Abstract
Objective
To determine whether the presence of definite osteophytes (in absence of joint space narrowing [JSN]) by radiograph is associated with (subregional) increases in cartilage thickness, in a within-person, between-knee cross-sectional comparison of participants in the Osteoarthritis Initiative (OAI). Based on previous results, external medial (ecMF) and external lateral weight-bearing femoral (ecLF) subregions were selected as primary endpoints.
Methods
Both knees of 61 (of 4798) OAI participants displayed definite tibial or femoral marginal osteophytes and no JSN in one knee, and no signs of radiographic OA in the contra-lateral knee; this being confirmed by an expert central reader. In these participants, cartilage thickness was measured in 16 femorotibial subregions of each knee, based on sagittal DESSwe magnetic resonance images. Location-specific joint space width from fixed flexion radiographs was determined using dedicated software. Location-specific associations of osteophytes with cartilage thickness were evaluated using paired t-tests and mixed effect models.
Results
Of the 61 participants, 48% had only medial, 36% only lateral, and 16% bi-compartmental osteophytes. Osteophyte knees had significantly thicker cartilage than contra-lateral non-osteophyte knees in the ecMF (+71±223μm, equivalent to +5.5%, p=0.015) and ecLF (+64±195μm, +4.1%, p=0.013). No significant differences between knees were noted in other subregions, nor in joint space width. Cartilage thickness in ecMF and ecLF was significantly associated with tibial osteophytes in the same (medial or lateral) compartment (p=0.003).
Conclusion
Knees with early radiographic OA display thicker cartilage than (contra-lateral) knees without radiographic findings of OA, specifically in the external femoral subregions of compartments with marginal osteophytes.
Keywords: Early osteoarthritis, Cartilage thickening, Osteophytes, Magnetic resonance imaging, Cartilage swelling
Introduction
Although cartilage loss is considered a hallmark of osteoarthritis (OA), an increase in cartilage thickness has been observed in animal models of OA during the early phase of disease; this has been attributed to either cartilage swelling [1-4] or hypertrophy [5, 6]. However, evidence for cartilage thickening in early human OA remains enigmatic. Studying clinical cohorts, in which some participants potentially display cartilage thickening whereas others show cartilage thinning (loss), may increase the variability of longitudinal observations and substantially reduce the capability of demonstrating efficacy for structure-modifying therapies. This applies, in particular, to novel non-weight-bearing imaging methodology, by magnetic resonance imaging (MRI) [7]. Clarification of whether cartilage thickening actually occurs in human OA, specifically, at which locations in the joint, at which phase of the disease (e.g. early), and at what magnitude, therefore is of great interest both from a basic- and from a clinical research perspective. Challenges in measurement of cartilage thickening in early human OA are that the actual onset of OA is not well defined, that limitations of cross-sectional analyses arise from the large inter-subject variability of (normal) cartilage thickness [8-11], and that it is difficult to identify the right “time window” in which cartilage thickening may occur, given the slow progression of the disease.
Recent cross-sectional studies, which have employed quantitative MRI, have reported a significantly greater (subregional) cartilage thickness in knees with Kellgren Lawrence grade (KLG2) radiographic knee OA (RKOA) compared with healthy reference participants [10, 12], KLG2 RKOA being characterized by the presence of definite osteophytes [OPs] but no joint space narrowing [JSN]. At a subregional level [13], the largest differences in cartilage thickness between RKOA and healthy reference participants were reported in the external aspect of the weight-bearing medial femoral condyle (ecMF) in both studies [10, 12]. However, the RKOA participants had a substantially greater body mass index (BMI) than the healthy participants [10, 12] so that the greater cartilage thickness may be explained by “adaptation” rather than disease. A longitudinal study in participants with anterior cruciate ligament injury (a risk factor of early RKOA) [14] found that within one year of the injury there was a significant increase in cartilage thickness in the medial weight-bearing femur (cMF), a significant reduction of cartilage thickness in the femoral trochlea, and no significant changes in other knee compartments. Further, a recent two-year longitudinal study of RKOA participants [15] reported that knees with early RKOA had a high likelihood of cartilage thickening in at least one medial femorotibial subregion, compared with changes observed in healthy knees; ecMF was the most commonly involved subregion medially, and the external weight-bearing lateral femoral subregion (ecLF) was the most commonly involved subregion laterally [15]. However, verification of significant cartilage thickening in longitudinal studies is challenging, given the precision (test-retest) errors involved in the measurement and the small changes observed over relatively short periods for time.
Within-person between-knee analyses have been shown to be very effective in removing between-person confounding from cross-sectional and longitudinal comparisons in RKOA [16, 17]. If the specific characteristic in question that differentiates both knees is rare, a between-knee, within-person comparison relies on large sample sizes for selecting the participants that display the specific between-knee differences of interest. Participants with marginal osteophytes and no JSN on radiograph in one knee, and with neither osteophytes nor JSN in the other knee, were selected from 4796 OAI subjects, to test the following hypotheses, based on the above study design:
Cartilage thickness in ecMF and ecLF is significantly greater in knees with definite OPs and no JSN than in contra-lateral knees without signs in RKOA,
The location of cartilage thickness increases (ecMF or ecLF) is related to the (medial or lateral) location of the OPs.
Based on previous reports [10, 12, 15], ecMF and ecLF were selected as primary endpoints, whereas other cartilage subregions were examined for exploratory purposes.
Methods
Osteoarthritis Initiative, radiographic grading and specific sample selection
The participants were selected from the Osteoarthritis Initiative (OAI) data base applying a between knee, within-person study design (http://www.oai.ucsf.edu/datarelease/; public use data sets 0.E.1 [imaging] and 0.2.2 [clinical]). The OAI includes 4798 participants aged 45-79 years and OAI inclusion/exclusion criteria have been published previously [18]. Fixed flexion radiographs [19, 20] (Fig. 1) and 3 Tesla MRIs [21, 22] (Fig. 2) were acquired as part of the OAI protocol, with baseline radiographic readings from the imaging sites being available for all 4796 participants. The site readers graded OPs in each knee according to the Osteoarthritis Research Society (OARSI) atlas [23], but the readings were not reported specific to compartment (medial or lateral) or bone (tibia or femur). JSN was also graded according to the OARSI atlas [23], and was reported as medial, or lateral, or bi-compartmental.
Figure 1.
Anterior-posterior fixed-flexion radiograph of a study participant. The right knee shows a grade 1 osteophyte on the lateral tibia (white arrow), but no medial or lateral joint space narrowing. In addition, there is a small osteophyte at the intercondylar notch that is not part of OARSI atlas-based grading (black arrow). The contra-lateral left knee shows neither osteophytes nor joint space narrowing.
Figure 3.
Graph showing the medial and lateral tibial, and the medial and lateral weight-bearing femoral cartilage plates. The 16 femorotibial subregions are shown. Percent differences between osteophyte versus contra-lateral non-osteophyte knees are given for each subregion. The values for the primary endpoints ecMF and ecLF are encircled and marked for significance (*). MT = medial tibia, cMF = weight-bearing medial femoral condyle, LT = lateral tibia, cLF = weight-bearing lateral femoral condyle; c= central, e=external, i=internal, a= anterior, p= posterior.
Figure 2.
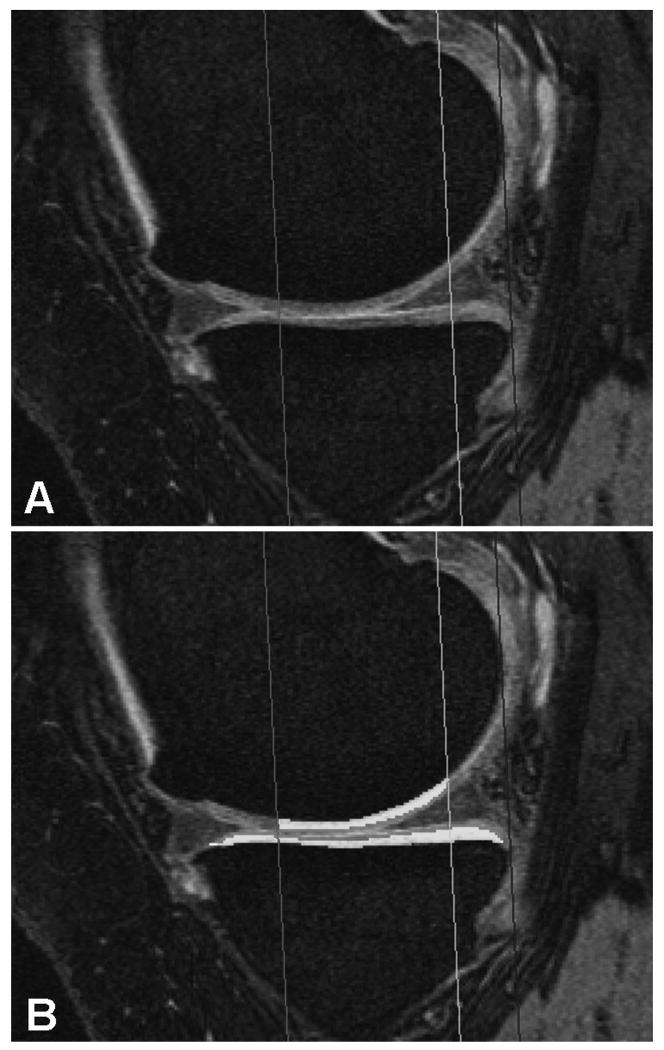
Sagittal double echo steady state (DESS) MR images with water excitation. Image A shows a MR image of the medial femorotibial compartment without and image B with segmentation of the cartilage in the defined region of interest.
From the clinical public-use data set 0.2.2 we selected participants that fulfilled the following criteria: definite OPs (OP grade > 0) in one knee, no definite or possible OPs in the contralateral knee (OP grade = 0), and no JSN in either knee (Fig. 1). Knees with JSN and without OPs were not included. The radiographs of these 84 cases were then centrally reviewed by an expert musculoskeletal radiologist reader (F.R.). The above radiographic inclusion criteria were confirmed in 61 of the 84 participants who were thus included in the current study. Additionally, the location (medial, lateral; tibial, femoral) and grade (1 to 3 [23]) of the OPs were recorded.
Image analysis of quantitative endpoints
The fixed flexion radiographs were used to quantitatively measure the minimum joint space width (mJSW) and JSW at fixed anatomical locations in the medial femorotibial compartment, as described previously [24, 25]. Baseline, double oblique, sagittal double echo steady state MR images with water excitation (DESSwe; Fig. 2) [21, 22, 26, 27] were obtained from the OAI coordinating center and processed at the image analysis center (Chondrometrics GmbH, Ainring, Germany). After initial image quality control (QC: S.C.), segmentation of paired left and right baseline images was performed by seven operators (Fig. 2), each with formal training and more than three years experience in cartilage segmentation. The images of the contra-lateral knee were uploaded, mirrored, and displayed during segmentation of the other knee, but the operators were blinded to the radiographic status of the knees. Segmentation of the femorotibial cartilage plates was performed as described previously [18, 26], with a 75% region of interest (trochlear notch to posterior end of femoral condyles) being used to separate the weight-bearing from the posterior portion of the femoral condyles [27] (Fig. 2). All segmentations were quality controlled (S.C.) and were corrected by the operators, if found necessary. The mean cartilage thickness (ThCtAB.Me) over the total area of subchondral bone (tAB) was then determined in the 4 femorotibial cartilage plates and in 16 subregions, as described previously [13, 28-30] (Fig. 1).
Univariate statistical analysis
Differences in JSW and in ThCtAB (in μm) were computed between (paired) OP knees versus non-OP knees. Descriptive summaries were obtained for a) all knee pairs, b) pairs with only medial (but not lateral) OPs, c) pairs with only lateral (but not medial) OPs, and d) pairs with medial and lateral (bi-compartmental) OPs. Differences between knee pairs were also expressed in percent (%), by relating side-differences to the mean values of the non-OP knees.
A two-sided paired t-test was applied to identify whether values for JSW and ThCtAB in ecMF and ecLF were significantly different (both effect possible: thickening and thinning) in OP than in contra-lateral non-OP knees. Given that tests were applied for each of the two external femoral subregions, a p-value of 0.025 was required to indicate significance. No further correction for multiple testing was applied, as analysis for all other plates and subregions was considered exploratory.
Multivariate statistical analysis (mixed effect models)
To confirm and further explore differences noted by the above tests, mixed effects models were constructed to examine cartilage thickness in OP vs. non-OP knees. Participants were treated as “random effects”, with different variances allowed for each sex. Three models were constructed which considered differences in cartilage thickness between knees with and without OPs for medial and lateral subregions simultaneously (e.g. ecMF OR ecLF = ecXF), in order to take advantage of the expected similarity in behavior (cartilage thickness increase in OP versus non-OP knees) and to maximize the number of OPs. A “compartment” covariate was included to distinguish between the medial and lateral femorotibial compartment. The intercept of the models was used to estimate the overall average difference in thickness between OP and non-OP knees. Model 1 only included “compartment” as a covariate to estimate the differences between effects in the medial vs. lateral compartment without further considering OP location. Model 2 explored whether an OP was found in the same compartment as the effect (e.g., medial OP for increases of cartilage thickness in ecMF and lateral OP for increases of cartilage thickness in ecLF.) The interaction term represents the difference between ecLF and ecMF in the effect of the presence of OP, i.e [OP(med) – NoOP(med)] – [OP(lat) – NoOP(lat)]. Finally, model 3 was used to explore whether OP grades at the tibial or femoral location had an impact on the side differences. Given small groups and large number of potential combinations, model 3 was confined to studying the primary endpoints.
Results
Demographics
The 61 participants selected based on the inclusion criteria including 32 women and 29 men, (age 60.8±9.6 yrs; BMI 27.8±4.7 kg/m2). In 32 of the 61 participants the right knee carried the OP, and in 29 participants the left. The specific locations and grades of the OPs are listed in Table 1 In brief 29 OP knees displayed only medial, 22 only lateral, and 10 bi-compartmental OPs: 49 knees displayed a single OP, 11 two OPs (in different locations), and 1 three OPs. Of these 74 OPs, 57 were grade 1 and 17 grade 2; 62 were tibial OPs and 12 femoral OPs (Table 1).
Table 1.
Frequency and location of the osteophytes (OP) stratified by 1 grades (1, 2) and compartment (scoring according to Altman et al. [23]).
| Medial Compartment | Lateral Compartment | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OP=1 | OP=2 | Total | OP=1 | OP=2 | Total | OP=1 | OP=2 | Total | |
| Tibia / n | 29 | 8 | 37 | 21 | 4 | 25 | 50 | 12 | 62 |
| Femur / n | 3 | 2 | 5 | 4 | 3 | 7 | 7 | 5 | 12 |
| Total / n | 32 | 10 | 42 | 25 | 7 | 32 | 57 | 17 | 74 |
Univariate comparison for primary endpoints (ecMF and ecLF)
When comparing cartilage thickness between both knees of all participants (paired t-test) OP knees displayed significantly greater ThCtAB.Me than contra-lateral non-OP knees in ecMF (+71±22μm, equivalent to an increase of +5.5%; p=0.015) and in ecLF (+64±20μm; +4.1%, p=0.013). When stratifying the results for OP grades, knees with OP grade 1 (n=45) displayed significantly thicker cartilage in ecMF (+96±239μm, +7.6%, p=0.01) and in ecLF (+63±184μm, +4.1%, p=0.027). In the few knees with OP grade 2 (n=16), a similar result was observed for ecLF (+68±230μm, +4.0%), albeit this did not reach statistical significance due to the smaller sample size (p=0.257). In contrast, cartilage thickness in ecMF did not appear to be greater in grade 2 OP knees than in contralateral non-OP knees (+3±152μm, +0.2%, p=0.943).
When restricting the analysis to knees with only tibial but no femoral OPs (n=49), the results were similar to those in the total cohort (ecMF: +5.0%, p=0.008; ecLF: +3.6%, p=0.03). At an observational level, knees with only medial OPs (n=29) displayed greater thickness differences between OP versus non-OP knees for ecMF (+4.8%) than for ecLF (-0.1%), and those with only lateral OPs (n=22) greater thickness differences for ecLF (+5.0%) than for ecMF (+1.7%). Knees with bi-compartmental OPs (n=10) displayed similar (and strong) thickening in ecMF and ecLF (+17%/+15%, respectively).
Univariate comparison for exploratory endpoints (other MRI regions and radiography)
No other femorotibial subregion displayed mean cartilage thickness differences between OP and contra-lateral non-OP knees of similar magnitude as ecMF and ecLF, or significant differences at p<0.025 for exploratory two-sided paired t-tests (Table 2). Amongst total cartilage plates, cMF and cLF displayed significantly greater cartilage thickness (+2.5%) in OP versus non-OP knees, but the difference only reached statistical significance in cLF (Table 2). Small (and non-significant) side differences were noted in radiographic measures of JSW, with mJSW rather displaying a trend towards a reduction (-2.6%) than an increase in OP vs. non-OP knees (Table 2).
Table 2. Difference in exploratory endpoints of subregional cartilage thickness (ThCtAB:me), total plate cartilage thickness, and radiographic joint space width (JSW) between knees with osteophytes (and without joint space narrowing) and contra-lateral knees without osteophytes or joint space narrowing.
(Note that results for the primary endpoints [ecMF and ecLF] are summarized in the text; + means thicker cartilage in osteophyte than in contralateral non-osteophyte knees, - mean thinner cartilage in osteophyte than in contralateral non-osteophyte knees.)
| Difference Mean (µm) | SD(mm) | Mean (%) | t-test paired | |
|---|---|---|---|---|
| Femorotibial subregion cartilage thickness (ThCtAB.Me) | ||||
| cMT | -9 | 242 | -0.4 | 0.78 |
| eMT | -16 | 189 | -1.1 | 0.52 |
| iMT | -8 | 337 | -0.4 | 0.86 |
| aMT | -5 | 144 | -0.4 | 0.77 |
| pMT | 20 | 145 | 1.5 | 0.29 |
|
| ||||
| ccMF | 37 | 284 | 1.7 | 0.31 |
| icMF | 38 | 313 | 1.8 | 0.35 |
|
| ||||
| cLT | 5 | 287 | 0.1 | 0.90 |
| eLT | 7 | 171 | 0.4 | 0.75 |
| iLT | -31 | 290 | -1.4 | 0.41 |
| aLT | 33 | 140 | 2.0 | 0.07 |
| pLT | 45 | 196 | 2.6 | 0.08 |
|
| ||||
| ccLF | 35 | 212 | 1.5 | 0.21 |
| icLF | 57 | 263 | 3.1 | 0.10 |
|
| ||||
| Femorotibial total plate cartilage thickness (ThCtAB.Me) | ||||
| MT | -1 | 134 | -0.1 | 0.93 |
| cMF | 45 | 215 | 2.5 | 0.11 |
| LT | 14 | 158 | 0.7 | 0.50 |
| cLF | 46 | 164 | 2.5 | 0.03 |
|
| ||||
| Radiographic joint space width (JSW) | ||||
| mJSW(m) | -125 | 651 | -2.6 | 0.14 |
| JSW(x=0.150) | -76 | 544 | -1.5 | 0.28 |
| JSW(x=0.175) | -46 | 590 | -0.9 | 0.54 |
| JSW(x=0.200) | -16 | 585 | -0.3 | 0.83 |
| JSW(x=0.225) | 3 | 610 | 0.1 | 0.97 |
| JSW(x=0.250) | 36 | 621 | 0.6 | 0.66 |
| JSW(x=0.275) | 24 | 682 | 0.4 | 0.79 |
| JSW(x=0.300) | 54 | 746 | 0.7 | 0.58 |
SD = standard deviation, JSW = joint space width, mJSW = minimum JSW; (x=0.X) = specific locations of JSW measurements; MT = medial tibia, cMF = weight-bearing medial femoral condyle, LT = lateral tibia, cLF = weight-bearing lateral femoral condyle; c= central, e=external, i=internal, a= anterior, p= posterior. A two-sided paired t-test was applied, without correcting for multiple comparisons for exploratory endpoints.
Multivariate comparison
All mixed effects models initially included age, sex and BMI, but these covariates were not significant (p-values >0.1) and were thus removed from the models.
Model 1 found the difference (i.e. the intercept of the model) between OP and non-OP knees averaged over external femoral subregions (ecXF) to be 68 μm (standard error=21μm), with the difference being significantly different from zero (p=0.002). There was no significant difference in this effect in the medial vs. lateral compartment (p=0.87). Other exploratory (combined medial and lateral) femorotibial subregions exhibited average differences between -20μm and +46μm (p=0.04 to 0.93), and there was no significant differences in the effect between compartments (p≥0.28)
Model 2 showed that the difference in ThCtAB.Me between OP vs. non-OP knees in ecXF was 104μm greater when the OP was located in the same rather than in the contralateral compartment (p=0.003). This effect appeared to be greater in the lateral than in the medial compartment, but the difference between compartments was not significant (p=0.40). No significant relationships were observed for other femorotibial subregions.
Model 3 showed that when an OP was present in the tibia the difference in ThCtAB.Me of ecXF between OP vs. non-OP knees was 0.116mm (p= 0.006). Noteworthy differences were observed in the effect observed in ecMF vs. ecLF with regard to the location (tibial vs. femoral) of the OP. No increased ThCtAB.Me was observed in ecLF when the OP was present only in the medial tibia and not laterally. In contrast, a greater ThCtAB.Me in ecMF was observed when the OP was present in medial or lateral tibia, regardless of the compartment. A trend towards a reduced cartilage thickness (in OP vs. non-OP knees), however, was observed in ecMF when the OP was located in the lateral femur.
Discussion
This study provides robust evidence that increases in cartilage thickness (swelling or hypertrophy) can occur in early human RKOA. A within-person, between-knee study design was employed to specifically test the hypothesis that cartilage thickness in the external aspects of the weight-bearing femoral condyles (ecMF and ecLF) is significantly greater in knees with definite OPs (and no JSN) than in contra-lateral knees without RKOA. Our observations confirm that significantly greater cartilage thickness is observed in these two subregions in OP knees, also when the analysis is confined to participants with only tibial (but not femoral) OPs. However, the effect appears to be limited to these two (out of 20) subregions and no significant differences were observed in fixed flexion radiographs. OP versus non-OP side-differences in cartilage thickness were greater in ecMF than in ecLF when knees displayed medial OPs, and greater in ecLF than ecMF when knees displayed lateral OPs. Albeit the differences were small, the findings are statistically significant and confirm previous cross sectional [10, 12] and longitudinal findings [14] in other subsamples and cohorts using univariate and multivariate statistical approaches.
A limitation of the study is the small number of knees, particularly those with femoral OPs. However, the participants were carefully selected from 4796 OAI participants, in order to support a paired-knee comparison that was intended to remove between-person confounding from covariates such as age, sex, BMI, genetics, physical activity, and others. Additionally, the study design excluded differences due to natural variation within a day, as both knees were always examined in the same session, and minimized potential differences of loading effects (prior to imaging) on the cartilage of both knees. This provides a considerable strength to the study but involves compromises in sample size, because only a small percentage of participants fulfilled the specific inclusion criteria in both knees. Another advantage of the between-knee, within-person comparison is that the variability in normal (physiological) cartilage thickness between left and right knees [31] is considerably less than that between subjects [11], and that therefore side-differences are easier to detect than inter-subject differences in cartilage thickness.
MRI was more sensitive in detecting side-differences in cartilage thickness between OP vs. contralateral non-OP knees than radiography. This is likely due to “thickening” being confined to the external femoral subregions, in which the cartilage can be directly visualized and quantified by MRI [13]. Radiographic JSW, in contrast, may rather be determined by the cartilage thickness in the central aspects of the joint, where both layers are in direct contact. Further, radiographic JSW is known to be not only associated with cartilage thickness, but also with meniscus extrusion [32, 33]. Also of note is that MRI is performed under non-weight-bearing conditions, under which cartilage “thickening” may be easier to detect than by weight-bearing radiography. When quantitative analysis of the cartilage is performed in knees with OPs, new cartilage associated with OP formation is excluded from the segmentation [34]. Given that ecMF and ecLF are the locations where marginal femoral OPs occur preferentially, and that the thickening appeared to be greater in the compartments where OPs occurred, one may suspect that the current findings reflect insufficient elimination of local (thickened) cartilage overlying OPs, given that osteophytes are osteo-cartilaginous formations. However, only few (<20%) of the knees displayed femoral OPs, and the analysis confirmed statistically significant thickening in ecMF and ecLF (but not in eMT and eLT) in the knees with only tibial (but not femoral) OPs (+5.0% in ecMF and +3.6% in ecLF). These findings support that cartilage thickening is not an artifact resulting from insufficient elimination of OP-associated cartilage during the segmentation process.
Potentially, the increase in cartilage thickness in the external subregions of the femur reflects an adaptive response to loss of meniscus function due to increased extrusion in OP knees, as suggested by the reduction in JSW in OP knees. However, this hypothesis needs to be tested in future studies that determine meniscus status in these knees by radiological scoring or quantitative measurement [35, 36]. The mixed effect models suggest that thickening in ecLF strongly depended on presence of an OP in the same compartment. Thickening in ecMF, in contrast, appeared to depend on presence of a medial or lateral tibial OP, and ecMF cartilage appeared to be thinner when a lateral femoral OP was present. Given the small sample size, particularly in participants with certain combinations of OPs across the four possible locations, the latter finding was not statistically significant and must hence be interpreted with caution. However, taken together and relying on the more robust compartment-specific models, there appears to be evidence that cartilage thickening in ecMF and ecLF may be locally mediated. This may, occur by increased mechanical stress affecting the opposite cartilage surface and/or may reflect an adaptive response to loss of meniscus function due to increased extrusion in OP knees, as mentioned above.
Whether cartilage thickening in human OA is caused by tissue hypertrophy (increase matrix production due to metabolic stimulation of cells by mechanical irritation) or tissue swelling (increased water content potentially originating from collagen cleavage) currently is unclear. In a surgical animal model of knee OA cartilage was shown to display a higher water content and higher transverse relaxation time (T2), with T2 and the water content being directly correlated [37]. T2 also was shown to be related to the water content of human cartilage [38] and to be increased in OA versus healthy cartilage [39-41]. Taken together, these studies indicate that higher water content may be responsible for cartilage swelling in human OA and future studies involving T2 analysis of articular cartilage may thus be focused on studying particularly the external aspects of the femoral condyles between OA and healthy knees. However, other animal models have observed tissue hypertrophy in early OA [5, 6], and the possibility that cartilage thickening in ecMF and ecLF may reflect an adaptive response to loss in meniscus function should not be excluded.
There is concern that inclusion of participants with cartilage thickening in clinical studies, which test the effect of disease modifying drugs (DMOADS), may dilute observations of cartilage loss occurring in other participants. This may increase the variability of longitudinal measurements and may make it challenging to demonstrate a drug effect on cartilage loss versus placebo in a reasonably sized cohort. Based on the current findings, certain approaches can be taken to circumvent these concerns: When choosing a primary endpoint, one may select a central subregion of the femur, tibia or central femorotibial compartment [28, 42, 43] rather than choosing a total cartilage plate such as the weight-bearing medial femur, as these are locations where no sign of cartilage thickening was observed in the current study. Alternatively, an approach could be chosen that does not focus at changes in specific (sub-)regions in each participants, but uses the region displaying the greatest longitudinal reduction in cartilage thickness in each knee and averages the observed difference across these ordered values (OV) [44, 45]. Using this approach, regions with cartilage thickening are excluded from the analysis, if the smallest OV is chosen as primary endpoint. If, in contrast, the effect of a drug on cartilage thickening (e.g. swelling or hypertrophy) is to be studied, then the above approach can be used by choosing the highest OV (the femorotibial subregion with the greatest longitudinal increase in cartilage thickness) as the primary endpoint. Finally, a study population can be enriched by participants who not only exhibit OPs but also JSN grades 1 or higher, since cartilage loss has been shown to be stronger in JSN versus contra-lateral no-JSN knees, and in participants with JSN (KLG3 or 4) versus those with no-JSN (KLG2) [45-47]. Also, a previous longitudinal study observed a much smaller proportion of participants with significant longitudinal cartilage thickening in JSN (KLG3) knees than in knees with OPs but without JSN (KLG2) [15].
In conclusion, we found knees with early radiographic OA had significantly thicker cartilage than contra-lateral knees without OPs. These differences appeared to be specific to external femoral subregions and to be associated with (tibial) osteophytes in the same compartment. In order to circumvent increased variability of longitudinal cartilage thickness changes in longitudinal trials in RKOA participants, it is suggested to use central (rather than external) femoral or tibial subregions as primary endpoints.
Significance and Innovation.
First compelling evidence that, in certain subregions of the femorotibial joint (external medial and lateral weight-bearing femur), articular cartilage undergoes thickening (swelling or hypertrophy) in early radiographic human OA, when analyzed in a within-person, between-knee cross-sectional comparison.
Acknowledgments
We would like to thank the following operators: Gudrun Goldmann, Linda Jakobi, Manuela Kunz, Dr. Susanne Maschek, Jana Matthes, Sabine Mühlsimer, Annette Thebis, and Dr. Barbara Wehr for dedicated data segmentation. We also would like to thank the OAI investigators and technicians and the staff of the OAI Coordinating Center for their working in generating the clinical data and images of this study and for making them publicly available. The MR image analysis component of this study was funded by a grant from the Paracelsus Medical University (PMU) Forschungsförderungsfond (FFF). The quantitative analysis of the fixed flexion radiographs and the statistical analysis was supported by a grant from MerckSerono SA with special thanks to Jeff Duryea.
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Financial support: The study and image acquisition was funded by the Osteoarthritis Initiative, a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262). The MR image analysis component of this study was funded by a grant from the Paracelsus Medical University (PMU) Forschungsförderungsfond (FFF). The quantitative analysis of the fixed flexion radiographs and the statistical analysis was supported by a grant from MerckSerono SA.
Footnotes
Competing interest disclosure: Felix Eckstein is CEO and co-owner of Chondrometrics GmbH, a company providing quantitative MR image analysis services. He provides consulting services to MerckSerono, Novartis, and SanofiAventis. Frank Roemer is co-owner of the Boston Imaging Core Lab (BICL), a company providing MR image reading service. Sebastian Cotofana and Wolfgang Wirth have part time appointments with Chondrometrics GmbH; Wolfgang Wirth is co-owner of Chondrometrics GmbH.
Reference List
- 1.Watson PJ, Carpenter TA, Hall LD, et al. Cartilage swelling and loss in a spontaneous model of osteoarthritis visualized by magnetic resonance imaging. Osteoarthritis Cartilage. 1996;4:197–207. doi: 10.1016/s1063-4584(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 2.Calvo E, Palacios I, Delgado E, et al. Histopathological correlation of cartilage swelling detected by magnetic resonance imaging in early experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12:878–86. doi: 10.1016/j.joca.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Calvo E, Palacios I, Delgado E, et al. High-resolution MRI detects cartilage swelling at the early stages of experimental osteoarthritis. Osteoarthritis Cartilage. 2001;9:463–72. doi: 10.1053/joca.2001.0413. [DOI] [PubMed] [Google Scholar]
- 4.Tessier JJ, Bowyer J, Brownrigg NJ, et al. Characterisation of the guinea pig model of osteoarthritis by in vivo three-dimensional magnetic resonance imaging. Osteoarthritis Cartilage. 2003;11:845–53. doi: 10.1016/s1063-4584(03)00162-6. [DOI] [PubMed] [Google Scholar]
- 5.Vignon E, Arlot M, Hartmann D, et al. Hypertrophic repair of articular cartilage in experimental osteoarthrosis. Ann Rheum Dis. 1983;42:82–8. doi: 10.1136/ard.42.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams ME, Brandt KD. Hypertrophic repair of canine articular cartilage in osteoarthritis after anterior cruciate ligament transection. J Rheumatol. 1991;18:428–35. [PubMed] [Google Scholar]
- 7.Cotofana S, Eckstein F, Wirth W, et al. In vivo measures of cartilage deformation: patterns in healthy and osteoarthritic female knees using 3T MR imaging. Eur Radiol. 2011;21:1127–35. doi: 10.1007/s00330-011-2057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudelmaier M, Glaser C, Hohe J, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001;44:2556–61. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Burgkart R, Glaser C, Hyhlik-Durr A, et al. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum. 2001;44:2072–7. doi: 10.1002/1529-0131(200109)44:9<2072::AID-ART357>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Frobell RB, Nevitt MC, Hudelmaier M, et al. Femorotibial subchondral bone area and regional cartilage thickness: a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1,003 knees from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010;62:1612–23. doi: 10.1002/acr.20262. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein F, Yang M, Guermazi A, et al. Reference values and Z-scores for subregional femorotibial cartilage thickness--results from a large population-based sample (Framingham) and comparison with the non-exposed Osteoarthritis Initiative reference cohort. Osteoarthritis Cartilage. 2010;18:1275–83. doi: 10.1016/j.joca.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellio Le Graverand MP, Buck RJ, Wyman BT, et al. Subregional femorotibial cartilage morphology in women - comparison between healthy controls and participants with different grades of radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2009 doi: 10.1016/j.joca.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–44. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 14.Frobell RB, Le Graverand MP, Buck R, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17:161–7. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Buck RJ, Wyman BT, Le Graverand MP, et al. An efficient subset of morphological measures for articular cartilage in the healthy and diseased human knee. Magn Reson Med. 2010;63:680–90. doi: 10.1002/mrm.22207. [DOI] [PubMed] [Google Scholar]
- 16.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckstein F, Wirth W, Hunter DJ, et al. Magnitude and regional distribution of cartilage loss associated with grades of joint space narrowing in radiographic osteoarthritis--data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2010;18:760–8. doi: 10.1016/j.joca.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckstein F, Maschek S, Wirth W, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68:674–9. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterfy C, Li J, Zaim S, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 20.Nevitt MC, Peterfy C, Guermazi A, et al. Longitudinal performance evaluation and validation of fixed-flexion radiography of the knee for detection of joint space loss. Arthritis Rheum. 2007;56:1512–20. doi: 10.1002/art.22557. [DOI] [PubMed] [Google Scholar]
- 21.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider E, NessAiver M, White D, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16:994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Duryea J, Li J, Peterfy CG, et al. Trainable rule-based algorithm for the measurement of joint space width in digital radiographic images of the knee. Med Phys. 2000;27:580–91. doi: 10.1118/1.598897. [DOI] [PubMed] [Google Scholar]
- 25.Neumann G, Hunter D, Nevitt M, et al. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis Cartilage. 2009;17:761–5. doi: 10.1016/j.joca.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckstein F, Hudelmaier M, Wirth W, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–41. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirth W, Nevitt M, Hellio Le Graverand MP, et al. Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols--comparative data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2010;18:547–54. doi: 10.1016/j.joca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirth W, Hellio Le Graverand MP, Wyman BT, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–7. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckstein F, Wirth W, Hudelmaier M, et al. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59:1563–70. doi: 10.1002/art.24208. [DOI] [PubMed] [Google Scholar]
- 30.Eckstein F, Guermazi A, Roemer FW. Quantitative MR imaging of cartilage and trabecular bone in osteoarthritis. Radiol Clin North Am. 2009;47:655–73. doi: 10.1016/j.rcl.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Eckstein F, Muller S, Faber SC, et al. Side differences of knee joint cartilage volume, thickness, and surface area, and correlation with lower limb dominance-an MRI-based study. Osteoarthritis Cartilage. 2002;10:914–21. doi: 10.1053/joca.2002.0843. [DOI] [PubMed] [Google Scholar]
- 32.Adams JG, McAlindon T, Dimasi M, et al. Contribution of meniscal extrusion and cartilage loss to joint space narrowing in osteoarthritis. Clin Radiol. 1999;54:502–6. doi: 10.1016/s0009-9260(99)90846-2. [DOI] [PubMed] [Google Scholar]
- 33.Gale DR, Chaisson CE, Totterman SM, et al. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–32. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 34.Eckstein F, Ateshian G, Burgkart R, et al. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthritis Cartilage. 2006;14:974–83. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Wirth W, Frobell RB, Souza RB, et al. A three-dimensional quantitative method to measure meniscus shape, position, and signal intensity using MR images: a pilot study and preliminary results in knee osteoarthritis. Magn Reson Med. 2010;63:1162–71. doi: 10.1002/mrm.22380. [DOI] [PubMed] [Google Scholar]
- 37.Chou MC, Tsai PH, Huang GS, et al. Correlation between the MR T2 value at 4.7 T and relative water content in articular cartilage in experimental osteoarthritis induced by ACL transection. Osteoarthritis Cartilage. 2009;17:441–7. doi: 10.1016/j.joca.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Lusse S, Claassen H, Gehrke T, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000;18:423–30. doi: 10.1016/s0730-725x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 39.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259–66. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 40.Yao W, Qu N, Lu Z, et al. The application of T1 and T2 relaxation time and magnetization transfer ratios to the early diagnosis of patellar cartilage osteoarthritis. Skeletal Radiol. 2009;38:1055–62. doi: 10.1007/s00256-009-0769-8. [DOI] [PubMed] [Google Scholar]
- 41.Stahl R, Blumenkrantz G, Carballido-Gamio J, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage. 2007;15:1225–34. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Eckstein F, Wirth W, Hudelmaier MI, et al. Relationship of compartment-specific structural knee status at baseline with change in cartilage morphology: a prospective observational study using data from the osteoarthritis initiative. Arthritis Res Ther. 2009;11:R90. doi: 10.1186/ar2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunter DJ, Bowes MA, Eaton CB, et al. Can cartilage loss be detected in knee osteoarthritis (OA) patients with 3-6 months' observation using advanced image analysis of 3T MRI? Osteoarthritis Cartilage. 2010;18:677–83. doi: 10.1016/j.joca.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buck RJ, Wyman BT, Le Graverand MP, et al. Does the use of ordered values of subregional change in cartilage thickness improve the detection of disease progression in longitudinal studies of osteoarthritis? Arthritis Rheum. 2009;61:917–24. doi: 10.1002/art.24613. [DOI] [PubMed] [Google Scholar]
- 45.Wirth W, Buck R, Nevitt M, et al. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography--data from the OA initiative. Osteoarthritis Cartilage. 2011;19:689–99. doi: 10.1016/j.joca.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Graverand MP, Buck RJ, Wyman BT, et al. Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls: a multicentre study using 3.0 Tesla MRI and Lyon-Schuss radiography. Ann Rheum Dis. 2010;69:155–62. doi: 10.1136/ard.2008.099762. [DOI] [PubMed] [Google Scholar]
- 47.Eckstein F, Nevitt M, Gimona A, et al. Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end-stage radiographic osteoarthritis: results from 831 participants from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2011;63:311–9. doi: 10.1002/acr.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]