Abstract
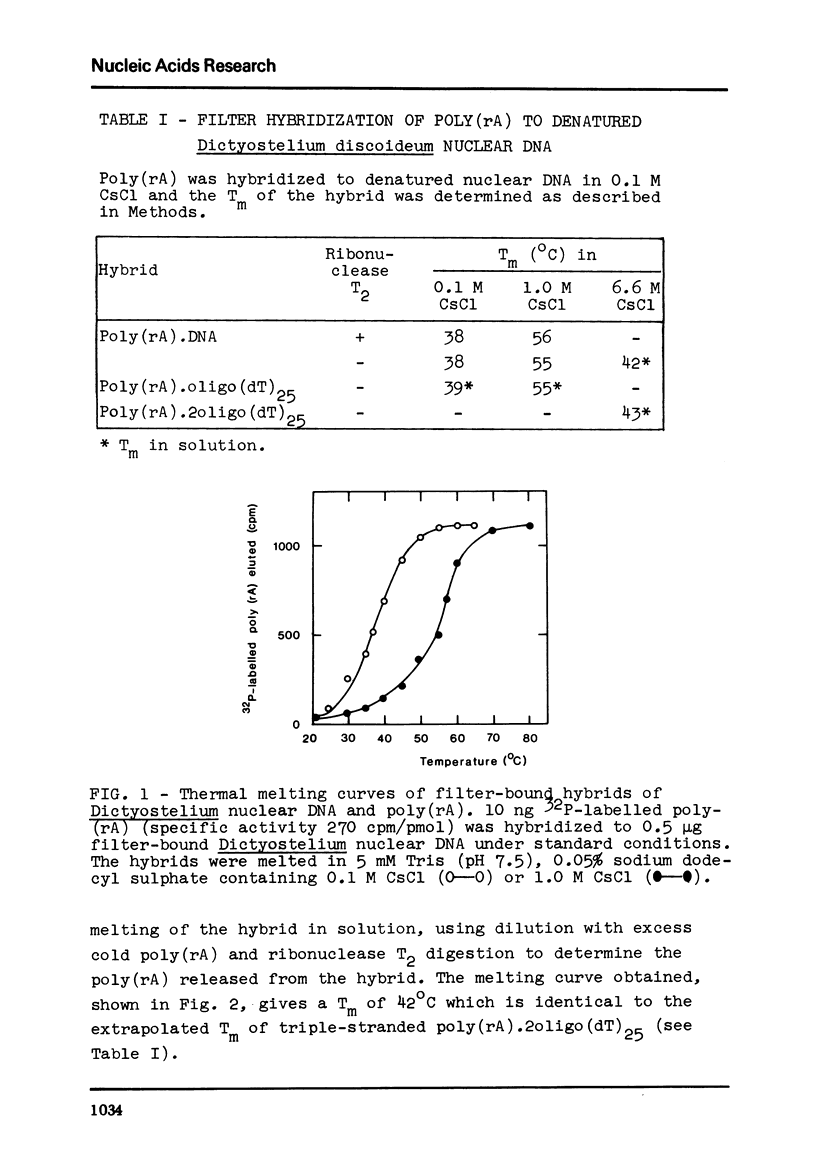
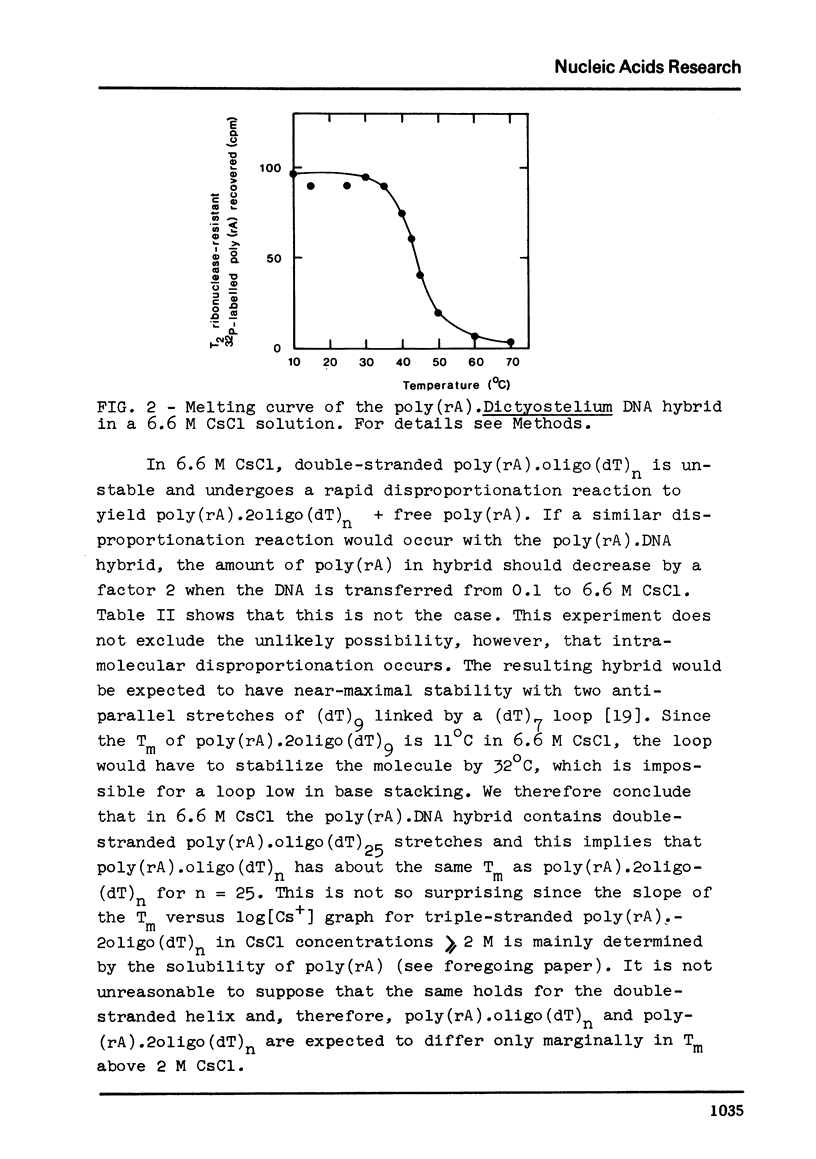
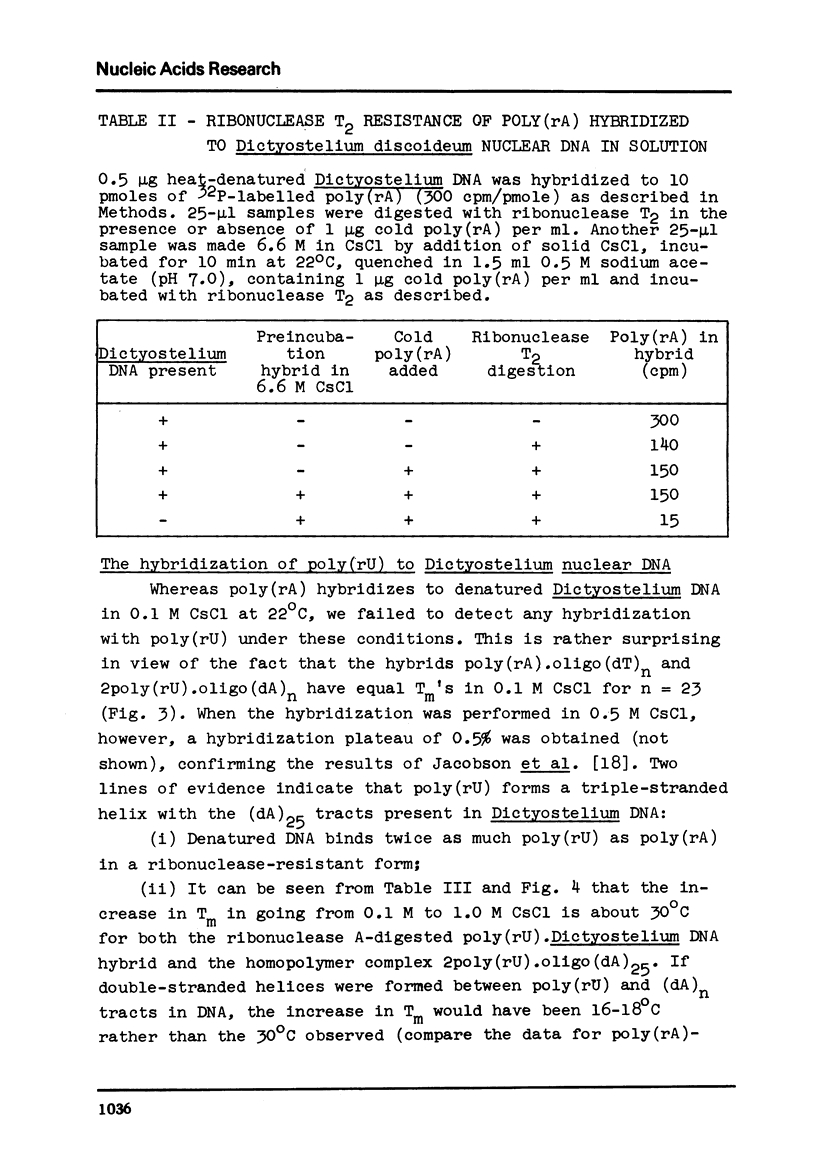
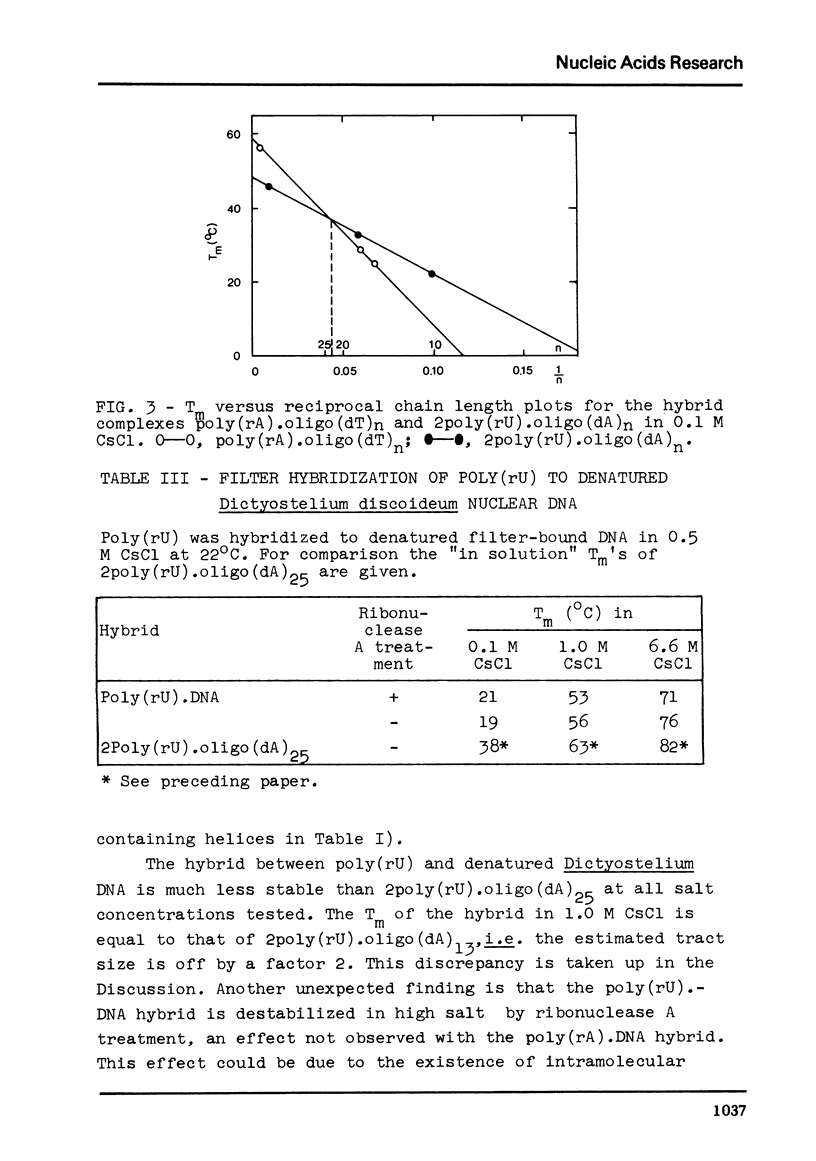
We have studied the interaction of poly(rA) and poly(rU) with natural DNAs containing (dA.dT)n sequences. The results indicate that hybridization of poly(rA) to denatured DNA can be used to estimate the size and frequency of large (dA.dT)n tracts, whereas hybridization with poly(rU) does not give reliable information on these points. In 6.6 M CsCl, poly(rU) can form stable complexes with denatured DNA containing short (dA)n tracts (n less than or equal to 6), whereas binding of poly(rA) to denatured DNA under these conditions requires much larger (dT)n tracts (estimated n greater than 13). Moreover, binding of poly(rA) requires pre-hybridization in low salt, because free poly(rA) precipitates in 6.6 M CsCl.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUTZ E. K., BAUTZ F. A. THE INFLUENCE OF NONCOMPLEMENTARY BASES ON THE STABILITY OF ORDERED POLYNUCLEOTIDES. Proc Natl Acad Sci U S A. 1964 Dec;52:1476–1481. doi: 10.1073/pnas.52.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Bonner J. Characterization of the genome of the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1972 May 28;66(3):339–361. doi: 10.1016/0022-2836(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice L. C., Baker R. F. Deoxyadenylate-rich sequences in sea urchin DNA during early development. Cell Differ. 1974 Jul;3(2):117–126. doi: 10.1016/0045-6039(74)90033-5. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Birfelder E. J., Sanders J. P., Borst P. DNA-DNA hybridization on nitrocellulose filters. 1. General considerations and non-ideal kinetics. Eur J Biochem. 1974 Sep 16;47(3):535–543. doi: 10.1111/j.1432-1033.1974.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Fouquet H., Bierweiler B., Sauer H. W. Reassociation kinetics of nuclear DNA from Physarum polycephalum. Eur J Biochem. 1974 May 15;44(2):407–410. doi: 10.1111/j.1432-1033.1974.tb03498.x. [DOI] [PubMed] [Google Scholar]
- HOHL H. R., RAPER K. B. Nutrition of cellular slime molds. I. Growth on living and dead bacteria. J Bacteriol. 1963 Jan;85:191–198. doi: 10.1128/jb.85.1.191-198.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramaru M., Uchida T., Egami F. Ribonuclease preparation for the base analysis of polyribonucleotides. Anal Biochem. 1966 Oct;17(1):135–142. doi: 10.1016/0003-2697(66)90016-9. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., Flavell R. A., van Kreijl C. F., van Bruggen E. F., Arnberg A. C. The unusual properties of mtDNA from a "low-density" petite mutant of yeast. Biochim Biophys Acta. 1972 Aug 16;277(1):44–58. doi: 10.1016/0005-2787(72)90350-4. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Firtel R. A., Lodish H. Transcription of polydeoxythymidylate sequences in the genome of the cellular slime mold, Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1974 May;71(5):1607–1611. doi: 10.1073/pnas.71.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnit D. M., Schildkraut C. L., Maio J. J. Single-strand interactions of mouse satellite DNA. Biochim Biophys Acta. 1972 Feb 15;259(3):297–312. doi: 10.1016/0005-2787(72)90305-x. [DOI] [PubMed] [Google Scholar]
- Marshall S., Gillespie D. Poly U tracts absent from viral RNA. Nat New Biol. 1972 Nov 8;240(97):43–45. doi: 10.1038/newbio240043a0. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Uhlenbeck O. C., Doty P. Self-complementary oligoribonucleotides: adenylic acid-uridylic acid block copolymers. J Mol Biol. 1971 Apr 28;57(2):201–215. doi: 10.1016/0022-2836(71)90341-x. [DOI] [PubMed] [Google Scholar]
- Mol J. N., Borst P., Grosveld F. G., Spencer J. H. The size of the repeating unit of the repetitive mitochondrial DNA from a "low-density" petite mutant of yeast. Biochim Biophys Acta. 1974 Dec 6;374(2):115–128. doi: 10.1016/0005-2787(74)90355-4. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Thomas W. L., Darnell J. E. Occurrence of uridylate-rich oligonucleotide regions in heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3684–3688. doi: 10.1073/pnas.69.12.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushynski W. E., Spencer J. H. Nucleotide clusters in deoxyribonucleic acids. V. The pyrimidine oligonucleotides of strands r and l of bacteriophage T7 DNA. J Mol Biol. 1970 Aug 28;52(1):91–106. doi: 10.1016/0022-2836(70)90179-8. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Kopp D. W., Edmonds M. Localization of the polyadenylate sequences in messenger ribonucleic acid and in the heterogeneous nuclear ribonucleic acid of HeLa cells. J Biol Chem. 1973 Feb 25;248(4):1472–1476. [PubMed] [Google Scholar]
- Pero R. W., Bryngelsson T., Bryngelsson C., Deutsch A., Nordén A., Norgren A. Hybridization of polyuridylic acid to human DNA immobilized onto nitrocellulose filters. Nucleic Acids Res. 1975 Jul;2(7):1163–1176. doi: 10.1093/nar/2.7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J. P., Flavell R. A., Borst P., Mol J. N. Nature of the base sequence conserved in the mitochondrial DNA of a low-density petite. Biochim Biophys Acta. 1973 Jul 13;312(3):441–457. doi: 10.1016/0005-2787(73)90443-7. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Base sequence and evolution of guinea-pig alpha-satellite DNA. Nature. 1970 Aug 22;227(5260):794–798. doi: 10.1038/227794a0. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Borst P. Erroneous transcription of mitochondrial DNA by RNA polymerase from Escherichia coli. Biochim Biophys Acta. 1970 Oct 15;217(2):356–364. doi: 10.1016/0005-2787(70)90533-2. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Borst P., Tabak A. J. Search for mitochondrial DNA sequences in chick nuclear DNA. Biochim Biophys Acta. 1973 Jan 19;294(1):184–191. [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Borer P. N., Dengler B., Tinoco I., Jr Stability of RNA hairpin loops: A 6 -C m -U 6 . J Mol Biol. 1973 Feb 5;73(4):483–496. doi: 10.1016/0022-2836(73)90095-8. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Martin F. H., Doty P. Self-complementary oligoribonucleotides: effects of helix defects and guanylic acid-cytidylic acid base pairs. J Mol Biol. 1971 Apr 28;57(2):217–229. doi: 10.1016/0022-2836(71)90342-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Hydroxyapatite chromatography of short double-helical DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):333–340. doi: 10.1016/0005-2787(73)90019-1. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]
- van Kreijl C. F., Borst P., Flavell R. A., Hollenberg C. P. Pyrimidine tract analysis of mtDNA from a "low-density" petite mutant of yeast. Biochim Biophys Acta. 1972 Aug 16;277(1):61–70. doi: 10.1016/0005-2787(72)90352-8. [DOI] [PubMed] [Google Scholar]



