Abstract
Accurate monitoring of respiration is often needed for neurophysiological studies, as either a dependent experimental variable or an indicator of physiological state. Current options for respiratory monitoring of animals held in a stereotaxic frame include EMG recordings, pneumotachograph measurements, inductance-plethysmography, whole-body plethysmography (WBP), and visual monitoring. While powerful, many of these methods prevent access to the animal’s body, interfere with experimental manipulations, or require deep anesthesia and additional surgery.
For experiments where these issues may be problematic, we developed a non-invasive method of recording respiratory parameters specifically for use with animals held in a stereotaxic frame. This system, ventilation pressure transduction (VPT), measures variations in pressure at the animal’s nostril from inward and outward airflow during breathing. These pressure changes are detected by a sensitive pressure transducer, then filtered and amplified. The output is an analog signal representing each breath.
VPT was validated against WBP using 10% carbon dioxide and systemic morphine (4 mg/kg) challenges in lightly anesthetized animals. VPT accurately represented breathing rate and tidal volume changes under both baseline and challenge conditions. This novel technique can therefore be used to measure respiratory rate and relative tidal volume when stereotaxic procedures are needed for neuronal manipulations and recording.
Keywords: stereotaxy, respiration, device, morphine, hypercapnia, plethysmography
1. Introduction
Many in vivo experiments in neuroscience employ stereotaxy, which allows manipulations or neuronal recordings in specifically targeted areas in the central nervous system (Subramanian and Holstege, 2009; Zhang et al., 2009; Depuy et al., 2011). Monitoring breathing of the anesthetized animal is critical for such studies, as animal health and anesthetic depth can drastically affect experiments. Non-invasive methods of respiratory monitoring are often preferred or even required, such as with non-terminal experiments.
The most commonly used methods for respiratory monitoring during stereotaxic procedures include EMG (electromyography) from respiratory muscles (Gray et al., 2001; Montandon et al., 2011), intubation or tracheotomy with pneumotachograph (Yasaki and Dyck, 1991; Weksler et al., 1994; Spoelstra et al., 2007), and capnography (Colman and Krauss, 1999). EMG electrodes can monitor the diaphragm, the intercostals, the genioglossus, or abdominal muscles, and depending on the muscle group, the resulting signal is associated with either inhalation or exhalation (Subramanian and Holstege, 2011). Although EMG is easily recorded in animals held in a stereotaxic frame, this method is subject to electrical artifacts and challenges of interpretation (O’Neil and Raub, 1984). Pneumotachograph accurately measures air flow, but to be compatible with stereotaxy it requires additional invasive procedures (tracheotomy or endotracheal intubation) and deep anesthesia. Capnography (monitoring carbon dioxide output) is a powerful method but is subject to environmental and metabolic interference (Bhavani-Shankar et al., 1992).
Less invasive methods of respiratory monitoring are available, although these methods also have limitations. One widely used non-invasive approach is respiratory plethysmography, which measures changes in volume of the chest and/or abdomen during breathing. Whole-body plethysmography (WBP) involves placing the animal in a closed chamber and measuring volume of air displaced during breathing (Dubois et al., 1956a; Dubois et al., 1956b; Palecek, 1969, O’Neil and Raub, 1984; Enhorning et al., 1998; Glaab et al., 2007). WBP allows absolute measurements of volume of air displaced, but the system is cumbersome when combined with stereotaxy, and prevents access to the animal’s body during the experiment. A newer, non-invasive method is accelerometry-based inductive plethysmography (ACC, Devonshire et al., 2009). Instead of measuring volume expansion, ACC uses a microchip to measure acceleration during movement of the chest or abdomen, and therefore has promise for noninvasively monitoring the breathing of animals in a stereotaxic frame. This method is similar to inductance plethysmography, which measures changes in the position of the chest and abdomen with breathing (Stromberg et al., 1993; Carry et al., 1997). However, acceleration as a surrogate for positional changes of the chest has not so far been validated by comparison to a standard method such as WBP.
Here we present a novel non-invasive method for measuring respiratory parameters during stereotaxic procedures. The method, ventilation pressure transduction (VPT), uses a sensor to pick up changes in air pressure at the nares during breathing. We compared VPT to whole-body plethysmography (WBP), as well as to accelerometry-based inductive plethysmography (ACC). We find that VPT provides an accurate, reliable, and easy-to-use solution for non-invasive measurement of breathing compatible with stereotaxy.
2. Material and Methods
2.1. Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University. Research methods followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (Zimmermann, 1983). Experiments were conducted on 6 male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing between 250 and 350 g.
2.2. Preparation and Surgery
Animals were initially anesthetized with inhaled 4% isoflurane in humidified oxygen (1.25 L/min) for 4–5 min (Martenson et al., 2005). An incision was made and a catheter (PE50) placed in the jugular vein for subsequent drug administration (morphine). The incision was covered with 5% lidocaine ointment and closed using wound clips. Animals were placed in a stereotaxic frame with a loose-fitting nasal mask for continuous flow isoflurane anesthesia, and the isoflurane concentration stepped down gradually to 1.25–1.5%. Waste gases were exhausted through a low pressure scavenger system.
2.3. Lightly Anesthetized Model
The lightly anesthetized model is an anesthetic preparation that permits stable in vivo stereotaxic electrophysiology experiments while maintaining important neuronal circuits, behavioral reflexes, and other characteristics of awake animals (Morel et al., 1987; Heinricher and Kaplan, 1991; Lanier et al., 1994; Reed et al., 2008). The experimental protocol was not started until anesthetic flow, heart rate, and breathing frequency were stable for least 25 minutes. For the duration of the experimental protocol, the anesthetic concentration and gas flow were maintained at a constant level, consistent with previous work in lightly anesthetized animals (Heinricher et al., 2010a, b).
2.4. Respiratory Monitoring
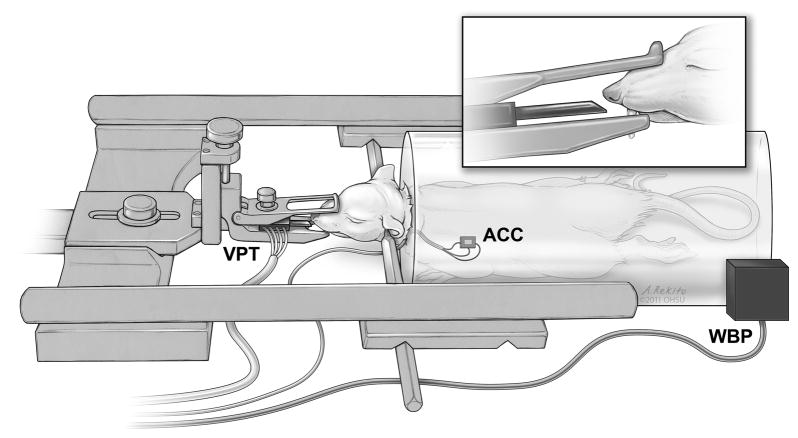
The three methods of respiratory monitoring, VPT, WBP and ACC, were used simultaneously in all experiments (Figure 1). The simultaneous use of these three non-invasive methods permitted within-subject comparisons of the characteristics of each approach.
Fig. 1.
Placement of respiratory monitoring devices. The measurement device for VPT was locked in place in the stereotaxic frame in front of the animal’s nose, so that the sensor was 1 to 5 mm from the nostril. The sensor for ACC was glued to the shaved chest wall using a flexible dental epoxy. WBP used an enclosed plastic chamber that completely surrounds the animal’s body, with subtle air displacements being picked up by pressure sensors in the system. (The isoflurane delivery method is not shown in this figure to highlight the positioning of the VPT sensor.)
2.4.1. Ventilation Pressure Transduction (VPT)
VPT is based on the idea that subtle changes in pressure fields from inhalation and exhalation can be detected externally as the animal breathes. The incisors and head were locked in place in the stereotactic frame, and a small piece of polyethylene tubing (2 mm outer diameter, 1 cm length) was connected to one port on the pressure transducer (BLVR-L01D, BLVR Series Low Pressure Sensors, All Sensors, Morgan Hill, CA). This sensor and tubing were placed in the frame such that the open end of the tubing was 1–5 mm from the nostrils, inside the nasal mask used for isoflurane delivery (Figure 1, nasal mask not shown in the figure for clarity). The sensor body was then set in place on the stereotaxic frame such that the tubing remained in a fixed position relative to the nostrils for the entire experiment.
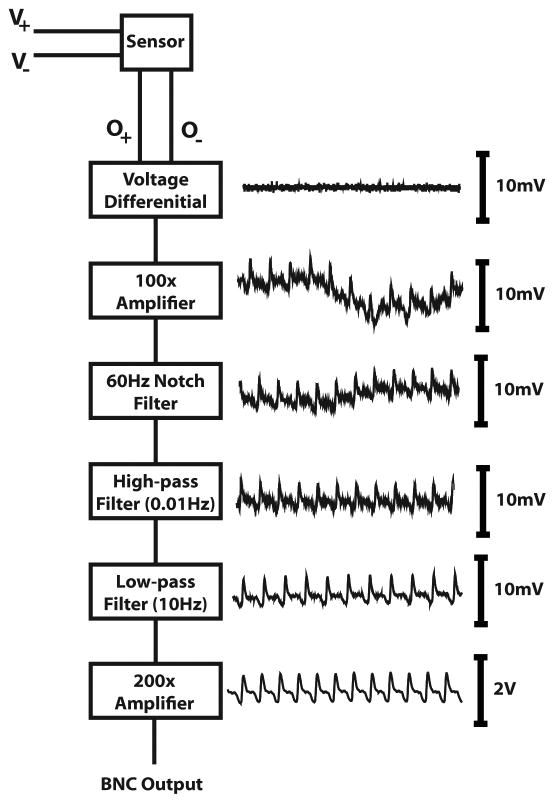
The conceptual design of the VPT system is represented in Figure 2. Changes in pressure were measured through physical displacement of an impermeable membrane inside the sensor body. The two-port sensor was positioned such that the port attached to the tubing was in close approximation to the animal’s nostril and the other port was open to the air. The electrical output of the sensor is relative to the pressure difference across the two ports, so leaving the second port open to air ensured that environmental changes would affect both ports equally and not disturb the recording. The sensor output was fed into a custom processing system, although any commercially available signal amplification and filtering unit would suffice. Our system used hardware-based band-pass filtering (0.01 to 10 Hz) to remove low- and high-frequency noise, and a 2-stage amplifier to boost the signal 10,000 to 20,000-fold. The final output signal was digitized, stored, and displayed using Spike2 software (CED, Cambridge, England). All data were digitized at a minimum of 1 kHz and saved for off-line analysis.
Fig. 2.
Schematic of VPT system. The VPT measurement device takes as power a voltage between 2.5 and 3.5 V, and outputs two signals whose differential represents the strength of the measured changes in pressure. Differential, amplification, and filters were all implemented through hardware processing. The final output was passed to an analog-to-digital converter, and the signal then fed into a computer.
2.4.2. Accelerometry-based induced plethysmography (ACC)
ACC constitutes a variant of inductance plethysmography, but instead of a chest or abdominal band to measure displacement, a small electronic accelerometer was used to quantify movement. This method provides only an indirect measure of chest movement, since the signal is not based on actual displacement but instead on acceleration, the second derivative of positional changes.
The ACC system was assembled similar to published circuit diagrams (Devonshire et al., 2009). In short, a commercially-available accelerometer chip (ADXL330K-CPZ, Analog Devices, Norwood, MA, USA) was hooked to ground and 3.5 V power source, and the output from the chip fed into an amplifier and filtering box (Grass General Purpose Amplifier, Grass Technologies, West Warwick, RI, USA). The chip was attached along the lateral chest wall caudal to the scapula (Figure 1) using quick-set dental impression material (Patterson Reflection, Patterson Dental, Saint Paul, MN). This material is non-toxic and adheres well to skin and hair, but is elastic enough to be easily peeled away at the end of the experiment to retrieve the accelerometer chip. The signal from the chip was high-pass filtered at 0.01 Hz to remove the DC offset, low-pass filtered at 15 Hz, notch filtered at 60 Hz, and amplified 1000-fold. The output signal was digitized and recorded as above.
2.4.3. Head-Out Whole-Body Plethysmography (WBP)
WBP measures changes in volume of an organ or body by quantifying pressure changes in a closed chamber (Dubois et al., 1956a; Cumming, 1961; Jacky, 1978; Bar-Yishay, 2009). In head-out whole-body plethysmography, an animal’s head is left free while the body is sealed inside the chamber, which is equipped with an external port for measuring pressure changes (Figure 1). Using Boyle’s law, measurements of pressure changes in the container can be converted to volumes of air displaced. The volume of air in one breath (tidal volume) is an integration of these measurements over time.
Here, WBP data were gathered using a single head-out chamber plethysmography system (Buxco Electronics, Sharon, CT, USA), as described previously (Nettleton et al., 2008; Wallisch et al., 2011). A flexible membrane was placed around the animal’s neck, tight enough to prevent airflow around the membrane when in the sealed chamber but not tight enough to occlude circulation or the airway. The line for the jugular catheter was passed out along the edges of the membrane. The animal was mounted in the stereotaxic apparatus and the rear portion of the chamber sealed around the animal’s body. Pressure changes in the body chamber were filtered and amplified by Buxco’s proprietary equipment and then digitized as above.
2.5. Experimental Design
The purpose of the experiment was to validate measurements of VPT against WBP, a well-established non-invasive method for respiratory monitoring (Coggins et al., 1981; Enhorning et al., 1998; Bar-Yishay, 2009), and to determine the relative merits of VPT and ACC. The comparisons were made under basal conditions and during increased and decreased respiratory drive.
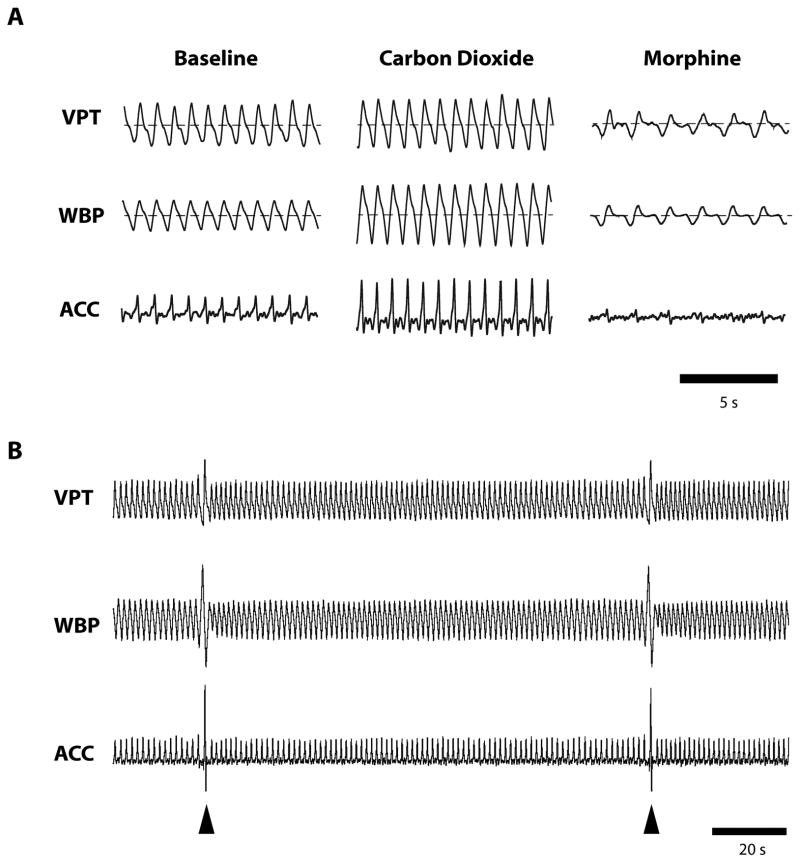
Baseline activity was first recorded for 10–15 min. Then, to increase respiratory drive, the pure oxygen in the gas anesthetic mix was switched to a 10% carbon dioxide and 90% oxygen mix for 3–5 minutes. Flow rate and anesthetic concentration were held constant during this process. The gas mixture was then switched back to pure oxygen, and the animal was given 10 minutes to recover. In the next part of the experiment, an intravenous injection of 4 mg/kg morphine was used to produce a decrease in respiration and periods of apnea. Figure 3A shows examples of the gross changes for the different treatments using the three recording methods.
Fig. 3.
Example data from experimental protocol. A) Following a baseline period, 10% carbon dioxide was administered for 5 minutes, which significantly increased respiratory drive. After a recovery period, morphine was administered at a dose (4 mg/kg) that produced respiratory depression. Dashed line indicates zero-point of signal. B) At intervals, anesthetized animals exhibited sighs (arrowheads), brief increases in inspiration that open alveoli and prevent atelectasis (Orem and Trotter, 1993). Recordings in this figure were taken from the same animal, using all three methods simultaneously.
2.6. Breathing Frequency
Breathing frequency for all three methods was measured using threshold detection followed by waveform matching. The thresholds for detection were often slightly off-set with the different methods, so for each successive breath detected, instead of using absolute time at the breath, the inter-breath interval was calculated and this value was converted to an instantaneous readout of breaths per second.
2.7. Tidal Volume
For measurements of tidal volume, the area under the curve for the WBP signal was integrated for each individual breath, and then normalized relative to the mean magnitude in the baseline period of the experiment. The normalized measurements thus represent the relative tidal volume, as compared to the baseline period. Although with calibration WBP can measure absolute tidal volume, normalized rather than absolute measurements were used here because future measures of tidal volume using only VPT or ACC alone could not likewise be calibrated for absolute measurements. Baseline amplitude of the VPT and ACC signals depend largely on placement of the device, which was slightly different for each experiment.
A number of different analyses of the VPT and ACC signals were carried out to identify the metric that best matched the WBP measurements of tidal volume. Absolute peak value, area under the curve, double integral, and peak-to-peak amplitude were all considered. A correlation analysis using all breaths during baseline in each individual experiment showed that peak-to-peak amplitude of VPT and ACC (difference between the highest point and lowest point for each individual breath) correlated best with measurements of WBP tidal volume (WBP tidal volume vs. ACC peak-to-peak: R2 = 0.65; WBP tidal volume vs. VPT peak-to-peak: R2 = 0.67). We therefore chose peak-to-peak measurement of VPT and ACC outputs for comparison to WBP in all analyses of tidal volume.
2.8. Statistical Analysis
The Bland-Altman method was used for assessing the level of agreement between methods (Bland and Altman, 1986). Mean difference (bias), the limits of agreement, and the standard error for the limits of agreement are reported for comparisons of WBP with VPT and with ACC. This analysis was conducted on data from 120 measurements of individual breaths from each of the three methods during a quiescent period of steady breathing in baseline (approximately 2–3 minutes of activity), with the required correction for repeated measures, giving n = 6 (Bland and Altman, 1999; 2007). With the correction for repeated measures, the traditional Bland-Altman plot does not accurately convey the limits of agreement, and a graphical plot is therefore not shown here.
Between-method comparisons of measured rate and tidal volume during increased and decreased respiration were made using a two-way ANOVA, with both time and method of measurement as within-subject factors. Averages of 30-s samples taken in baseline, during CO2, and after morphine administration were compared. A Bonferroni post-hoc test was used to test for specific differences. Results are expressed as mean ± SEM.
3. Results
3.1. Measurements of Respiration
All three methods (WBP, VPT, and ACC) provided reliable baseline respiratory signals (Figure 3A). Signals were steady, regular, and consistent, and events such as sighs were clearly visible across all three methods (Figure 3B). Occasionally, an adjustment in the ACC placement would have to be made before the start of the experiment, but once the protocol was started, no further adjustments were made to any of the devices.
3.2. Breathing Frequency
A Bland-Altman analysis of the three different methods for measuring breathing frequency showed strong agreement between WBP and each of the other two methods. The mean difference in measurements of breathing frequency between WBP and VPT was 0.0025 Hz, and the 95% limits of agreement were −0.084 Hz and 0.089 Hz, with a standard error of 0.016 Hz for this confidence interval. Since the confidence interval crosses zero, measurements of breathing frequency for VPT showed no bias relative to WBP.
For ACC, measurements of breathing frequency also agreed with WBP. The mean difference in measurements of individual breaths between WBP and ACC was 0.00002 Hz, and the limits of agreement were from −0.086 Hz to 0.086 Hz, with a standard error for the confidence interval of 0.04 Hz. Measurements of breathing frequency made using ACC therefore have no significant bias relative to WBP.
3.3. Relative Tidal Volume
Low systematic bias of VPT or ACC relative to WBP was also found for tidal volume. For measurements of relative tidal volume, the mean bias of VPT compared to WBP was 1.4%. The 95% limits of agreement ranged between −18.7 and 21.5%, and the standard error for these limits was 6.9%. Similarly, ACC was not significantly biased relative to WBP, with a mean difference of measurement of −3.9%. The 95% limits of agreement were from −30.1 to 23.1%. The standard error for these limits was 7.6%.
3.4. Detection and Quantification of Changes in Respiration
A carbon dioxide challenge drove an increase in respiration, while systemically administered morphine severely depressed respiration. Both changes were grossly evident in output from all three systems (Figure 3A).
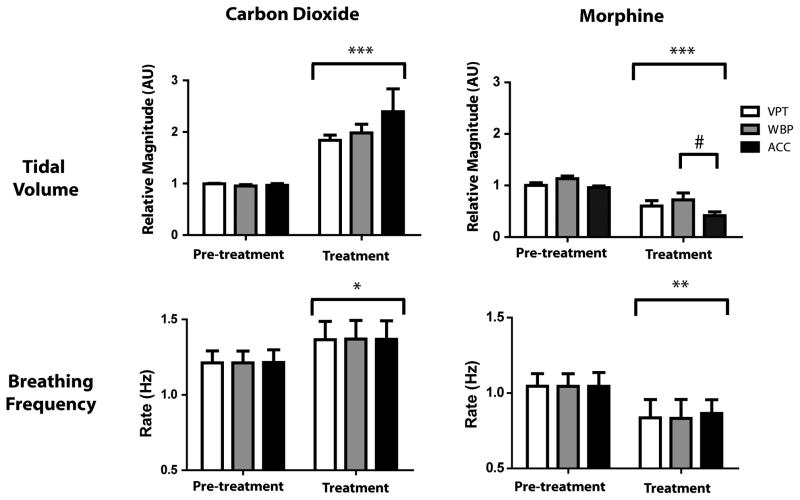
Carbon dioxide produced significant increases in both breathing frequency and tidal volume, as measured using WBP, VPT, and ACC (Figure 4). For breathing frequency, we saw a significant effect of treatment (F2,15 = 8.40, p = 0.011), but no difference among measurement methods (F2,15 = 0.00, p = 0.99). Relative tidal volume showed a similar pattern, with a significant effect of treatment (F2,15 = 44.72, p < 0.001), and no effect of method (F2,15 = 1.04, p = 0.38).
Fig. 4.
Mean effect of 10% carbon dioxide and 4 mg/kg morphine on breathing frequency and tidal volume. Administration of carbon dioxide produced an increase in rate and tidal volume detected by all three methods. Morphine produced a decrease in breathing frequency and tidal volume evident with all three methods, but ACC significantly overestimated the effect of morphine on tidal volume. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to pre-treatment; #p < 0.05 compared to WBP.
Respiratory depression was next induced by administration of morphine (4 mg/kg, i.v.). Morphine produced a significant decrease in breathing frequency (F2,15 = 14.11, p < 0.01), with no difference among measurement methods (F2,15 = 0.01, p = 0.99). Although relative tidal volume was significantly reduced by morphine treatment (F2,15 = 47.72, p < 0.001), the three methods were not equivalent (F2,15 = 3.99, p < 0.05). Post-hoc tests showed that VPT accurately tracked WBP measurement of tidal volume after morphine, whereas ACC significantly overestimated the decrease in tidal volume relative to WBP (Figure 4). This difference in measurement occurred because some lower-amplitude breaths after morphine were indistinguishable from noise when using ACC (Figure 5).
Fig. 5.
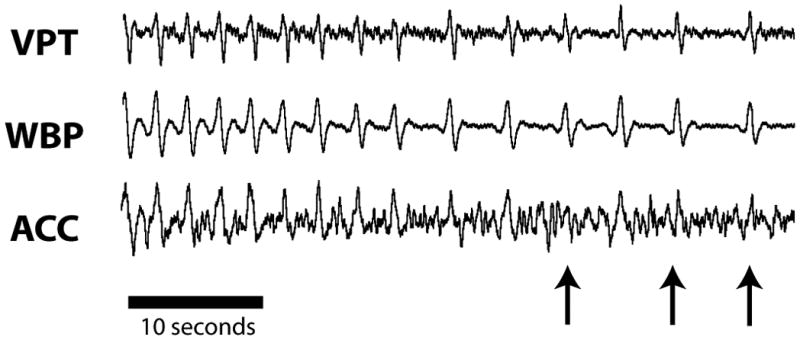
Breaths lost to detection by ACC during respiratory depression. Administration of morphine produced respiratory depression bordering on apnea. While the periodic breaths were still detectable using VPT and WBP, the waveform for ACC was indistinguishable from noise (arrows indicate breaths lost to detection).
4. Discussion
We present a novel method for measuring respiratory parameters compatible with stereotaxy. This method, VPT, provides an accurate and reliable measure of breathing frequency and relative changes in tidal volume. The low profile and unobtrusive design allow surgical and behavioral perturbations of the body without concern over disturbing the respiratory signal. VPT is therefore a useful tool for measuring respiration, either as an experimental dependent variable or as an indicator of anesthetic stability (Steffey et al., 2003).
4.1. Sensitivity of VPT
With VPT, the relative amplitude of the respiratory signal depends on the detection of small pressure changes, and filtering and amplification are required to extract a workable signal from the noise. The sensors used in this experiment have a working pressure range of ±1 inH2O (~250 Pas or ~2.5 mBar), and the sensor’s normal electrical output for this pressure range is from 4.5 to 11.5 mV. The output is graded relative to pressure, and the sensor manufacturer claims excellent linearity in the pressure-to-signal ratio (0.3% linearity). In the present experiments, the raw electrical output with the animal’s breathing was in the tens of μV for each breath. Assuming the sensors have consistent linearity, the pressure changes being measured by these sensors were in the range of single Pascals or tens of μBar. In an open environment, these small pressure perturbations from airflow at the nostril drop off exponentially with distance from the source to the sensor, so the sensor should be placed as close to the source as possible, although we were able to obtain a usable signal at distances of up to 5 mm from the nostril. Sensors less sensitive than those used here may not be able to register a signal or may need to be placed much closer to the nostril.
4.2. VPT compared to WBP
VPT showed good agreement with WBP in measuring respiratory frequency and relative tidal volume, as demonstrated by the Bland-Altman analysis. The negligible difference in measurements of rate between these two methods was reflected in the average rates seen during increased and decreased respiratory drive, with no detectable difference in the response to carbon dioxide or morphine obtained with these two methods.
While the estimates of relative tidal volume obtained with VPT did not systematically over-or underestimate tidal volume determined using WBP, the Bland-Altman analysis of individual breaths showed variation of as much as 25% on some individual breaths between the two measures. However, this variation should not pose a problem in most experimental applications. Because the VPT system shows no systematic bias, aggregate measures that average tidal volume over a period of time would substantially reduce this source of error. This idea was applied here in the practical measurements of effects of morphine and carbon dioxide, where we used the average of a 30-second sample. With this approach, measures obtained using WBP and VPT were not significantly different.
Finally, the waveform for VPT closely matched that of WBP for individual breaths. The similarity of waveform would allow advanced analyses on VPT signals using the same techniques that are applied to WBP, including measuring relative inspiratory and expiratory times, quantifying number of sighs in a given period, or considering irregularities in breathing patterns (Zhang et al., 2009; Subramanian and Holstege, 2011).
4.3. VPT compared to other methods
Our data show that for rate and relative volume, VPT measurements were in close agreement with those obtained using WBP, a well-validated method of measuring respiration (Hantos and Brusasco, 2002; Glaab et al., 2007). The principal advantage of VPT over WBP is the ease and simplicity of obtaining respiratory signals in the stereotaxic environment. WBP requires the animal’s entire body be enclosed in a sealed chamber, and opening the chamber to manipulate the animal or change the configuration affects measurements. By contrast, VPT uses only one sensor placed in close proximity to the head, fixed to the stereotaxic frame. Shifts in the animal’s body, surgical procedures, sensory testing, or repeated injections do not perturb the signal obtained from the animal. Further, since the transducer measures the pressure difference across two ports, the system is resistant to ambient changes in environmental pressures, such as a door opening or an experimenter moving around the room.
We also compared VPT to ACC, since both methods provide non-invasive respiratory monitoring in a manner compatible with stereotaxic frames or other head restraint. The two methods perform comparably in detecting respiratory rate, but VPT provides a more accurate measure of tidal volume during respiratory depression. In addition, interpretation of the ACC signal in terms of inspiration and expiration would not be possible. Further, with ACC any movement of the animal, the sensor, or the connecting wires can change the placement and distort further measurements of relative tidal volume, a problem that does not arise with VPT. Lastly, since animals can use both chest and abdominal muscles for breathing (O’Neil and Raub, 1984), the placement of a single accelerometer in relation to specific muscle groups increases possible error in measurement.
For survival surgeries or experiments that need to minimize surgical procedures, other non-invasive methods are also available for monitoring respiration. Hot-wire anemometry measures changes in wire impedance from flow of gas over a small, heated filament. This method provides an accurate representation of airflow under physiological conditions, but the direction of flow (inspiration vs. expiration) cannot be discriminated without additional equipment (Godal et al., 1976; Lundsgaard et al., 1979; Yoshiya et al., 1979). Capnography is a commonly used clinical method for monitoring breathing, but has shortcomings that limit its use in the research environment. The accuracy of carbon dioxide readings are affected by atmospheric pressure, gas anesthetic, oxygen concentration, and water vapor, and metabolic activity will change the actual excretion of carbon dioxide (Bhavani-Shankar et al., 1992).
Invasive methods of respiratory measurement in anesthetized animals include EMG recordings, pneumotachograph measurement, or even deriving respiratory rate from blood pressure. EMG recordings offer advantages in dissecting the neuromuscular components of respiration, although for simply monitoring physiological state EMG does not necessarily represent tidal volume well (Campbell et al., 1995; Hodges et al., 2001). EMG recordings are also subject to electrical noise from the heart, animal movements, and the environment (Bartolo et al., 1996). Deriving respiratory rate from blood pressure avoids having an additional measurement device for respiration, but the method requires an arterial cannula and is limited to measurements of breathing rate (Mason et al., 2007). Pneumotachograph measurements via tracheotomy or an endotracheal tube is a powerful approach that can be employed with stereotaxy in deeply anesthetized animals. However, while possibly more accurate, invasive pneumotachograph measurements are incompatible with light anesthesia and survival experiments. Also, in some cases the maintenance of the laryngeal valve is preferred, such as with the investigation of airway dysfunction related to neurodegeneration (Dutschmann et al., 2010; Menuet et al., 2011).
4.4. Limitations of VPT
VPT does not provide an absolute measure of tidal volume, so tidal volume and minute volume could only be compared between subjects by calculating change relative to baseline. However, the inability to calculate absolute tidal volumes for these systems is not necessarily a significant experimental limitation. With a treatment, the change in absolute tidal volume may differ between animals, but the relative change may be the more consistent and relevant value (e.g. with a treatment, a large animal may have a larger absolute change in tidal volume than a small animal, but the relative change could be equal between the two animals). The variability in agreement of VPT with WBP measures of tidal volume is largely inconsequential, although WBP is slightly better for detecting subtle changes in tidal volume when only sampling a small number of breaths. Additionally, variations in experimental conditions, such as changes in anesthetic gas viscosity or ambient humidity, as well as altered airway patency, could also increase the variance in the measurements of tidal volume. However, these variables should not affect measurements of breathing frequency.
5. Conclusion
Various methods of measuring respiratory function provide quantitative and qualitative information related to breathing rate, tidal volume, and gas exchange. Depending on the specific question addressed and the experimental paradigm, some technologies may be more appropriate. Here we present and validate a novel, non-invasive method for use with anesthetized animals in a stereotaxic frame. Respiratory rate and relative tidal volume are quantified via detection of small pressure variations that occur with the inward and outward flow of air during breathing. This method is compatible with stereotaxy, allows surgical and experimental access to the entire animal, and accurately measures breathing rate and relative tidal volume.
VPT is a low-profile, non-invasive respiratory monitor used with a stereotaxic frame
VPT detects small fluctuations in pressure from outflow of breath
This technique measures rate and relative tidal volume
VPT is accurate, reliable, and compares well against whole-body plethysmography
Acknowledgments
Andy Rekito of OHSU Dept. of Neurological Surgery provided illustrations. We thank John Hunt of the OHSU Dept. of Biomedical Engineering for circuit design assistance.
Grants
Supported by grants from NINDS (NS066159, NS070374) and NIDA (DA022492). DRC was supported in part by a Neurobiology of Disease Fellowship from OHSU Brain Institute.
Abbreviations
- ACC
accelerometry-based inductive plethysmography
- VPT
ventilation pressure transduction
- WBP
whole body plethysmography
Footnotes
Author Contributions
Author contributions: DRC designed, built, and tested the system. RSP contributed to design and testing. All authors contributed to data analysis, writing, and editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bar-Yishay E. Whole-body plethysmography. The human factor Chest. 2009;135:1412–4. doi: 10.1378/chest.09-0115. [DOI] [PubMed] [Google Scholar]
- Bartolo A, Dzwonczyk RR, Roberts C, Goldman E. Description and validation of a technique for the removal of ECG contamination from diaphragmatic EMG signal. Med Biol Eng Comput. 1996;34:76–81. doi: 10.1007/BF02637025. [DOI] [PubMed] [Google Scholar]
- Bhavani-Shankar K, Moseley H, Kumar AY, Delph Y. Capnometry and anaesthesia. Can J Anaesth. 1992;39:617–32. doi: 10.1007/BF03008330. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–82. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- Campbell C, Weinger MB, Quinn M. Alterations in diaphragm EMG activity during opiate-induced respiratory depression. Respiration Physiol. 1995;100:107–17. doi: 10.1016/0034-5687(94)00119-k. [DOI] [PubMed] [Google Scholar]
- Carry PY, Baconnier P, Eberhard A, Cotte P, Benchetrit G. Evaluation of respiratory inductive plethysmography: accuracy for analysis of respiratory waveforms. Chest. 1997;111:910–5. doi: 10.1378/chest.111.4.910. [DOI] [PubMed] [Google Scholar]
- Coggins CR, Duchosal F, Musy C, Ventrone R. Measurement of respiratory patterns in rodents using whole-body plethysmography and a pneumotachograph. Lab Anim. 1981;15:137–40. doi: 10.1258/002367781780959125. [DOI] [PubMed] [Google Scholar]
- Colman Y, Krauss B. Microstream capnograpy technology: a new approach to an old problem. J Clin Monit Comput. 1999;15:403–9. doi: 10.1023/a:1009981115299. [DOI] [PubMed] [Google Scholar]
- Cumming G. The body plethysmorgraph. Postgraduate medical journal. 1961;37:257–8. doi: 10.1136/pgmj.37.427.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–90. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire IM, Preston MJ, Dommett EJ, Murphy KL, Greenfield SA. Design and evaluation of a low-cost respiratory monitoring device for use with anaesthetized animals. Lab Anim. 2009;43:382–9. doi: 10.1258/la.2009.0080124. [DOI] [PubMed] [Google Scholar]
- Dubois AB, Botelho SY, Bedell GN, Marshall R, Comroe JH., Jr A rapid plethysmographic method for measuring thoracic gas volume: a comparison with a nitrogen washout method for measuring functional residual capacity in normal subjects. J Clin Invest. 1956a;35:322–6. doi: 10.1172/JCI103281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois AB, Botelho SY, Comroe JH., Jr A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. J Clin Invest. 1956b;35:327–35. doi: 10.1172/JCI103282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Menuet C, Stettner GM, Gestreau C, Borghgraef P, Devijver H, Gielis L, Hilaire G, Van Leuven F. Upper airway dysfunction of Tau-P301L mice correlates with tauopathy in midbrain and ponto-medullary brainstem nuclei. J Neurosci. 2010;30:1810–21. doi: 10.1523/JNEUROSCI.5261-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enhorning G, van Schaik S, Lundgren C, Vargas I. Whole-body plethysmography, does it measure tidal volume of small animals? Can J Physiol Pharmacol. 1998;76:945–51. doi: 10.1139/cjpp-76-10-11-945. [DOI] [PubMed] [Google Scholar]
- Glaab T, Taube C, Braun A, Mitzner W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res. 2007;8:63. doi: 10.1186/1465-9921-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal A, Belenky DA, Standaert TA, Woodrum DE, Grimsrud L, Hodson WA. Application of the hot-wire anemometer to respiratory measurements in small animal. J Appl Physiol. 1976;40:275–7. doi: 10.1152/jappl.1976.40.2.275. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–30. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantos Z, Brusasco V. Assessment of respiratory mechanics in small animals: the simpler the better? J Appl Physiol. 2002;93:1196–7. doi: 10.1152/japplphysiol.00526.2002. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: role in nociceptive modulation in the lightly anesthetized rat. Pain. 1991;47:105–13. doi: 10.1016/0304-3959(91)90017-R. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Maire JJ, Lee D, Nalwalk JW, Hough LB. Physiological basis for inhibition of morphine and improgan antinociception by CC12, a P450 epoxygenase inhibitor. J Neurophysiol. 2010a;104:3222–30. doi: 10.1152/jn.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Martenson ME, Nalwalk JW, Hough LB. Neural basis for improgan antinociception. Neuroscience. 2010b;169:1414–20. doi: 10.1016/j.neuroscience.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW, Heijnen I, Gandevia SC. Postural activity of the diaphragm is reduced in humans when respiratory demand increases. J Physiol. 2001;537:999–1008. doi: 10.1111/j.1469-7793.2001.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacky JP. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J Appl Physiol. 1978;45:644–7. doi: 10.1152/jappl.1978.45.4.644. [DOI] [PubMed] [Google Scholar]
- Lanier WL, Iaizzo PA, Milde JH, Sharbrough FW. The cerebral and systemic effects of movement in response to a noxious stimulus in lightly anesthetized dogs. Possible modulation of cerebral function by muscle afferents. Anesthesiology. 1994;80:392–401. doi: 10.1097/00000542-199402000-00019. [DOI] [PubMed] [Google Scholar]
- Lundsgaard JS, Gronlund J, Einer-Jensen N. Evaluation of a constant-temperature hot-wire anemometer for respiratory-gas-flow measurements. Med Biol Eng Comput. 1979;17:211–5. doi: 10.1007/BF02440931. [DOI] [PubMed] [Google Scholar]
- Martenson M, Houts J, Heinricher M, Ogden B. A simple device for humidification of inspired gases during volatile anesthesia in rats. Contemp Top Lab Anim Sci. 2005;44:46–8. [PubMed] [Google Scholar]
- Mason P, Gao K, Genzen JR. Serotonergic raphe magnus cell discharge reflects ongoing autonomic and respiratory activities. J Neurophysiol. 2007;98:1919–27. doi: 10.1152/jn.00813.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet C, Borghgraef P, Matarazzo V, Gielis L, Lajard AM, Voituron N, Gestreau C, Dutschmann M, Van Leuven F, Hilaire G. Raphe tauopathy alters serotonin metabolism and breathing activity in terminal Tau. P301L mice: possible implications for tauopathies and Alzheimer’s disease. Respir Physiol Neurobiol. 2011;178:290–303. doi: 10.1016/j.resp.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, Rouiller E, de Ribaupierre Y, de Ribaupierre F. Tonotopic organization in the medial geniculate body (MGB) of lightly anesthetized cats. Exp Brain Res. 1987;69:24–42. doi: 10.1007/BF00247026. [DOI] [PubMed] [Google Scholar]
- Nettleton RT, Wallisch M, Olsen GD. Respiratory effects of chronic in utero methadone or morphine exposure in the neonatal guinea pig. Neurotoxicol Teratol. 2008;30:448–54. doi: 10.1016/j.ntt.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil JJ, Raub JA. Pulmonary function testing in small laboratory mammals. Environ Health Perspect. 1984;56:11–22. doi: 10.1289/ehp.845611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem J, Trotter RH. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetized cats. J Appl Physiol. 1993;74:761–9. doi: 10.1152/jappl.1993.74.2.761. [DOI] [PubMed] [Google Scholar]
- Palecek F. Measurement of ventilatory mechanics in the rat. J Appl Physiol. 1969;27:149–56. doi: 10.1152/jappl.1969.27.1.149. [DOI] [PubMed] [Google Scholar]
- Reed JL, Pouget P, Qi HX, Zhou Z, Bernard MR, Burish MJ, Haitas J, Bonds AB, Kaas JH. Widespread spatial integration in primary somatosensory cortex. Proc Natl Acad Sci U S A. 2008;105:10233–7. doi: 10.1073/pnas.0803800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra EN, Ince C, Koeman A, Emons VM, Brouwer LA, van Luyn MJ, Westerink BH, Remie R. A novel and simple method for endotracheal intubation of mice. Lab Anim. 2007;41:128–35. doi: 10.1258/002367707779399400. [DOI] [PubMed] [Google Scholar]
- Steffey MA, Brosnan RJ, Steffey EP. Assessment of halothane and sevoflurane anesthesia in spontaneously breathing rats. Am J Vet Res. 2003;64:470–4. doi: 10.2460/ajvr.2003.64.470. [DOI] [PubMed] [Google Scholar]
- Stromberg NO, Dahlback GO, Gustafsson PM. Evaluation of various models for respiratory inductance plethysmography calibration. J Appl Physiol. 1993;74:1206–11. doi: 10.1152/jappl.1993.74.3.1206. [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. Midbrain and medullary control of postinspiratory activity of the crural and costal diaphragm in vivo. J Neurophysiol. 2011;105:2852–62. doi: 10.1152/jn.00168.2011. [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. The nucleus retroambiguus control of respiration. J Neurosci. 2009;29:3824–32. doi: 10.1523/JNEUROSCI.0607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallisch M, Subban CV, Nettleton RT, Olsen GD. Chronic in utero buprenorphine exposure causes prolonged respiratory effects in the guinea pig neonate. Neurotoxicol Teratol. 2011;32:398–405. doi: 10.1016/j.ntt.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B, Ng B, Lenert J, Burt M. A simplified method for endotracheal intubation in the rat. J Appl Physiol. 1994;76:1823–5. doi: 10.1152/jappl.1994.76.4.1823. [DOI] [PubMed] [Google Scholar]
- Yasaki S, Dyck PJ. A simple method for rat endotracheal intubation. Lab Anim Sci. 1991;41:620–2. [PubMed] [Google Scholar]
- Yoshiya I, Shimada Y, Tanaka K. Evaluation of a hot-wire respiratory flowmeter for clinical applicability. J Appl Physiol. 1979;47:1131–5. doi: 10.1152/jappl.1979.47.5.1131. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid micro-receptors in medullary raphe depresses sighs. Am J Physiol. 2009;296:R1528–37. doi: 10.1152/ajpregu.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]