Abstract
Schimke immuno-osseous dysplasia (SIOD) is a multisystemic disorder with prominent skeletal, renal, immunological, and ectodermal abnormalities. It is caused by mutations of SMARCAL1 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a-like 1), which encodes a DNA stress response protein. To determine the relationship of this function to the SIOD phenotype, we profiled the cancer prevalence in SIOD and assessed if defects of nucleotide excision repair (NER) and of nonhomologous end joining (NHEJ), respectively, explained the ectodermal and immunological features of SIOD. Finally, we determined if Smarcal1del/del mice had hypersensitivity to irinotecan (CPT-11), etoposide and hydroxyurea (HU) and whether exposure to these agents induced features of SIOD. Among 71 SIOD patients, three had non-Hodgkin lymphoma (NHL) and one had osteosarcoma. We did not find evidence of defective NER or NHEJ; however, Smarcal1-deficient mice were hypersensitive to several genotoxic agents. Also, CPT-11, etoposide and HU caused decreased growth and loss of growth plate chondrocytes. These data, which identify an increased prevalence of NHL in SIOD and confirm hypersensitivity to DNA damaging agents in vivo, provide guidance for the management of SIOD patients.
Keywords: Schimke immuno-osseous dysplasia, non-Hodgkin lymphoma, T-cell immunodeficiency, genotoxic agents
INTRODUCTION
Schimke immuno-osseous dysplasia (SIOD) is an autosomal recessive multisystem disorder characterized by the prominent features of dysmorphic facies, growth failure from spondyloepiphyseal dysplasia, renal failure from focal segmental glomerulosclerosis (FSGS), and T-cell immunodeficiency [Schimke et al., 1971; Spranger et al., 1991]. Additional less prominent features include hyperpigmented macules, sparse hair, migraine-like headaches, atherosclerosis with cerebral ischemia, deficiency of other blood cell lineages, hypothyroidism, opportunistic infections, dental anomalies, corneal opacities, autoimmune enteropathy, and pulmonary hypertension [Boerkoel et al., 2000; da Fonseca 2000; Kilic et al., 2005; Spranger et al., 1991]. The mean age of death among SIOD patients is 10.8 years (range: 3-28 years).
SIOD is caused by biallelic mutations of SMARCAL1, which encodes an enzyme with annealing helicase activity; this enzyme participates in the DNA stress response to genotoxic agents such as hydroxyurea, camptothecin and aphidicolin and in reactivation of stalled replication forks [Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai and Kadonaga 2008; Yusufzai et al., 2009]. To test the potential contribution of defective DNA repair to the phenotype of SIOD, we compared SIOD to other disorders of DNA repair, assessed cancer prevalence, tested for NER and NHEJ defects and measured the sensitivity of Smarcal1-deficient mice to genotoxic agents. We find that SIOD patients have a high frequency of non-Hodgkin lymphoma (NHL) and that Smarcal1-deficient mice are hypersensitive to several genotoxic agents.
MATERIALS AND METHODS
Human subjects
Patients referred to this study gave informed consent. The study was approved by the Institutional Review Boards of Baylor College of Medicine (Houston, TX USA), the Hospital for Sick Children (Toronto, ON, Canada), and the University of British Columbia (Vancouver, BC Canada). Clinical data were obtained from questionnaires completed by the attending physician as well as medical summaries.
Animal subjects
Mice used in this study were housed, bred, and euthanized in accordance with accepted ethical guidelines. These procedures were approved by the Institutional Review Board of Baylor College of Medicine (Houston, TX, USA, IRB protocol: AN-2983) or the University of British Columbia (Vancouver, BC, Canada, Animal Care Certificate: A10-0296).
Immunoglobulin switch junction amplification and sequencing
Genomic DNA was extracted from peripheral blood cells of 7 SIOD patients and 4 unaffected controls. Amplification, sequencing and analysis of the switch Sμ-Sα1 and Sμ-Sγ1 junctions were performed as previously described with minor modifications [Pan-Hammarstrom et al., 2005]. In brief, nested polymerase chain reaction (PCR) strategy was used to amplify Sμ-Sα1 and Sμ-Sγ1 junctions. In the first round of PCR reactions, 50 ng or 150 ng of genomic DNA from each individual was amplified in 8 parallel reactions using either Sμ1 and Sα-common-1 primers for a total of 35 cycles of 94°C for 1 minute, 60°C for 1 minute, and 72°C for 90 seconds, or Sμ1 and Sγ-common primers for a total of 35 cycles of 94°C for 1 minute, 66°C for 1 minute, and 72°C for 30 seconds, respectively. In order to amplify Sμ-Sα1 junctions, 1 μl of each Sμ1-Sα-common-1 reaction was amplified using Sμ5 and Sα1-specific primers in reactions consisting of 35 cycles of 94°C for 1 minute, 65°C for 1 minute, and 72°C for 1 minute. Also, in order to amplify Sμ-Sγ1 junctions, 3 μl of each Sμ1-Sγ-common reaction was amplified using Sμ5 and Sγ1-specific primers in reactions consisting of 40 cycles of 94°C for 1 minute, 66°C for 1 minute, and 72°C for 1 minute. PCR products from the second round of reactions were gel purified with QIAEX II Gel Extraction Kit (Qiagen, Toronto, ON, Canada) and sequenced using the Sμ5, Sα1-specific, and Sγ1-specific primers. The switch fragment sequences were aligned with Sμ (X54713) /Sα1 (L19121) or Sμ /Sγ1 (U39737) reference sequences and microhomology at each switch junction was defined as the longest region of identity with both the Sμ and Sα1 or Sμ and Sγ1 reference sequences. The primers are listed in Supplementary eTable S1 (See Supporting Information online).
Smarcal1del/del mice and treatment with CPT-11, etoposide, and HU
The Smarcal1del/del mice have a deletion of the first two coding exons of Smarcal1 (NM_018817.2:c.172_989del), which includes the replication protein A (RPA) binding site, nuclear localization signal and the first HARP domain. They were generated and analyzed as described elsewhere [Baradaran-Heravi et al., 2012]. Smarcal1-deficient (Smarcal1del/del) and wild-type (Smarcal1+/+) male mice were divided into 4 groups (3-5 mice in each group) at the age of 4-weeks. Mice in each group were given intra-peritoneal (ip) injections of CPT-11 (40 mg/kg, Sandoz, Boucherville, QC, Canada), etoposide (20 mg/kg, Sigma, Oakville, ON, Canada), HU (100 mg/kg, Sigma, Oakville, ON, Canada), or carrier (PBS, Gibco, Burlington, ON, Canada). After 5 consecutive daily injections, the mice were followed for 3 days and then their spleens were harvested. To analyze the long-term effect of these drugs, mice were injected daily for 8 weeks. To assess the recovery of growth retardation, CPT-injected mice were observed for another 12 weeks without further injections. At the end of the treatment, radiography of mice was performed using the Faxitron X-ray cabinet. The density of the distal femur was measured using a Scanco μCT35 scanner (Scanco Medical, Bassersdorf, Switzerland) as previously described [Arama and Steller 2006].
To compare the toxicity of each drug among Smarcal1del/del and Smarcal1+/+ mice, 3-4 mice of each genotype were given daily ip injections with CPT-11 (80 mg/kg), etoposide (40 mg/kg), HU (500 mg/kg) or PBS for 5 consecutive days and followed for two weeks unless sacrificed earlier.
Urinary creatinine and albumin assays
Spot urine was collected from Smarcal1del/del and Smarcal1+/+ mice injected with PBS, CPT-11, etoposide or HU. Urinary creatinine was measured using the creatinine assay kit from Cayman Chemical Company (Ann Arbor, MI, USA) following the manufacturer’s instructions. Urinary albumin was measured using the mouse albumin ELISA kit from Immunology Consultants Laboratory (Portland, OR, USA) following the manufacturer’s instructions. Urinary albumin excretion was normalized to urinary creatinine for each sample.
Histopathology and TUNEL assay
Mouse tissues for histopathology and TUNEL assay were fixed in 4% paraformaldehyde (PFA) in PBS, processed, paraffin embedded, and cut into 5 μm sections according to standard protocols as previously described [Hirano et al., 2007]. TUNEL detection of apoptotic cells in the spleens was carried out using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (S7101, Chemicon, Billerica, MA, USA) and the tissue was counterstained with Mayer’s hematoxylin. Harris hematoxylin and eosin (H&E) staining of the femurs and Masson trichrome staining (Sigma, Oakville, ON, Canada) of the kidneys were performed following the manufacturer’s instructions. Images of the processed tissues were captured using a Zeiss Axiovert 200 microscope. Chondrocytes within the proliferative and hypertrophic zones of the distal femoral growth plates were counted as previously described [Baradaran-Heravi et al., 2012].
Cell culture
The isolation and culturing of human dermal fibroblast cell lines were performed as described by Clewing et al. [Clewing et al., 2007b]. The SIOD patient fibroblasts (N859) were derived from patient SD31, who had a homozygous deletion of the first 5 exons of SMARCAL1 (NT_005403.17:g.[67482574_67497178del]+[67482574_67497178del]), and cultured in DMEM (Gibco, Burlington, ON, Canada) containing 15% fetal bovine serum (FBS; Gibco, Burlington, ON, Canada) and 1% antibiotic/antimycotic (penicillin, streptomycin and amphotericin B; Gibco, Burlington, ON, Canada) at 37°C and 5% CO2. Other cell lines including normal human fibroblasts (C5RO) and Cockayne syndrome B fibroblasts (CS188TOR and CS163TOR) were also cultured in DMEM containing 15% FBS and 1% antibiotic/antimycotic at 37°C and 5% CO2.
Cell survival assays
Cell survival following UV or illudin S treatment was determined using a modified assay based on tritiated thymidine incorporation [Jaspers et al., 2002]. In brief, triplicate sparse cultures (5 × 103 to 1 × 104 cells/6-cm dish) of primary C5RO, N859, CS188TOR, and CS163TOR fibroblasts were grown for 48 hours and exposed to illudin S (0, 0.2, 0.4, 0.6, 0.8, and 1ng/ml) or UV (0, 5, 10, and 15 J/m2). After 3–5 days additional culture, the cells were pulse-labelled with tritiated thymidine (40–60 Ci/mmol, 5 μCi/ml) for 3 hours in presence of 20 mM HEPES pH 7.3. Following 30 minutes incubation in unlabelled medium, the cells were harvested and the incorporated radioactivity was measured in a liquid scintillation counter.
Unscheduled DNA synthesis and recovery following UV-induced transcription inhibition
Global-genome and transcription-coupled NER activities were determined by the Unscheduled DNA Synthesis (UDS) assay and recovery following UV-induced transcription inhibition as described by Jaspers et al. [Jaspers et al., 2007]. For measurement of UDS, C5RO and N859 fibroblasts were cultured on coverslips and exposed to 16 J/m2 of UV light and subsequently incubated for 3 hours in media containing 10 μCi/mL [3H-1′,2′]-thymidine (120 Ci/mmol). Cells were fixed and processed for autoradiography by dipping in Ilford K2 photographic emulsion. After 3 days slides were developed, Giemsa staining was performed and nuclear silver grains were counted in 50 non-S-phase cells. To assess the ability to recover from UV-induced inhibition of transcription, C5RO and N859 primary fibroblasts were exposed to 16 J/ m2 of UV for 16 hours, and then were cultured for 1 h in medium containing [3H-5′,6′]-uridine (40 Ci/mmol). Cells were fixed and processed for autoradiography as described above.
RESULTS
SIOD patients have features observed in other disorders of DNA repair
Comparison of SIOD patients to individuals with Bloom syndrome, Fanconi anemia, Cockayne syndrome, ataxia telangiectasia, Nijmegen breakage syndrome, and xeroderma pigmentosum showed that SIOD shares many features with these disorders including poor postnatal growth, immune defects, and premature atherosclerosis (Table I) [Boerkoel et al., 2000; Clewing et al., 2007a]. Unlike these disorders, however, SIOD is not reported to predispose to cancer although there are anecdotal reports of patients with non-Hodgkin lymphoma (NHL) [Basiratnia et al., 2011; Boerkoel et al., 2000; Taha et al., 2004]. To ascertain better the prevalence of cancer in SIOD, we reviewed the medical records of 71 SIOD patients with identified biallelic SMARCAL1 mutations. Three individuals developed B-cell non-Hodgkin lymphoma (NHL) in the first ten years of their life and two had EBV positive tumors. One individual developed osteosarcoma. No other cancers were reported for this cohort.
TABLE I.
Comparison of the prominent clinical features of SIOD to those of some DNA repair disorders [Ciccia and Elledge 2010; Friedberg et al., 2006; Kubota et al., 1993; Scriver et al., 2001].
| Clinical Feature | BS | FA | CS | AT | NBS | XP | WS | RTS | RS | SIOD |
|---|---|---|---|---|---|---|---|---|---|---|
| Growth deficiency | + | + | + | +/− | + | + | + | + | + | + |
| Radial ray defects | − | + | − | − | − | − | − | + | + | − |
| Cognitive deficits | − | + | + | − | + | + | − | − | − | − |
| Ataxia | − | − | + | + | − | +/− | − | − | − | − |
| Corneal or lens defects |
+ | + | + | − | − | + | + | + | − | + |
| Immunodeficiency | + | − | − | + | + | − | − | +/− | − | + |
| Single cytopenias | + | + | − | + | + | − | − | +/− | − | + |
| Pancytopenia | − | + | − | − | − | − | − | +/− | − | + |
| Malignancy predisposition |
+ | + | − | + | + | + | + | + | + | − |
| Photosensitivity | + | − | + | − | − | + | − | + | + | − |
| Radiosensitivity | − | +/− | − | + | + | − | − | + | NR | − |
| Abnormal pigmentation |
+ | + | + | +/− | + | + | + | + | + | + |
| Telangiectasias | + | − | − | + | − | +/− | − | + | − | − |
| Thin hair | − | + | − | − | − | + | + | − | + | |
| Premature atherosclerosis |
− | − | + | + | − | − | + | − | NR | + |
| Hypogonadism | + | + | + | + | + | + | + | + | NR | + |
| Chromosomal instability |
+ | + | − | + | + | − | + | + | NR | − |
| Poor lymphocyte mitogenic response |
+ | − | − | + | + | − | − | +/− | − | + |
| DNA repair defect | HR | ICL repair, HR |
TC- NER |
DSB repair |
DSB reapair, replication fork repair |
NER | HR, BER, telomere maintenance |
BER, HR? |
Resolution of stalled replication/ transcription intermediates |
Replication fork repair |
Abbreviations: BS, Bloom syndrome; FA, Fanconi anemia; CS, Cockayne syndrome; AT, Ataxia telangectasia; NBS, Nijmegen breakage syndrome; XP, Xeroderma pigmentosum; WS, Werner syndrome; RTS, Rothmund-Thomson syndrome; RS, RAPADILINO syndrome; +, common feature; −, uncommon feature; +/−, presented as case reports; NR, not reported; HR, Homologous recombination; ICL, interstrand crosslink; TC-NER, Transcription-coupled nucleotide excision repair; DSB, Double-strand break; BER, Base excision repair.
Ectodermal features in SIOD do not arise from a defect of NER
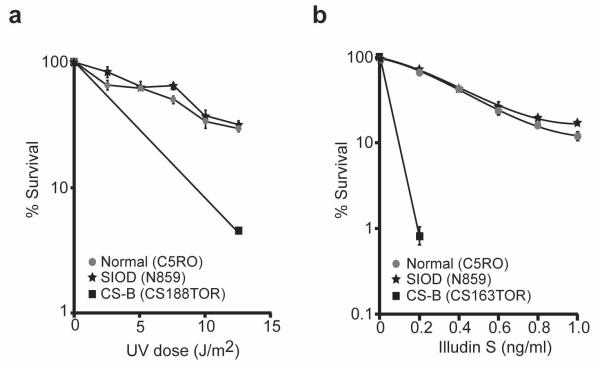
Reminiscent of disorders of NER such as xeroderma pigmentosum and Cockayne syndrome, prominent ectodermal features of SIOD include hyperpigmented macules as well as fine sparse hair. Therefore, to test if a defect of NER might contribute to SIOD, we measured hypersensitivity of dermal fibroblasts from a patient with a homozygous deletion of SMARCAL1 promoter (SD31) [Elizondo et al., 2009] to ultraviolet light (UV) and to the anti-tumor antibiotic illudin S. The latter agent causes damage that is repaired exclusively by transcription coupled (TC)-NER. In contrast to Cockayne syndrome (CS) cells, which have a defect of TC-NER [Jaspers et al., 2002], SMARCAL1-deficient fibroblasts were not hypersensitive to either UV or to illudin S treatment (Fig. 1a,b). Also, both UV-induced unscheduled DNA synthesis, a measure of global genomic (GG)-NER activity, and recovery of RNA synthesis inhibition, a measure of TC-NER activity, were unaffected in the SIOD cells (data not shown).
FIG. 1.
Analysis of SMARCAL1 participation in nucleotide excision repair. a: Survival curve comparing the sensitivity of SIOD (N859), unaffected (C5RO), and Cockayne syndrome B (CS-B, CS188TOR) skin fibroblasts to killing by UV radiation (254 nm). This is a measure of both TC-NER and global NER. b: Survival curve comparing the sensitivity of SIOD (N859), unaffected (C5RO), and CS-B (CS163TOR) skin fibroblasts to killing by illudin-S. This is a measure of TC-NER.
Immunological defects in SIOD do not arise from a defect of NHEJ
The efficient generation of antibodies and T-cell receptors requires competency for double strand break repair, particularly NHEJ [Gellert 2002; Schatz and Ji 2011]. Therefore, to test if SIOD patients had defects of NHEJ that could account for their prominent T-cell immunodeficiency and occasional immunoglobulin deficits [Boerkoel et al., 2000], we checked for a NHEJ defect altering immunoglobulin switching in SIOD patients. We analyzed 25 Sμ-Sα1 and 22 Sμ-Sγ1 immunoglobulin switch recombination junctions amplified from the peripheral blood DNA of 7 SIOD patients including one who died of NHL and had hypersensitivity to chemotherapy [Basiratnia et al., 2011]. Comparison of the junctions from these patients to those from 4 control individuals did not identify abnormalities such as increased switch junction homology or breakpoint mutations (Supplementary eFigures. S1 and S2 – See Supporting Information online).
Smarcal1-deficient mice are hypersensitive to genotoxic agents
Recent findings have indicated SMARCAL1 is a component of the DNA stress response and that SMARCAL1-deficient cells are hypersensitive to genotoxic agents hydroxyurea and camptothecin [Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009]. Nonetheless, with one exception [Basiratnia et al., 2011], SIOD patients treated for cancer or bone marrow ablation have been given doses of genotoxic agents standard for individuals with immunodeficiency without reports of increased mortality [CFB, unpublished data; Petty et al. 2000]. Therefore to clarify this issue better, we asked if Smarcal1del/del mice were hypersensitive to genotoxic agents (Table II). Using growth and splenic cell apoptosis as outcome measures, Smarcal1del/del mice were more sensitive to CPT-11, etoposide, or HU than were Smarcal1+/+ mice (Fig. 2a-l and Fig. 3a-j).
TABLE II.
Response of Smarcal1+/+ and Smarcal1del/del mice to exposure to CPT-11, etoposide and hydroxyurea.
| Compound | Dose (mg/kg) |
Frequency | Phenotype |
|
|---|---|---|---|---|
| Smarcal1+/+ | Smarcal1del/del | |||
|
| ||||
| CPT-11 | 40 | daily × 56 days | Intermittent diarrhea | Chronic diarrhea, weakness, severe growth arrest |
| 80 | daily × 5 days (1 week rest) daily × 10 days |
Weight loss (2:3), mild diarrhea (2:3) |
Weight loss (3:3), diarrhea (3:3), severe weakness and death (2:3) |
|
|
| ||||
| Etoposide | 20 | daily × 56 days | Normal | Severe growth arrest |
| 40 | daily × 5 days | Weight loss (3:3), severe weakness (2:3), death (1:3) |
Weight loss and severe weakness (4:4), death (2:4) |
|
|
| ||||
| Hydroxyurea | 100 | daily × 56 days | Normal | Mild growth arrest |
| 500 | daily × 10 days | Weight loss (4:4), death (1:4) |
Weight loss (4:4), severe weakness and death (3:4) |
|
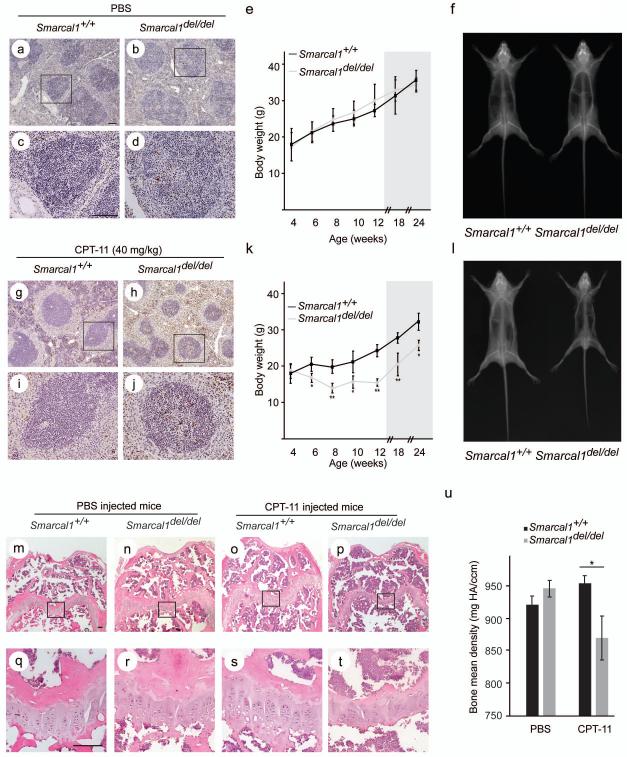
FIG. 2.
Hypersensitivity of Smarcal1del/del mice to CPT-11. a-d: Photomicrographs of TUNEL-positive nuclei in splenic tissue from postnatal day (P) 36 Smarcal1del/del and Smarcal1+/+ mice following 5 daily ip-injections with PBS. Panels c and d are higher magnification photomicrographs of the boxed areas in panels a and b, respectively. e: Graph showing the average weight gain of Smarcal1del/del (n = 4) and Smarcal1+/+ (n = 4) mice receiving daily ip-injections of PBS from P28 through P84 and during 90 days following treatment (shaded area). f: Representative skeletal radiographs of Smarcal1del/del and Smarcal1+/+ mice at P174 following 8 weeks of daily injections with PBS. g-j: Photomicrographs of TUNEL-positive nuclei in splenic tissue from P36 Smarcal1del/del and Smarcal1+/+ mice following 5 daily ip-injections with CPT-11. Panels i and j are higher magnification photomicrographs of the boxed areas in panels g and h, respectively. k: Graph showing the average weight gain of Smarcal1del/del (n = 3) and Smarcal1+/+ (n = 4) mice receiving daily ip-injections of CPT-11 from P28 through P84 and during 90 days following treatment (shaded area). l: Representative skeletal radiographs of Smarcal1del/del and Smarcal1+/+ mice at P173 following 8 weeks of daily injections with CPT-11. m-t: Photomicrographs of representative sections of the distal femoral growth plate of Smarcal1+/+ and Smarcal1del/del male mice treated with PBS or CPT-11 for 8 weeks. The tissue was stained with H&E. Panels q-t are higher magnification photomicrographs of the boxed areas in panels m-p, respectively. Note the poorly organized columns of chondrocytes in the smaller hypocellular growth plates of the CPT-11 treated Smarcal1del/del mice. u: Graph showing the mean bone density of the distal femur of Smarcal1+/+ and Smarcal1del/del mice treated either with PBS or CPT-11. Scale bars = 100 μm. Error bars represent one standard deviation. For statistically significant differences between Smarcal1del/del and Smarcal1+/+ mice, a * indicates P<0.05 and a ** indicates P<0.01. Abbreviations: CPT-11, irinotecan; ip, intraperitoneal; mg HA/ccm, milligrams hydroxyapatite per cubic centimetre.
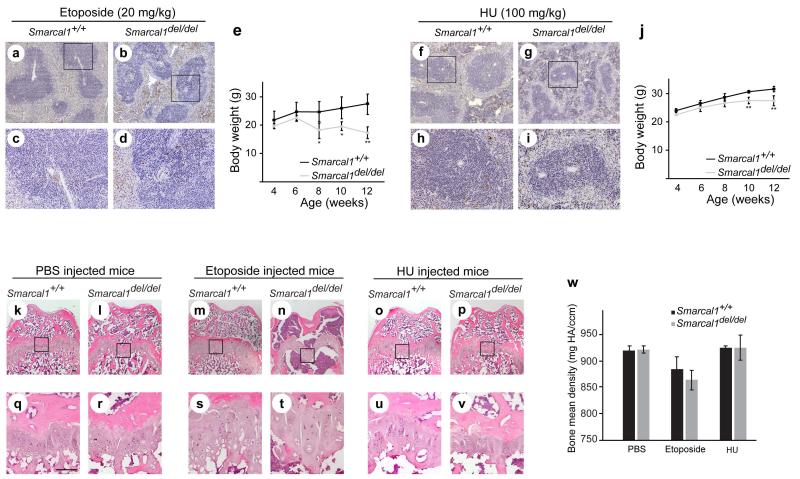
FIG. 3.
Hypersensitivity of Smarcal1del/del mice to etoposide and HU. a-d: Photomicrographs of TUNEL-positive nuclei in splenic tissue from P36 Smarcal1del/del and Smarcal1+/+ mice following 5 daily ip-injections with etoposide. Panels c and d are higher magnification photomicrographs of the boxed areas in panels a and b, respectively. e: Graph showing the average weight gain of Smarcal1del/del (n = 4) and Smarcal1+/+ (n=3) mice receiving daily ip-injections of etoposide from P28 through P84. f-i: Photomicrographs of TUNEL-positive nuclei in splenic tissue from P36 Smarcal1del/del and Smarcal1+/+ mice following 5 daily ip-injections with hydroxyurea (HU). Panels h and i are higher magnification photomicrographs of the boxed areas in panels f and g, respectively. j: Graph showing the average weight gain of Smarcal1del/del (n = 5) and Smarcal1+/+ (n = 4) mice receiving daily ip-injections of HU from P28 through P84. k-v: Photomicrographs of representative sections of the distal femoral growth plate of Smarcal1+/+ and Smarcal1del/del male mice treated with PBS, etoposide, or HU for 8 weeks. The tissue was stained with H&E. Panels q-v are higher magnification photomicrographs of the boxed areas in panels k-p, respectively. Note the poorly organized columns of chondrocytes in the hypocellular growth plates of the etoposide and HU treated Smarcal1del/del mice. Also note the near absence of trabeculae in the etoposide treated Smarcal1del/del mice. w: Graph showing the bone mean density of the distal femur of Smarcal1+/+ and Smarcal1del/del mice treated with PBS, etoposide or HU. Scale bars = 100 μm. Error bars represent one standard deviation. For statistically significant differences between Smarcal1del/del and Smarcal1+/+ mice, a * indicates P<0.05 and a ** indicates P<0.01. Abbreviations: HU, hydroxyurea; ip, intraperitoneal; mg HA/ccm, milligrams hydroxyapatite per cubic centimetre.
CPT-11, etoposide and HU exposure partially recapitulate the bone phenotype of SIOD in Smarcal1del/del mice
We have previously shown that Smarcal1del/del mice do not recapitulate SIOD without environmental or genetic factors further compromising gene expression [Baradaran-Heravi et al., 2012]. We asked therefore if this exposure to genotoxic agents would partially or fully recapitulate SIOD in the Smarcal1del/del mice. Reminiscent to the growth plates of SIOD patients [Clewing et al., 2007a; Spranger et al., 1991], the distal femur growth plates of Smarcal1del/del mice treated with genotoxic agents were hypocellular, and chondrocytes in the proliferation and hypertrophic zones formed less organized columns (Fig. 2m-t, Fig. 3k-v, and Supplementary eFigure S3a ). Also, like the reduced bone density observed in some SIOD patients [Hunter et al., 2010; Tylki-Szymanska et al., 2003], micro-computed tomography scanning of the distal femurs revealed significantly reduced bone density in Smarcal1del/del mice treated with CPT-11 (Fig. 2u); the bone density was not significantly different from controls after treatment with etoposide or HU (Fig. 3w). Additionally, we hypothesized that if CPT-11 treatment moves chondrocytes past a threshold required for expression of skeletal dysplasia in Smarcal1del/del mice, then the growth rate of Smarcal1del/del mice should not return to normal when CPT exposure is removed but remain reduced. To test this, we monitored CPT-11-injected mice for another 12 weeks after discontinuation of CPT-11 injections; interestingly, the Smarcal1del/del and Smarcal1+/+ mice had the same growth rates (Figs. 2e and k).
CPT-11, etoposide and HU exposure do not recapitulate the renal phenotype of SIOD in Smarcal1del/del mice
Besides skeletal dysplasia, renal failure is another cardinal feature of SIOD and one SIOD patient developed renal failure following treatment with genotoxic agents [Petty et al., 2000]. We hypothesized therefore that the Smarcal1del/del mice treated with genotoxic agents might also develop renal failure. Measurement of urine albumin excretion as well as Masson trichrome staining of the kidneys after 8 weeks of exposure to CPT-11, etoposide, and HU did not show increased albuminuria in Smarcal1del/del mice or signs of sclerosis compared to controls (Supplementary eFigure S3b-c – See Supporting Information online).
DISCUSSION
Despite superficial similarities to classical disorders of DNA repair, SIOD is a distinct disorder. The ectodermal defects cannot be ascribed to an obvious defect of NER as in Cockayne syndrome or xeroderma pigmentosum, and the immune defects cannot be ascribed to molecular defects of NHEJ as in ataxia telangiectasia and Nijmegen breakage syndrome.
SIOD is not a classical cancer predisposition syndrome in which there is increased occurrence of multiple cancers although there is an increased prevalence of NHL and possibly osteosarcoma. The detection of EBV in two of the three NHL tumors suggests that the increased prevalence of NHL is attributable to the immunodeficiency. NHL is a heterogeneous group of malignancies with variable etiologic, morphologic, immune, genetic, and clinical features [Alexander et al., 2007]. Risk factors for NHL include congenital and acquired immunodeficiency, chronic antigen stimulation, autoimmune disorders, environmental factors, and genetic deficiency of NHEJ enzymes [Grulich and Vajdic 2005; Hill et al., 2006]. Since the prevalence of NHL in the general population during the first ten years of life is 0.0026% [Howlader et al., 2012], the relative risk for NHL in our cohort of SIOD patients is 1,625. Although defective DNA repair might in an undefined manner contribute to this, the absence of recurrent cancers during the lifetime of SIOD patients suggests that the immunodeficiency is likely the major contributor to the increased risk of NHL in SIOD.
Whether the single SIOD patient with osteosarcoma represents an increased risk for osteosarcoma or is a coincidental co-occurrence is unclear. Assuming that this is not coincidental co-occurrence, the relative risk for osteosarcoma in our cohort of SIOD patients is 150 since the prevalence of osteosarcoma in the general population during the first twenty years of life is 0.0093% [Howlader et al., 2012]. An increased risk for osteosarcoma is also observed in the DNA repair disorders Rothmund-Thompson syndrome, RAPADILINO syndrome and Werner syndrome [Goto et al., 1996; Lindor et al., 2000; Siitonen et al., 2003]. Again, the absence of recurrent cancers during the lifetime of SIOD patients questions whether SIOD is a cancer predisposition disorder unless one assumes the patients die too early to manifest recurrent tumors.
SMARCAL1 does nonetheless play a role in genome maintenance and the DNA stress response [Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009]. As an annealing helicase, SMARCAL1 resolves single to double-stranded DNA transitions [Yusufzai and Kadonaga 2008] and contributes to the maintenance of genomic integrity [Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009]. It also participates in the reactivation of stalled replication forks, associates with double-strand DNA breaks, and facilitates cell cycle progression from the G2 to the M phase [Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009]. Four domains within the SMARCAL1 enzyme are critical to these functions: a nuclear localization signal (NLS), a replication protein A (RPA) binding domain, two tandem HARP domains with annealing helicase activity, and a SWI/SNF ATPase and DNA binding domain [Bansbach et al., 2009; Ciccia et al., 2009; Coleman et al., 2000; Elizondo et al., 2009; Ghosal et al., 2011; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009]. The deletion of the NLS, RPA and HARP domains is associated with SIOD in humans [Boerkoel et al., 2002; Clewing et al., 2007b], and herein we show that mice with deletion of the homologous domains are hypersensitive to genotoxic agents. Extension of these observations to humans shows that one SIOD patient was hypersenstive to anticancer agents [Basiratnia et al., 2011], another with NHL tolerated standard doses of R-CHOP chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) but then developed severe infection and delayed neutrophil recovery when treated with one cycle of ifosfamide (76.5ml/hr × 24 hours) and etoposide (61mg) for relapse and another with osteosarcoma tolerated and survived chemotherapeutic regimens adjusted for immunodeficiency. Definitive evidence of whether SIOD patients are hypersensitive to genotoxic agents will therefore only come as more patients are treated for cancer; however, in the interim, caution in the use of such agents in SIOD patients seems advisable.
Since our earlier studies have shown that the Smarcal1del/del mice did not express features of SIOD unless gene expression was compromised [Baradaran-Heravi et al., 2012], we hypothesized that exposure to genotoxic agents also induced features of SIOD. Indeed, treatment with genotoxic agents did cause growth retardation and skeletal features similar to those observed in SIOD. Additionally, exposure to CPT-11 did not induced a continued loss of growth potential after its removal suggesting that the growth of SIOD patients will be retarded by exposure to genotoxic agents during bone marrow ablation or treatment for cancer but that their growth rate will likely return to baseline when the genotoxic therapy is removed.
In the management of SIOD, bone marrow transplantation is often considered for the immunodeficiency. Of concern, however, is the one SIOD patient who developed renal failure shortly after bone marrow transplantation [Petty et al., 2000]. The question arises therefore as to whether this was the natural progression of SIOD or prematurely induced by the exposure to the genotoxic agents. The failure of genotoxic agents to induce albuminuria and renal sclerosis in Smarcal1del/del mice suggests that despite the potential hypersensitivity of SIOD patients to chemotherapy or bone marrow ablation, short-term treatment with genotoxic agents is unlikely to hasten renal failure. Nonetheless, since the four other SIOD patients treated with bone marrow transplantation died from EBV infection, cerebral haemorrhage, pulmonary embolism or pancreatitis shortly after transplant (C. F. B., unpublished data), definitive evidence of whether genotoxic agents cause long term sequelae in SIOD will only come as more patients survive cancer therapy and bone marrow transplantation. In the interim, the hypersensitivity of the Smarcal1del/del mice suggests caution in the use of such agents in SIOD patients.
Regarding screening of SIOD patients for cancer, extra hematologic surveillance or imaging appear unnecessary. Patients should maintain close contact with their physicians, and symptoms that cannot be accounted for otherwise should be evaluated promptly as potential early indicators of cancer.
In summary, SIOD patients have an increased risk for NHL that is likely attributable to their T-cell immunodeficiency and possibly for osteosarcoma; therefore patients should be evaluated promptly for cancer when they have unexplained symptoms. SIOD patients do not have a detectable defect of NER or NHEJ to explain their ectodermal or immunological features, but as shown by the hypersensitivity of Smarcal1del/del mice to some DNA damaging agents, some SIOD patients are likely to be hypersensitive to genotoxic drugs. We conclude therefore that extra caution should be observed during bone marrow ablation and administration of genotoxic agents to SIOD patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Jan M. Friedman, Catherine Pallen, Thomas Lücke, and Shirin Kalyan for critical review of this manuscript. This work was supported in part by grants from the March of Dimes (CF Boerkoel), the Gillson Longenbaugh Foundation (CF Boerkoel), the Dana Foundation (CF Boerkoel) and the New Investigator Development Award, Microscopy, and Administrative Cores of the Mental Retardation and Developmental Disabilities Research Center at Baylor College of Medicine (CF Boerkoel), the Burroughs Wellcome Foundation (CF Boerkoel), the National Institute of Diabetes, Digestive, and Kidney Diseases, NIH (R21DK065725, CF Boerkoel), and the Association Autour D’Emeric et D’Anthony (CF Boerkoel) and The Little Giants Foundation. This work is supported in part by the Intramural Research Program of the National Institute on Aging (Z01 AG000657-08), National Institute of Health (US). CF Boerkoel is a scholar of the Michael Smith Foundation for Health Research.
Footnotes
COMPETING INTERESTS No authors have conflicts of interest to report.
REFERENCES
- Alexander DD, Mink PJ, Adami HO, Chang ET, Cole P, Mandel JS, Trichopoulos D. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- Arama E, Steller H. Detection of apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and acridine orange in Drosophila embryos and adult male gonads. Nat Protoc. 2006;1:1725–1731. doi: 10.1038/nprot.2006.235. [DOI] [PubMed] [Google Scholar]
- Bansbach CE, Betous R, Lovejoy CA, Glick GG, Cortez D. The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev. 2009;23:2405–2414. doi: 10.1101/gad.1839909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran-Heravi A, Cho KS, Tolhuis B, Sanyal M, Morozova O, Morimoto M, Elizondo LI, Bridgewater D, Lubieniecka J, Beirnes K, Myung C, Leung D, Fam HK, Choi K, Huang Y, Dionis KY, Zonana J, Keller K, Stenzel P, Mayfield C, Lucke T, Bokenkamp A, Marra MA, van Lohuizen M, Lewis DB, Shaw C, Boerkoel CF. Penetrance of biallelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression. Hum Mol Genet. 2012;21:2572–2587. doi: 10.1093/hmg/dds083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basiratnia M, Baradaran-Heravi A, Yavarian M, Gerami zadeh B, Karimi M. Non-Hodgkin Lymphoma in A Child with Schimke immuno osseous dysplasia. IJMS. 2011;36:222–225. [PMC free article] [PubMed] [Google Scholar]
- Boerkoel CF, O’Neill S, Andre JL, Benke PJ, Bogdanovic R, Bulla M, Burguet A, Cockfield S, Cordeiro I, Ehrich JH, Frund S, Geary DF, Ieshima A, Illies F, Joseph MW, Kaitila I, Lama G, Leheup B, Ludman MD, McLeod DR, Medeira A, Milford DV, Ormala T, Rener-Primec Z, Santava A, Santos HG, Schmidt B, Smith GC, Spranger J, Zupancic N, Weksberg R. Manifestations and treatment of Schimke immuno-osseous dysplasia: 14 new cases and a review of the literature. Eur J Pediatr. 2000;159:1–7. doi: 10.1007/s004310050001. [DOI] [PubMed] [Google Scholar]
- Boerkoel CF, Takashima H, John J, Yan J, Stankiewicz P, Rosenbarker L, Andre JL, Bogdanovic R, Burguet A, Cockfield S, Cordeiro I, Frund S, Illies F, Joseph M, Kaitila I, Lama G, Loirat C, McLeod DR, Milford DV, Petty EM, Rodrigo F, Saraiva JM, Schmidt B, Smith GC, Spranger J, Stein A, Thiele H, Tizard J, Weksberg R, Lupski JR, Stockton DW. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat Genet. 2002;30:215–220. doi: 10.1038/ng821. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Bredemeyer AL, Sowa ME, Terret ME, Jallepalli PV, Harper JW, Elledge SJ. The SIOD disorder protein SMARCAL1 is an RPA-interacting protein involved in replication fork restart. Genes Dev. 2009;23(20):2415–25. doi: 10.1101/gad.1832309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewing JM, Antalfy BC, Lücke T, Najafian B, Marwedel KM, Hori A, Powel RM, Safo Do AF, Najera L, SantaCruz K, Hicks MJ, Armstrong DL, Boerkoel CF. Schimke immuno-osseous dysplasia: a clinicopathological correlation. J Med Genet. 2007a;44:122–130. doi: 10.1136/jmg.2006.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewing JM, Fryssira H, Goodman D, Smithson SF, Sloan EA, Lou S, Huang Y, Choi K, Lucke T, Alpay H, Andre JL, Asakura Y, Biebuyck-Gouge N, Bogdanovic R, Bonneau D, Cancrini C, Cochat P, Cockfield S, Collard L, Cordeiro I, Cormier-Daire V, Cransberg K, Cutka K, Deschenes G, Ehrich JH, Frund S, Georgaki H, Guillen-Navarro E, Hinkelmann B, Kanariou M, Kasap B, Kilic SS, Lama G, Lamfers P, Loirat C, Majore S, Milford D, Morin D, Ozdemir N, Pontz BF, Proesmans W, Psoni S, Reichenbach H, Reif S, Rusu C, Saraiva JM, Sakallioglu O, Schmidt B, Shoemaker L, Sigaudy S, Smith G, Sotsiou F, Stajic N, Stein A, Stray-Pedersen A, Taha D, Taque S, Tizard J, Tsimaratos M, Wong NA, Boerkoel CF. Schimke immunoosseous dysplasia: suggestions of genetic diversity. Hum Mutat. 2007b;28:273–283. doi: 10.1002/humu.20432. [DOI] [PubMed] [Google Scholar]
- Coleman MA, Eisen JA, Mohrenweiser HW. Cloning and characterization of HARP/SMARCAL1: a prokaryotic HepA-related SNF2 helicase protein from human and mouse. Genomics. 2000;65(3):274–82. doi: 10.1006/geno.2000.6174. [DOI] [PubMed] [Google Scholar]
- da Fonseca MA. Dental findings in the Schimke immuno-osseous dysplasia. Am J Med Genet. 2000;93:158–160. doi: 10.1002/1096-8628(20000717)93:2<158::aid-ajmg14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Elizondo LI, Cho KS, Zhang W, Yan J, Huang C, Huang Y, Choi K, Sloan EA, Deguchi K, Lou S, Baradaran-Heravi A, Takashima H, Lucke T, Quiocho FA, Boerkoel CF. Schimke immuno-osseous dysplasia: SMARCAL1 loss-of-function and phenotypic correlation. J Med Genet. 2009;46:49–59. doi: 10.1136/jmg.2008.060095. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. ASM Press; Washington, DC: 2006. [Google Scholar]
- Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–132. doi: 10.1146/annurev.biochem.71.090501.150203. [DOI] [PubMed] [Google Scholar]
- Ghosal G, Yuan J, Chen J. The HARP domain dictates the annealing helicase activity of HARP/SMARCAL1. EMBO Rep. 2011;12:574–580. doi: 10.1038/embor.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Miller RW, Ishikawa Y, Sugano H. Excess of rare cancers in Werner syndrome (adult progeria) Cancer Epidemiol Biomarkers Prev. 1996;5(4):239–46. [PubMed] [Google Scholar]
- Grulich AE, Vajdic CM. The epidemiology of non-Hodgkin lymphoma. Pathology. 2005;37:409–419. doi: 10.1080/00313020500370192. [DOI] [PubMed] [Google Scholar]
- Hill DA, Wang SS, Cerhan JR, Davis S, Cozen W, Severson RK, Hartge P, Wacholder S, Yeager M, Chanock SJ, Rothman N. Risk of non-Hodgkin lymphoma (NHL) in relation to germline variation in DNA repair and related genes. Blood. 2006;108:3161–3167. doi: 10.1182/blood-2005-01-026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano R, Interthal H, Huang C, Nakamura T, Deguchi K, Choi K, Bhattacharjee MB, Arimura K, Umehara F, Izumo S, Northrop JL, Salih MA, Inoue K, Armstrong DL, Champoux JJ, Takashima H, Boerkoel CF. Spinocerebellar ataxia with axonal neuropathy: consequence of a Tdp1 recessive neomorphic mutation. EMBO J. 2007;26:4732–4743. doi: 10.1038/sj.emboj.7601885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KAe. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2009_pops09/ based on November 2011 SEER data submission, posted to the SEER web site, April 2012. [Google Scholar]
- Hunter KB, Lucke T, Spranger J, Smithson SF, Alpay H, Andre JL, Asakura Y, Bogdanovic R, Bonneau D, Cairns R, Cransberg K, Frund S, Fryssira H, Goodman D, Helmke K, Hinkelmann B, Lama G, Lamfers P, Loirat C, Majore S, Mayfield C, Pontz BF, Rusu C, Saraiva JM, Schmidt B, Shoemaker L, Sigaudy S, Stajic N, Taha D, Boerkoel CF. Schimke immunoosseous dysplasia: defining skeletal features. Eur J Pediatr. 2010;169:801–811. doi: 10.1007/s00431-009-1115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers NG, Raams A, Kelner MJ, Ng JM, Yamashita YM, Takeda S, McMorris TC, Hoeijmakers JH. Anti-tumour compounds illudin S and Irofulven induce DNA lesions ignored by global repair and exclusively processed by transcription- and replication-coupled repair pathways. DNA Repair (Amst) 2002;1:1027–1038. doi: 10.1016/s1568-7864(02)00166-0. [DOI] [PubMed] [Google Scholar]
- Jaspers NG, Raams A, Silengo MC, Wijgers N, Niedernhofer LJ, Robinson AR, Giglia-Mari G, Hoogstraten D, Kleijer WJ, Hoeijmakers JH, Vermeulen W. First reported patient with human ERCC1 deficiency has cerebro-oculo-facio-skeletal syndrome with a mild defect in nucleotide excision repair and severe developmental failure. Am J Hum Genet. 2007;80:457–466. doi: 10.1086/512486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic SS, Donmez O, Sloan EA, Elizondo LI, Huang C, Andre JL, Bogdanovic R, Cockfield S, Cordeiro I, Deschenes G, Frund S, Kaitila I, Lama G, Lamfers P, Lucke T, Milford DV, Najera L, Rodrigo F, Saraiva JM, Schmidt B, Smith GC, Stajic N, Stein A, Taha D, Wand D, Armstrong D, Boerkoel CF. Association of migraine-like headaches with Schimke immuno-osseous dysplasia. Am J Med Genet A. 2005;135:206–210. doi: 10.1002/ajmg.a.30692. [DOI] [PubMed] [Google Scholar]
- Kubota M, Yasunaga M, Hashimoto H, Kimata H, Mikawa H, Shinya A, Nishigori C. IgG4 deficiency with Rothmund-Thomson syndrome: a case report. Eur J Pediatr. 1993;152:406–408. doi: 10.1007/BF01955898. [DOI] [PubMed] [Google Scholar]
- Lindor NM, Furuichi Y, Kitao S, Shimamoto A, Arndt C, Jalal S. Rothmund-Thomson syndrome due to RECQ4 helicase mutations: report and clinical and molecular comparisons with Bloom syndrome and Werner syndrome. Am J Med Genet. 2000;90:223–228. doi: 10.1002/(sici)1096-8628(20000131)90:3<223::aid-ajmg7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Pan-Hammarstrom Q, Jones AM, Lahdesmaki A, Zhou W, Gatti RA, Hammarstrom L, Gennery AR, Ehrenstein MR. Impact of DNA ligase IV on nonhomologous end joining pathways during class switch recombination in human cells. J Exp Med. 2005;201:189–194. doi: 10.1084/jem.20040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty EM, Yanik GA, Hutchinson RJ, Alter BP, Schmalstieg FC, Levine JE, Ginsburg D, Robillard JE, Castle VP. Successful bone marrow transplantation in a patient with Schimke immuno-osseous dysplasia. J Pediatr. 2000;137:882–886. doi: 10.1067/mpd.2000.109147. [DOI] [PubMed] [Google Scholar]
- Postow L, Woo EM, Chait BT, Funabiki H. Identification of SMARCAL1 as a component of the DNA damage response. J Biol Chem. 2009;284(51):35951–61. doi: 10.1074/jbc.M109.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol. 2011;11:251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- Schimke RN, Horton WA, King CR. Chondroitin-6-sulphaturia, defective cellular immunity, and nephrotic syndrome. Lancet. 1971;2(7733):1088–1089. doi: 10.1016/s0140-6736(71)90400-4. [DOI] [PubMed] [Google Scholar]
- Scriver CR, Sly WS, Childs B, Beaudet AL, Valle D, Kinzler KW, Vogelstein B. The metabolic and molecular bases of inherited disease. 8 ed McGraw-Hill; New York: 2001. [Google Scholar]
- Siitonen HA, Kopra O, Kaariainen H, Haravuori H, Winter RM, Saamanen AM, Peltonen L, Kestila M. Molecular defect of RAPADILINO syndrome expands the phenotype spectrum of RECQL diseases. Hum Mol Genet. 2003;12:2837–2844. doi: 10.1093/hmg/ddg306. [DOI] [PubMed] [Google Scholar]
- Spranger J, Hinkel GK, Stoss H, Thoenes W, Wargowski D, Zepp F. Schimke immuno-osseous dysplasia: a newly recognized multisystem disease. J Pediatr. 1991;119(1 ( Pt 1)):64–72. doi: 10.1016/s0022-3476(05)81040-6. [DOI] [PubMed] [Google Scholar]
- Taha D, Boerkoel CF, Balfe JW, Khalifah M, Sloan EA, Barbar M, Haider A, Kanaan H. Fatal lymphoproliferative disorder in a child with Schimke immuno-osseous dysplasia. Am J Med Genet A. 2004;131:194–199. doi: 10.1002/ajmg.a.30356. [DOI] [PubMed] [Google Scholar]
- Tylki-Szymanska A, Pyrkosz A, Krajewska-Walasek M, Michalkiewicz J, Kowalska A, Rokicki D. Schimke immuno-osseous dysplasia: two cases. Pediatr Radiol. 2003;33:216–218. doi: 10.1007/s00247-002-0852-y. [DOI] [PubMed] [Google Scholar]
- Yuan J, Ghosal G, Chen J. The annealing helicase HARP protects stalled replication forks. Genes Dev. 2009;23:2394–2399. doi: 10.1101/gad.1836409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai T, Kadonaga JT. HARP is an ATP-driven annealing helicase. Science. 2008;322(5902):748–750. doi: 10.1126/science.1161233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai T, Kong X, Yokomori K, Kadonaga JT. The annealing helicase HARP is recruited to DNA repair sites via an interaction with RPA. Genes Dev. 2009;23:2400–2404. doi: 10.1101/gad.1831509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.