedonic responses to sweet taste and preference for higher amounts of sweetness by children than by adults have been observed in many countries and cultures.32 Such heightened preferences, which persist throughout childhood and adolescence, may have an ecological basis because, in nature, sweet-tasting foods are associated with energy-producing sugars, minerals and vitamins. Such preferences may have evolved to solve a nutritional problem of attracting children to sources of energy during periods of growth.33,34 In the present study, children seemed to be cueing in on the sweetness of the puddings; they preferred the puddings with higher sweet content and those with less fat content. In complex solid food mixtures, sweet and fatty tastes interact with increasing fat concentrations suppressing the perception of sweetness.35,36 In this experiment, the sugar content was held constant in the fat puddings, so puddings with a lower fat content tasted sweeter despite a constant amount of sugar in the recipe. The puddings did differ in caloric density but we emphasize that during the psychophysical testing, subjects did not swallow the stimuli and rated the stimuli immediately after tasting, thus minimizing post-ingestive effects on liking.
Another potential explanation for why children and not mothers preferred the low-fat pudding may relate to body weight or fat patterning. Obese adults are less able to perceive low concentrations of fatty acids37 and prefer fat more in some studies17 than do lean adults. Likewise, adults with larger waist circumferences are less able to discriminate fat in salad dressing than are those with smaller waist circumference.38 The effects of obesity or fat distribution may be cumulative, and mothers have been obese longer than comparably overweight children. It could be that chronic obesity or the accumulation of abdominal fat increases fat preference but that these children are not yet affected.
We found that genetic variation in the sweet receptor was related to sweet preference for the mothers but not for the children. The TAS1R3 gene has a common variant in the promoter region which has three genotypes, CC, CT and TT, and the T allele was previously linked with sucrose sensitivity in adult populations.18 Here for the mothers, it had a similar effect, with each T allele increasing sucrose preference. From these data, we suggest that the desire for sweetness during development33 is stronger than effects of genotype on taste perception when children are growing. These genetic differences may emerge in adulthood, once full stature has been achieved. Furthermore, the effects of genotype were apparent only for sucrose in water and not for sucrose in pudding, which suggests that genotype effects may have real-world significance for simpler items such as sweet juices or sodas rather than for more complex foods.
In addition to differences between children and their mothers, we also found that mothers who smoked preferred higher concentrations of sucrose in water, more similar to those preferred by children, than did the other mothers. However, smoking did not affect ranking ability, so these effects on preference are unlikely to be secondary to effects of smoking on sensitivity to sucrose.39 The determinants of sweet-preference differences between smokers and nonsmokers may lie instead in the ability of sweet solutions to reduce pain and increase pleasure: smoking may be a sign of an addiction-prone phenotype and elevated sweet preference is also a sign of this biology.40
Although mothers and children performed the same procedure for ranking and preference, children made more errors in sweetness ranking, but not in creaminess ranking. Ranking is a more difficult task than reporting preference, but deficiencies in general task comprehension are an unlikely explanation for the observed difference in sweetness ranking, because children did not differ in creaminess ranking, a task that is equally or more difficult. We hypothesize instead that children may have found it harder to separate their positive hedonic response to the sample from objective sensations of sweetness.
Our results have implications for the treatment of childhood obesity. A goal of most health advisory panels in developed countries is to reduce the amount of sugar and fat in the diets of children to reduce obesity. Recommendations put forth by the Committee of Nutrition of the American Academy of Pediatrics,41,42 American Heart Association,43 and the Institute of Medicine44 describe drastic changes in children’s diet, for example, replacing whole-fat milk with reduced-fat milk for weaned infants beginning at 1 year of age, reducing added sugars and salts, and offering nutritious snacks, such as vegetables and fruits, low-fat dairy foods and whole grains. Knowledge gleaned from chemosensory research in children suggests that limiting sugars and salt may be particularly difficult for pediatric populations because children’s strong hedonic appeal for more intense sweet2 and salty45 tastes than adults.
In the present study, we found that although children preferred higher concentrations of sweetness in water and pudding, they preferred fat less than did mothers and were less able to detect low-fat foods (as measured by the ranking task). In this context, our data suggest a new direction, to focus on fat reduction. Whether our laboratory-based results extend to common sources of fat in the diets of children such as fried foods and processed meat products is not known but is an important area of future research. Likewise, limits need to be placed on the consumption of low-fat but sweet-tasting foods because of the contribution of sugar to overweight and diabetes. A better knowledge of what children prefer to eat and why will better inform policy on the prevention of childhood obesity.
ACKNOWLEDGEMENTS
This project was funded by the Pennsylvania Department of Health, by award R01HD37119 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and an ARRA supplement to this grant (3R01HD037119-10S1) and by awards R01DC011287 and P30DC011735 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. We acknowledge the valuable technical assistance of Aleida Silva-Garcia, Fiona Crowley, Kristi Roberts and Dr Stacie Miller, whose positions were created by supplement 3R01HD037119-10S1 received under the American Recovery and Reinvestment Act (ARRA). The technical help of Fujiko Duke, Alexis Burdick Will, Rebecca James, Anna Lysenko and Liang-Dar (Daniel) Hwang with the DNA purification, extraction and genotyping and Ryan Crawford for subject testing is gratefully acknowledged. Joseph H Lee provided statistical advice and Ms Patricia Watson provided expert editorial assistance. We acknowledge the valuable comments provided by Dr Gary K Beauchamp on an earlier version of the manuscript.
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS JAM designed the experiment, analyzed the data and wrote the manuscript. SF collected the data and assisted in manuscript preparation. DRR assayed genotypes and assisted in data analysis and assisted in writing the manuscript.
REFERENCES
- 1.Gidding SS, Lichtenstein AH, Faith MS, Karpyn A, Mennella JA, Popkin B, et al. Implementing American Heart Association Pediatric and Adult Nutrition Guidelines: a scientific statement from the American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular Disease in the Young, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, and Council for High Blood Pressure Research. Circulation. 2009;119:1161–1175. doi: 10.1161/CIRCULATIONAHA.109.191856. [DOI] [PubMed] [Google Scholar]
- 2.Mennella JA, Lukasewycz LD, Griffith JW, Beauchamp GK. Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chem Senses. 2011;36:345–355. doi: 10.1093/chemse/bjq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr. 2011;93:836–843. doi: 10.3945/ajcn.110.000141. [DOI] [PubMed] [Google Scholar]
- 4.Drewnowski A, Brunzell JD, Sande K, Iverius PH, Greenwood MR. Sweet tooth reconsidered: taste responsiveness in human obesity. Physiol Behav. 1985;35:617–622. doi: 10.1016/0031-9384(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 5.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 6.Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes (Lond) 2009;33(Suppl 2):S44–S48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes JE, Duffy VB. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol Behav. 2008;95:77–87. doi: 10.1016/j.physbeh.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattes RD. Is there a fatty acid taste? Annu Rev Nutr. 2009;29:7.1–7.23. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drewnowski A. Why do we like fat? J Am Diet Assoc. 1997;97(7 Suppl):S58–S62. doi: 10.1016/s0002-8223(97)00732-3. [DOI] [PubMed] [Google Scholar]
- 11.Mattes RD. Accumulating evidence supports a taste component for free fatty acids in humans. Physiol Behav. 2011;104:624–631. doi: 10.1016/j.physbeh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mennella J. The sweet taste of childhood. In: Basbaum A, Kaneko A, Shephers C, Westheimer G, editors. The Senses: A Comprehensive Reference. Olfaction and Taste. vol. 4. Academic Press; San Diego: 2008. pp. 183–188. [Google Scholar]
- 13.Pepino MY, Mennella JA. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain. 2005;119:210–218. doi: 10.1016/j.pain.2005.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blass EM. Milk-induced hypoalgesia in human newborns. Pediatrics. 1997;99:825–829. doi: 10.1542/peds.99.6.825. [DOI] [PubMed] [Google Scholar]
- 15.Lanfer A, Knof K, Barba G, Veidebaum T, Papoutsou S, de Henauw S, et al. Taste preferences in association with dietary habits and weight status in European children: results from the IDEFICS study. In J Obesity. 2012;36:7–34. doi: 10.1038/ijo.2011.164. [DOI] [PubMed] [Google Scholar]
- 16.Monneuse MO, Bellisle F, Louis-Sylvestre J. Impact of sex and age on sensory evaluation of sugar and fat in dairy products. Physiol Behav. 1991;50:1111–1117. doi: 10.1016/0031-9384(91)90569-a. [DOI] [PubMed] [Google Scholar]
- 17.Pepino MY, Finkbeiner S, Mennella JA. Similarities in food cravings and mood states between obese women and women who smoke tobacco. Obesity (Silver Spring) 2009;17:1158–1163. doi: 10.1038/oby.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol. 2009;19:1288–1293. doi: 10.1016/j.cub.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattes RD. Fat preference and adherence to a reduced-fat diet. Am J Clin Nutr. 1993;57:373–381. doi: 10.1093/ajcn/57.3.373. [DOI] [PubMed] [Google Scholar]
- 20.Low EC, Ong MC, Tan M. Breath carbon monoxide as an indication of smoking habit in the military setting. Singapore Med J. 2004;45:578–582. [PubMed] [Google Scholar]
- 21.Cowart BJ, Beauchamp GK. Early development of taste perception. In: McBride R, MacFie H, editors. Psychological Basis of Sensory Evaluation. Elsevier; London: 1990. pp. 1–17. [Google Scholar]
- 22.Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104:145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002:1–190. [PubMed] [Google Scholar]
- 24.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults – The evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 25.Sankar P, Cho MK. Genetics. Toward a new vocabulary of human genetic variation. Science. 2002;298:1337–1338. doi: 10.1126/science.1074447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner J, Suitor C, Witshci J, Wang J. Dietary assessment methodology for use in the special supplemental food program for women, infants and children. (WIC)Agriculture UDo; Washington, DC: 1991. 58-3198-0-048. [Google Scholar]
- 27.Blum RE, Wei EK, Rockett HR, Langeliers JD, Leppert J, Gardner JD, et al. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health J. 1999;3:167–172. doi: 10.1023/a:1022350023163. [DOI] [PubMed] [Google Scholar]
- 28.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:e216–e222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anonymous . Philadelphia. Pew Chartitable Trust; Philadelphia: 2011. 2011. [Google Scholar]
- 30.Freedman DS. Obesity — United States, 1988–2008. MMWR Surveill Summ. 2011;60(Suppl):73–77. [PubMed] [Google Scholar]
- 31.Austin SB. The blind spot in the drive for childhood obesity prevention: bringing eating disorders prevention into focus as a public health priority. Am J Public Health. 2011;101:e1–e4. doi: 10.2105/AJPH.2011.300182. [DOI] [PubMed] [Google Scholar]
- 32.Liem DG, Mennella JA. Sweet and sour preferences during childhood: role of early experiences. Dev Psychobiol. 2002;41:388–395. doi: 10.1002/dev.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coldwell SE, Oswald TK, Reed DR. A marker of growth differs between adolescents with high vs low sugar preference. Physiol Behav. 2009;96:574–580. doi: 10.1016/j.physbeh.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drewnowski A. Sensory control of energy density at different life stages. Proc Nutr Soc. 2000;59:239–244. [PubMed] [Google Scholar]
- 35.Drewnowski A, Schwartz M. Invisible fats: sensory assessment of sugar/fat mixtures. Appetite. 1990;14:203–217. doi: 10.1016/0195-6663(90)90088-p. [DOI] [PubMed] [Google Scholar]
- 36.Hayes JE, Duffy VB. Revisiting sugar-fat mixtures: sweetness and creaminess vary with phenotypic markers of oral sensation. Chem Senses. 2007;32:225–236. doi: 10.1093/chemse/bjl050. [DOI] [PubMed] [Google Scholar]
- 37.Stewart JE, Newman LP, Keast RS. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin Nutr. 2011;30:838–844. doi: 10.1016/j.clnu.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Liang LC, Sakimura J, May D, Breen C, Driggin E, Tepper BJ, et al. Fat discrimination: A phenotype with potential implications for studying fat intake behaviors and obesity. Physiol Behav. 2011;105:470–475. doi: 10.1016/j.physbeh.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–1899. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mennella JA, Pepino MY, Lehmann-Castor SM, Yourshaw LM. Sweet preferences and analgesia during childhood: effects of family history of alcoholism and depression. Addiction. 2010;105:666–675. doi: 10.1111/j.1360-0443.2009.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krebs NF, Jacobson MS. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112:424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- 42.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 43.Gidding SS, Dennison BA, Birch LL, Daniels SR, Gillman MW, Lichtenstein AH, et al. Dietary recommendations for children and adolescents: a guide for practitioners: consensus statement from the American Heart Association. Circulation. 2005;112:2061–2075. doi: 10.1161/CIRCULATIONAHA.105.169251. [DOI] [PubMed] [Google Scholar]
- 44.McGuire S. Adv Nutr (Bethesda) Vol. 112. The National Academies Press; Washington, DC: 2010. Institute of Medicine. Strategies to reduce sodium intake in the United States; pp. 49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beauchamp GK, Moran M. Acceptance of sweet and salty tastes in 2-year-old children. Appetite. 1984;5:291–305. doi: 10.1016/s0195-6663(84)80002-1. [DOI] [PubMed] [Google Scholar]


