Abstract
Background
China faces a major increase in cardiovascular disease, yet there is limited population-based data on risk factors, particularly in children.
Methods and Results
Fasting blood samples, anthropometry and blood pressure were collected on 9,244 children and adults aged ≥7 years in late 2009 as part of the national China Health and Nutrition Survey. Prevalent overweight, elevated blood pressure, and cardiometabolic risk factors: glucose, HbA1c, triglycerides (TG), total cholesterol (TC), high and low density lipoprotein cholesterol (HDL-C and LDL-C), and C-reactive protein (CRP) are presented.
Results
11% of Chinese children and 30% of Chinese adults are overweight. Rates of diabetes, dyslipidemia, hypertension, and inflammation are high and increased with age and were associated with urbanization. Approximately 42% of children have at least one of the following: pre-diabetes or diabetes, hypertension, high TC, LDL-C, TG, and CRP and low HDL-C, as do 70% males and 60% females aged 18–40 years and >86% of males and females ≥40 years.
Conclusions
HbA1c findings suggest that as many as 29.4 million Chinese children and 415.8 million Chinese adults may be prediabetic or diabetic. The high prevalence in less urban areas and across all income levels suggests that cardiometabolic risk is pervasive across rural and urban China.
Keywords: China, children, diabetes, cardio-metabolic, adults
Introduction
Obesity rates in China across all age-gender groups have risen greatly in the past two decades with particularly profound increases in BMI in children at the upper portion of the distribution over the past decade1, 2. Concurrently, China has seen a major increase in cardiometabolic risk, including high rates of hypertension, stroke and diabetes 3–5. Underlying factors, such as diet and physical activity have also changed, including increased consumption of refined carbohydrates, saturated fat, and added sugars, and declines in work, leisure and transport-related physical activity 1, 6. These changes extend to poor and rural areas, where the most rapid increases in overweight and obesity have recently occurred 7. With China’s rapid economic growth, the burden of obesity has shifted to lower income groups, mirroring what is typically seen in high income countries 8. Further complicating the disease profile in China is the relatively higher cardiometabolic risk at relatively low body mass index (BMI) 9 and at younger ages 10 in Asian populations as well as higher insulin resistance relative to Western populations, even at the same level of BMI 11.
Using a representative, randomly selected sample from nine provinces across China, with anthropometry, blood pressure measures and fasting blood samples from 9,244 individuals aged 7 and older, we examine age and sex-specific prevalence of overweight, hypertension, and biomarkers of diabetes, dyslipidemia, and inflammation. In addition, we examine differences in cardiometabolic risk factors across levels of urbanization and income. Further, we provide a comparative analysis of cardiometabolic risk relative to patterns in the US and in other Asian countries.
Methods
The China Health and Nutrition Survey (CHNS) is the only large-scale longitudinal, household-based survey in China 12. The CHNS has followed individuals randomly selected from 228 communities and designed to represent a set of large provinces with a range of economic and demographic variation, covering approximately 56% of China’s population, including Liaoning, Shandong, Heilongjiang, Henan, Jiangsu, Hubei, Hunan, Guizhou, and Guangxi (from north to south). The CHNS age and gender distribution is slightly older than that of the Chinese census for 2009, since Muslim provinces which have higher fertility rates that other parts of China were not included in the CHNS. Thus, CHNS includes approximately 3% more individuals aged 60 and older and 3% fewer children and adolescents compared to the national China census 12. A multistage, random cluster process was used to draw the sample in each province, with the baseline survey in 1989, and fasting blood collected for the first time in 2009. The analysis sample includes 9,244 individuals aged 7 and older who provided fasting blood and anthropometry in 2009. Unlike other surveys, the number of individuals who provided anthropometry is slightly greater (79 more individuals) than those providing blood samples, likely due to the ordering of data collection. There were no statistically significant differences in overweight and age between individuals providing blood and anthropometry data. The sample of 2009 participants represented 85.6% of the 2006 participants. Survey protocols, instruments, and the process for obtaining informed consent for this study were approved by the institutional review committees of the University of North Carolina at Chapel Hill, the National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, and the China-Japan Friendship Hospital, Ministry of Health.
During home visits spanning 3 days, survey data were collected through interviews with each household member, including anthropometrics (height, weight, skinfold thicknesses and body circumferences), detailed individual and household level dietary data, measures of time spent in a wide range of daily activities and detailed individual, household and community socio-demographic data 12. Individuals older than 7 years visited a neighbourhood clinic to have trained physicians collect fasting blood samples. Individuals unable to attend the clinic had blood samples collected at home. To avoid missing children in boarding schools and migrant workers, special efforts were made to schedule visits in early morning or in the weekend when these participants were at home. All interviewers had 7 days of training provided by the collaborating teams and were overseen via site visits to monitor data collection in each site visit by the University of North Carolina at Chapel Hill, the China Centers for Disease Control and Prevention and the China-Japan Friendship Hospital at selected locations in each province. Measurement of cardiometabolic disease risk factors.
Following an overnight fast, blood was collected by venipuncture and tested immediately for glucose and hemoglobin A1c (HbA1c). Plasma and serum samples were then frozen, and stored at −86 °C for later laboratory analysis. All samples were analyzed in a national central lab in Beijing (medical laboratory accreditation certificate ISO 15189:2007) with strict quality control. We focus on blood pressure [systolic and diastolic BP] and biomarkers of cardiometablic disease risk related to diabetes [fasting glucose measured with the GOD-PAP method (Randox Laboratories Ltd., UK), HbA1c via high-performance liquid chromatography system (model HLC-723 G7; Tosoh Corporation, Tokyo, Japan)]; dyslipidemia [total cholesterol (TC), high and low density lipoprotein cholesterol (HDL-C and LDL-C) all measured using glycerol-phosphate oxidase method and the PEG-modified enzyme method respectively by determiner regents (Kyowa Medex Co., Ltd, Tokyo, Japan ) and triglycerides (TG) using glycerol-phosphate oxidase method and the PEG-modified enzyme method respectively by determiner regents (Kyowa Medex Co., Ltd, Tokyo, Japan ). All lipids measures were on the Hitachi 7600 automated analyzer (Hitachi Inc., Tokyo, Japan); inflammation [high sensitivity C-reactive protein (CRP)] via the immunoturbidimetric method with Denka Seiken, Japan reagents (Hitachi 7600 automated analyzer, Hitachi Inc., Tokyo, Japan). Levels of biomarkers were categorized to represent risk using cutoff points recommended by the International Diabetes Federation (IDF) 13 or in other published literature, with separate cutoff points for adults and children, and for males and females where appropriate (Table 1).
Table 1.
Biomarkers, laboratory analyses methods and definitions of cardiometabolic risk, 2009 China Health and Nutrition Survey fasting blood samples
| Biomarker | Risk Indicator | Definition |
|---|---|---|
| Glucose | Impaired fasting glucose | Glucose ≥ 100 & < 126 mg/dL |
| Diabetes | Glucose ≥ 126 mg/dL or taking diabetes medication | |
| HbA1C | Impaired glucose control | HbA1c ≥ 5.7 & < 6.5% |
| Diabetes | HbA1c ≥ 6.5% or taking diabetes medication | |
| Total Cholesterol | High TC | ≥ 200 mg/dL |
| HDL-C | Low HDL | Male: < 40 mg/dL; female < 50 mg/dL; pediatric < 40 mg/dL |
| LDL-C | High LDL | > 130 mg/dL |
| Triglycerides | High TG | ≥ 150 mg/dL |
| High Sensitivity CRP | High GRP | ≥ 3 mg/dL |
Systolic (SBP) and diastolic (DBP) blood pressure were measured on the right arm, using mercury sphygmomanometers with appropriate cuff sizes. Measures were collected in triplicate after a 10 minute seated rest and the mean of the three measurements was used in analyses. Adult hypertension was determined (according to the IDF cut point (SBP/DBP ≥140/90 mmHg) or taking blood pressure medication. For youth <18 years, hypertension risk was defined as blood pressure above the 85th age, sex, and height-specific reference 14.
Overweight. Height was measured without shoes to the nearest 0.1 cm using a portable SECA stadiometer; weight was measured without shoes and in light clothing to the nearest 0.1 kg on a calibrated beam balance. For adults, overweight is defined as BMI ≥25 kg/m2 and obesity as BMI ≥30 kg/m2 15. For youth, age- and sex-specific reference data for the BMI ≥25 kg/m2 and BMI ≥30 kg/m2 equivalent cut-points from the International Obesity Task Force were used to classify overweight and obesity to be consistent with the adult definitions 16.
We also present population-specific BMI percentile distribution data by age to characterize trends in BMI in children and adolescents in China over the past two decades. Using quantile regression 17, 18, we characterize the mean BMI value associated with the population-specific 95th percentile in 10 and 15 year olds from 1991 to 2009 based on a regression of BMI data against age and age squared.
Other variables
Age was recorded as the respondent’s age on the date of exam and categorized into the following groups: 7–11, 12–17, 18–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years). Urbanicity is defined using a multidimensional 12 component urbanization index that captures community-level physical, social, cultural, and economic environments and which represents the heterogeneity that would be otherwise missed in a urban/rural measure based only on population density (mean=67.2; SD=19.5) 19. A high urbanization index represents a large population living closely together in a physical environment providing an efficient transport system; a communication network; good-quality health care; higher-level education; water, sewer, and electric lines. Household income is based on detailed measures of all income-earning activities of all household members (mean=37,979, SD=45,584 yuan). Tertiles of the urbanization index are used to define low, medium, and high urbanicity and household income per capita.
Comparative cardiometabolic risk data
For wider comparison with the US, we used most recently published data from the National Health and Nutrition Survey20, 21, 22 and from the SEARCH for Diabetes in Youth study.23 We used nationally representative data from the Korean Health and Nutrition Survey for individuals 10 and older collected in 2009, which we analyzed for this paper. We conducted a systematic other literature search for using the following key words alone and in combination: non–insulin-dependent diabetes mellitus, type 2 diabetes mellitus, inflammation, children, adolescents, youth, and then for adults to obtain nationally representative studies from Asia on this topic. We limited our review only to national or very large-scale studies, which limited the number or included studies.
Statistical Analyses
Statistical analyses were conducted using Stata (Release 11.0 SE, Stata Corporation, College Station, TX). For descriptive analyses, percentages were calculated for categorical variables, while means were calculated for continuous variables. The nonparametric test for trend was used to test for age differences in cardiometabolic risk factors. Logistic regression models were used to test for differences in risk factor prevalence by income or urbanization in age and sex-stratified models.
Results
Approximately 12% of children and adolescents were overweight (Table 2). Obesity was higher among the 7–11 year olds (3%) than among the 12–17 year olds (1%). Among adults, overweight increased with age with highest rates in middle aged adults; approximately 30% were overweight and 4% obese in the full sample.
Table 2.
Mean BMI and percent overweight and obese in CHNS participants
| Age Group | N 4,417 | Mean BMI (SD) | Overweight [BMI ≥ 25 (or pediatric equivalent18)]; % (95% CI) | Obesity [BMI ≥ 30 (or pediatric equivalent18)]; % (95% CI) |
|---|---|---|---|---|
| MALES | ||||
| 7–11* y | 211 | 17.0 (2.9) | 12.8 (10.5, 15.1) | 3.2 (2.0, 4.4) |
| 12–17* y | 205 | 19.0 (3.4) | 11.7 (9.5, 14.0) | 1.4 (0.6, 2.2) |
| Total 7–17 y | 416 | 18.0 (3.4) | 12.3 (10.6, 13.9) | 2.3 (1.6, 3.0) |
| 18–29 y | 374 | 22.2 (3.5) | 20.8 (18.7, 22.9) | 2.3 (1.5, 3.1) |
| 30–39 y | 611 | 24.0 (3.5) | 31.0 (29.1, 32.9) | 4.5 (3.7, 5.3) |
| 40–49 y | 857 | 24.0 (3.3) | 35.2 (33.5, 36.8) | 4.8 (4.1, 5.5) |
| 50–59 y | 973 | 23.5 (3.2) | 29.8 (28.4, 31.3) | 3.4 (2.8, 3.9) |
| 60–69 y | 659 | 23.2 (3.4) | 30.0 (28.2, 31.7) | 3.3 (2.6, 4.0) |
| ≥70 y | 431 | 22.5 (4.0) | 22.6 (20.6, 24.6) | 4.1 (3.2, 5.0) |
| Total ≥18 y | 3905 | 23.3 (3.4) | 29.5 (28.8, 30.3) | 3.8 (3.5, 4.1) |
| Age Group | N 4,827 | Mean BMI (SD) | Overweight [BMI ≥ 25 (or pediatric equivalent18)]; % (95% CI) | Obesity [BMI ≥ 30 (or pediatric equivalent18)]; % (95% CI |
|---|---|---|---|---|
| FEMALES | ||||
| 7–11 y* | 160 | 16.3 (3.0) | 12.2 (9.6, 14.8) | 3.0 (1.7, 4.3) |
| 12–17 y* | 184 | 19.0 (3.0) | 8.2 (6.2, 10.2) | 1.1 (0.3, 1.8) |
| Total 7–17y | 344 | 18.0 (3.2) | 10.1 (8.5, 11.7) | 2.0 (1.2, 2.7) |
| 18–29 y | 392 | 21.0 (3.0) | 9.4 (8.0, 10.9) | 0.8 (0.3, 1.2) |
| 30–39 y | 665 | 23.0 (3.4) | 21.7 (20.1, 23.3) | 3.9 (3.1, 4.6) |
| 40–49 y | 1039 | 24.0 (3.3) | 32.7 (31.2, 34.1) | 4.4 (3.8, 5.1) |
| 50–59 y | 1098 | 24.0 (3.3) | 36.4 (35.0, 37.9) | 4.6 (4.0, 5.2) |
| 60–69 y | 697 | 24.0 (4.0) | 36.1 (34.3, 38.0) | 5.7 (4.8, 6.6) |
| ≥70 y | 519 | 23.1 (4.0) | 28.2 (26.2, 30.2) | 5.6 (4.6, 6.5) |
| Total ≥18 y | 4410 | 23.4 (4.0) | 29.9 (29.2, 30.6) | 4.4 (4.1, 4.7) |
| Age Group | N | Mean BMI (SD) | Overweight [BMI ≥ 25 (or pediatric equivalent18)]; % (95% CI) | Obesity [BMI ≥ 30 (or pediatric equivalent18)]; % (95% CI) |
|---|---|---|---|---|
| TOTAL POPULATION | ||||
| Pediatric | 760 | 17.7 (3.3) | 11.3 (10.1, 12.4) | 2.2 (1.6, 2.7) |
| Adult | 8315 | 23.4 (3.5) | 29.7 (29.2, 30.2) | 4.1 (3.9, 4.3) |
7–17 year old age group broken into two categories given variation in growth for children and adolescents
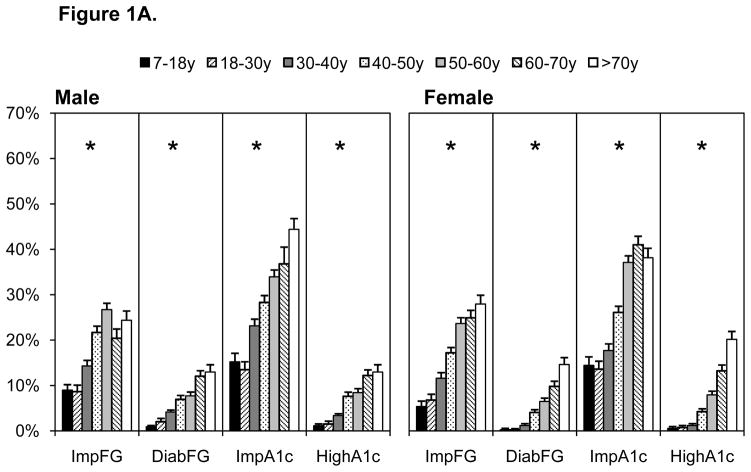
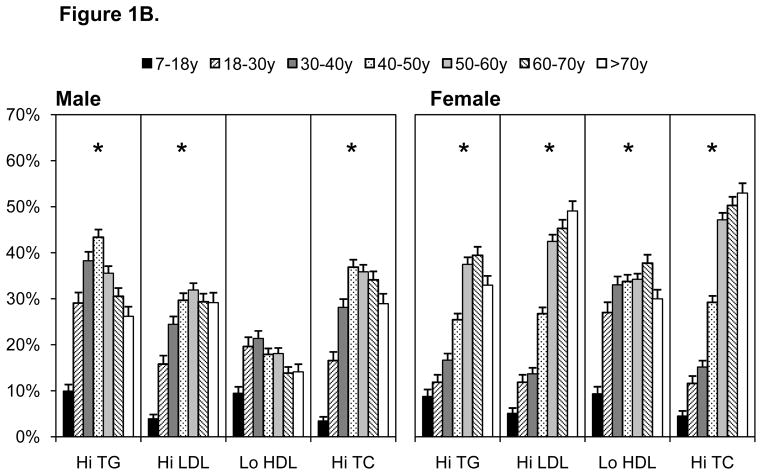
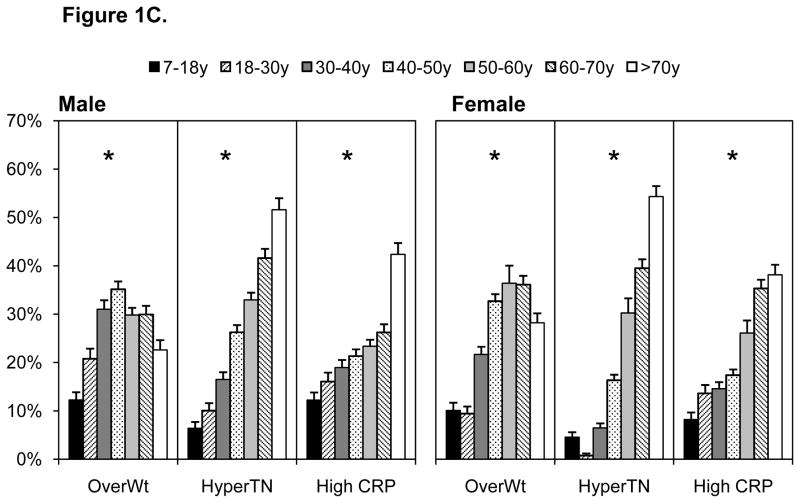
All cardiometabolic risk factors (except low HDL-C in males) increased with age (p for trend, p<0.0001), Figures 1A–1C. Pre-diabetes (IFG and impaired HbA1c) and diabetes were highest in individuals older than 50 years (Figure 1A). Diabetes prevalence was similar when based on fasting glucose or HbA1c, although pre-diabetes was higher using HbA1c. Markers of dyslipidemia were comparatively high in females than males, with particularly high prevalence of dyslipidemia in individuals over the age of 40 years (Figure 1B). Similarly, elevated blood pressure and inflammation were comparatively higher in individuals over the age of 40 years (Figure 1C). Values for all risk markers are shown in Supplemental Table 1.
Figure 1.
A–C. Cardiometabolic Risk Across Several Indicators by Age, 2009 China Health And Nutrition Survey
Cutpoints shown in Table 1, abbreviations below
Over WT overweight
Imp FG impaired fasting glucose
Diab FG, diabetes based on fasting glucose
Imp A1c, impaired HbA1c
High A1c, hemoglobin HbA1c
High TG, triglycerides
High LDL, high low density lipoprotein cholesterol
Low HDL, low high density lipoprotein cholesterol
High TC, high total cholesterol
Hyper TN hypertension
High CRP, Inflammation measured by C-reactive protein
Of major public health relevance is the fact that in the pediatric population, 42% of children aged 7–17 had at least one of the following cardiometabolic risk factors: pre-diabetes/diabetes (HbA1c ≥5.7%), hypertension, high TC, high LDL, low HDL, high TG, high CRP. In contrast, among adults aged 18 to 40 years, 70% of males and 60% of females had at least one risk factor, whereas approximately 83% of 40–60 year olds and over 90% of those 60 years and older had at least one elevated risk factor (Figure 2).
Figure 2.
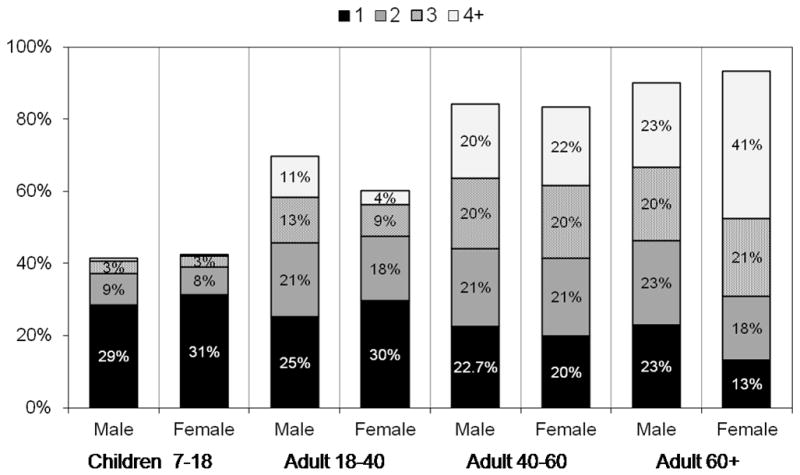
Proportion of The CHNS Sample with at Least one Elevated Cardiometabolic Risk Factor by Age Group (age 7–17 Years; age 18–39 Years, age 40–60 years; and age ≥60 Years
Proportion of the population with one or more of the following cardiometabolic risk factors: impaired/diabetic (HbA1c ≥5.7%), hypertension (140/90, or age-sex specific percentiles for pediatric population), high TC (≥200 mg/dL), high LDL (>130 mg/dL), low HDL(<40 mg/dL), high TG (≥150 mg/dL), high CRP High CRP (≥3).
The observed elevated cardiometabolic risk factors at younger ages are consistent with distribution of overweight in the CHNS pediatric population. Elsewhere, we have shown that the mean BMI at the Chinese BMI 95th population-specific percentile at age 6 in 2006 was 24.8 kg/m2, whereas the US 95th population-specific percentile for 6 year olds was 22.2 kg/m2.1 Looking across the past two decades, the increase in mean BMI at the population-specific BMI for China was biggest between 1991 and 2000 and between 2006 and 2009 and that the increase has been comparatively larger in 10 year olds than 13 year olds.
After adjusting for age, higher urbanization was associated with higher levels of most risk factors, although there were some sex differences in which specific markers related to urbanicity, particularly in individuals over the age of 40 years (Table 4). Urbanization was unrelated to impaired HbA1c and prehypertension (males and females). Comparing older (≥40 yr) to younger (<40) individuals, some interesting relationships emerged. The positive association of urbanization was stronger or seen only among older individuals for overweight, IFG, diabetes (males and females), and low HDL (males only). The direction of association of urbanization with risk indicators was opposite in older versus younger females for high TG, and prehyptertension. For these risk factors, prevalence was lower in the highest urbanization category among younger women, but higher in the highest urbanization category among older women.
Table 4.
Comparison of CHNS findings to findings in the United States and other Asian countries using fasting blood and C-reactive protein
| Adolescent Males | CHNS (Aged 12–18 years) | United States Publications (Aged 12–19 years) |
|---|---|---|
| A. Adolescent Males only: CHNS and US adolescents | ||
| HbA1c≥6.5% | 1.9% | 0.5% NHANES20 |
| CRP ≥3 mg/dL | 12.1% | 8.5% NHANES21 |
| Glucose ≥ 126 mg/dL | 0.9% | 0.28% NHANES22; 0.22% SEARCH**23 |
| IFG (100–126 mg/dL) | 8.4% | 20% NHANES22 |
| Country | Glucose ≥ 126 mg/dL Plus Medication | IFG (100–126 mg/dL) |
|---|---|---|
| B. Adolescents: CHNS and other Asian countries | ||
| CHNS ages 12–17 | 0.9% | 8.4% |
| South Korea, KHANES 10–17** | 0.1% | 5.3% |
| Taiwan 10–1838 | <0.0 | -- |
| Country | Glucose ≥ 126 mg/dL Plus Medication | IFG (100–126 mg/dL) |
|---|---|---|
| C. Adults: CHNS, US, and other Asian countries | ||
| CHNS ages 18 and older | 6.9% | 20.0% |
| NHANES 18 and over 201122* | 8.8% | -- |
| South Korea, KHANES 10–17** | 8.1% | 17.8% |
| Hong Kong39 | 9.8% | -- |
| Malaysia39 | 11.0% | -- |
| Thailand 200439 | 6.7 | -- |
| Singapore 200440 | 8.9 (men); 7.6 (women) | -- |
| Malaysia41 | 12.6% | 22.1% |
--No data available
Source: NHANES weighted to be national representative22
Use of weighted data from the Korean Health and Nutrition Examination Survey 2009
For both males and females
Associations of elevated cardiovascular risk factors with income were less consistent. Higher income was associated with higher prevalence of overweight, diabetes and high TG only in males. Income was unrelated to lipids (except LDL in highest tertile), prehypertension, or CRP in males, and unrelated (positively) to all markers except overweight in the highest tertile in females. Higher income was associated with lower risk of high TG and high CRP in younger women, but with lower risk of diabetes and hypertension in older women (Table 4).
Comparison of CHNS findings to findings in the United States and other Asian countries
We compare our CHNS findings with other nationally representative studies from the US and the few published studies in Asia (Table 4). In Table 4A we show that diabetes and inflammation prevalence was higher in CHNS adolescents than in US adolescents. Although in the CHNS study we are unable to distinguish Type 1 and Type 2 diabetes, available evidence for East Asia suggests almost no Type 1 insulin dependent diabetes among youth 24–26. Comparisons of diabetes across China, South Korea and Taiwan (the only other Asian countries with nationally representative data), suggests higher rates in China (Table 4B). For adults, on the other hand, diabetes prevalence is higher in South Korea, Hong Kong, Singapore, and Malaysia than in China (Table 4C).
Discussion
The observed findings indicate strong age-related differences in overweight and cardiometabolic risk. These estimates highlight the huge burden that the health care system will need to confront. Of particular concern is the relatively high risk in the pediatric population, important not only because of current impact on child health, but also because risk tends to track into adulthood and even worsen. Based on HbA1c, the currently recommended indicator for diabetes 27, we observed rates of diabetes of 0.9% and pre-diabetes of 14.9% in those aged 7–17 years. Although the CHNS data do not match the Chinese census (the CHNS sample has 3% more adults aged 60 and older and 3% fewer under the age of 19), we can approximate the numbers of individuals affected using Chinese census data from 2009 28. Using the census data, our findings suggest that about 1.7 million Chinese children under the age of 18 are diabetic and about 27.7 million are pre-diabetic. The high rates of pre-diabetes as measured by HbA1c in youth (15%) and adults (30%) portend a public health problem of massive proportion. Furthermore, over one-third of children under the age of 18 had high levels of at least one cardiometabolic risk factor and rates increase to 85% in those ≥40 years. Of additional concern is the high prevalence of cardiometabolic risk in less urban areas and across all income levels.
Of additional concern is the observation that diabetes and inflammation rates are higher in the Chinese pediatric population than in the US pediatric population and in other Asian countries. In contrast, diabetes rates among adults were comparable to those in the US and other Asian countries. Furthermore, obesity and pre-diabetes measured by HbA1c were twice as high in 7–11 versus 12–18 year olds, it is possible that the younger cohorts who are growing up in a very different environmental setting than the adolescents and adults grew up in and are thus experiencing more dramatic health consequences than the adolescent Chinese population. Coupled with the higher BMI values at the upper end of the distribution in Chinese youth, relative to that in the US, UK and Australia1, the future health of the current pediatric generation of Chinese youth is concerning, particularly given the higher cardiometabolic risk at lower BMI in Asians 29–31.
We also observed age-related increases in cardiometabolic risk across all markers (with the exception of low HDL in males), paralleling the increase in overweight in this population. Diabetes, hypertension and inflammation were higher at older ages, despite a drop off in overweight. More than three quarters of adults had at least one elevated cardiometabolic risk factor, which increased to over 90% in individuals aged 60 and older. A recent study of diabetes in a large national study of Chinese adults, ages 20 yr and older 5, used fasting and 2-hour glucose levels to define impaired fasting glucose and diabetes. We calculated age- and sex-adjusted estimates (not shown) in the same age range as Yang to provide comparison between our results and theirs. Our overall age and sex-adjusted estimate of diabetes for adults aged 20 and older based on HbA1c (6.7%) is lower than the Yang age- and sex-standardized estimate of 9.7% for the same age group. In comparison with Yang et al., we observed an age- and sex-adjusted estimate of pre-diabetes of 30%, whereas Yang et al., found 15.5%. Compared to the fasting glucose cutoff point of 100 mg/dl (5.6 mmol/l), the HbA1c cutoff point of 5.7% is less sensitive but more specific and has a higher positive predictive value to identify people at risk for later development of diabetes 32. Consistent with their study, we find similar age distributions and higher rates of diabetes in men compared to women. Our population study of cardiometabolic risk factors in Chinese adults is the most current data available.
In addition to age-related increases in risk and high rates of cardiometabolic risk in youth, we observed high prevalence of risk in less urban areas and in across all income levels, suggesting pervasive risk across all parts of China. This is of major concern as these risk factors were heretofore less common in rural China. In prior work, we have shown that the burden of overweight is shifting from the rich to the poor in China and other countries, and we have found comparatively rapid increases in obesity in rural and lower income populations 7. In addition, we have shown an increased burden of obesity among women, but an opposite trend in men 8. In the past in low in middle income countries, higher levels of chronic disease risk were associated with affluence. Our findings suggest that as cardiometabolic risk increases in China, even rural populations will be affected.
There are a few limitations to this analysis. This cross-sectional analysis does not examine temporal changes in cardiometabolc risk factors owing to the fact that biomarker data were only collected in the 2009 round of the CHNS. Despite the overall large sample size, some age and sex-specific subgroups are small, and thus may provide unstable estimates of prevalence for rarer outcomes. Our main objective was to determine the prevalence of a wide range of cardiometabolic risk factors by age and gender, and across urbanization and income. To provide comparability, we used conventional, anthropometric and cardiometabolic cutpoints. Nevertheless they may not represent true risk in this population if, for example, disease develops at lower BMI in Asian versus other populations, which is possible given higher visceral fat at the same level of BMI in Asians versus Europeans 9. We used the BMI cutoff points of 25 and 30 kg/m2 for adults and the IOTF 25 and 30 kg/m2 equivalent for youth to allow for comparison of youth and adults. We did not exclude obese individuals from this analysis as we were interested in estimating risk in the full Chinese population; nonetheless when we exclude the 369 obese individuals in the sample from the analysis we find very similar results that with rounding are almost identical. We are not able to distinguish type 1 from type 2 diabetes in our sample, although no children reported insulin injection use. Whereas it is possible that the comparatively higher rates of pre-diabetes as measured by HbA1c in children versus adolescents could relate to type 1 diabetes, the rate of type 1 diabetes in China is among the lowest in the world, with an estimate of 0.1 per 100,000/year 33. The differences in pre-diabetes and diabetes as measured by HbA1c versus fasting glucose in youth, could possibly relate to differential meaning of these measures in youth or transient increases in insulin resistance with puberty 34–37. While we include comparative data from several countries, we were limited in the availability of published data, particularly in South East and South Asian countries.
Important strengths of the study include its population based design, representation of a wide range of urban and rural communities across China, its inclusion of fasting blood data for children down to the age of 7 year, and the wide range of biomarkers collected. The data from this national study suggests a high prevalence of overweight and elevated cardiometabolic risk factors in China that is higher across age groups and is evident even in the pediatric population. Further, rates were markedly high even in less urban and lower income groups within China. Our HbA1c results suggest a very high burden of chronic disease risk starting at a young age, with 1.7 million Chinese children 7–18 having diabetes (HbA1c), another 27.7 million considered pre-diabetic. For adults, the observed findings that suggest 82.1 million adults have diabetes and 334 million are pre-diabetic, are higher than that of Yang 5. Further we provide evidence that this is just the beginning of high prevalence of elevated cardiometabolic risk factors, given the increase in overweight with age. Elsewhere we have discussed some of the economic burden of this coming crisis 6. The observed findings foreshadow substantial and increasing chronic disease burden in China over the next several decades.
Figure 3.
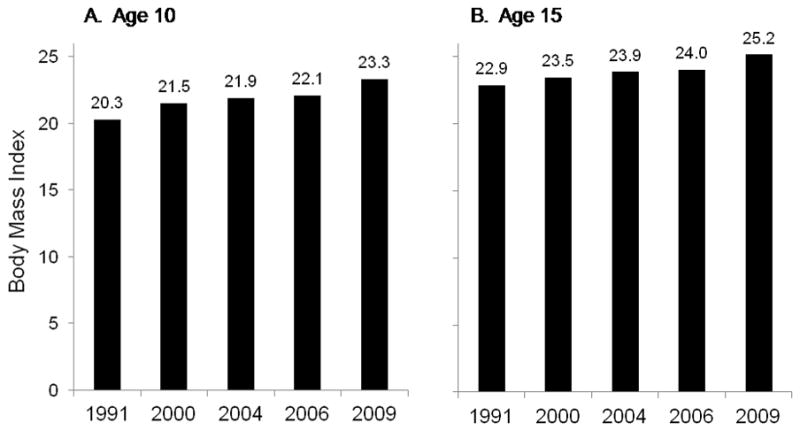
Mean BMI value associated with the population-specific 95th percentile in 10 and 15 year olds from 1991 to 2009
Based on quantile regression models of BMI data against age and age squared
Table 3.
Association of urbanization (first panel) and income (second panel) with cardiometabolic risk in younger and older adults.
| Prevalence Odds ratios from logistic regression models, stratified by age | |||||
|---|---|---|---|---|---|
| Urbanization | Males | Females | |||
| Age 18–40 years n=1,014 |
Age ≥40 years n=2,970 |
Age 18–40 years n=1,068 |
Age ≥40 years n=3,406 |
||
| Overweight | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.16 (0.82, 1.64) | 1.70 (1.39, 2.08) | 0.84 (0.58, 1.24) | 1.23 (1.03, 1.47) | |
| Hi | 1.22 (0.86, 1.73) | 1.86 (1.53, 2.27) | 0.67 (0.45, 1.00) | 1.20 (1.01, 1.43) | |
| IFG | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.84 (0.51, 1.38) | 1.45 (1.16, 1.82) | 1.31 (0.75, 2.30) | 1.55 (1.24, 1.93) | |
| Hi | 1.17 (0.73, 1.87) | 1.72 (1.38, 2.14) | 1.84 (1.08, 3.15) | 1.88 (1.52, 2.33) | |
| Diabetic | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.48 (0.62, 3.51) | 1.42 (1.03, 1.96) | 0.97 (0.14, 6.92) | 1.50 (1.06, 2.10) | |
| Hi | 1.53 (0.64, 3.62) | 1.75 (1.28, 2.38) | 2.41 (0.46, 12.50) | 2.01 (1.45, 2.78) | |
| Impaired A1c | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.83 (0.57, 1.20) | 0.89 (0.73, 1.08) | 0.80 (0.54, 1.18) | 0.97 (0.81, 1.16) | |
| Hi | 0.75 (0.51, 1.10) | 0.87 (0.72, 1.05) | 0.62 (0.41, 0.93) | 0.96 (0.80, 1.15) | |
| High A1c | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 2.69 (0.95, 7.65)* | 1.35 (0.98, 1.86) | 2.92 (0.30, 28.26)* | 1.86 (1.37, 2.53) | |
| Hi | 2.12 (0.72, 6.27)* | 1.77 (1.31, 2.39) | 6.80 (0.83, 55.59)* | 2.01 (1.49, 2.71) | |
| High TG | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.36 (0.98, 1.88) | 1.43 (1.18, 1.73) | 0.75 (0.50, 1.12) | 1.12 (0.94, 1.34) | |
| Hi | 1.96 (1.42, 2.70) | 1.89 (1.57, 2.27) | 0.64 (0.42, 0.97) | 1.24 (1.04, 1.48) | |
| High LDL | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.71 (1.16, 2.53) | 2.07 (1.70, 2.54) | 1.16 (0.74, 1.83) | 1.47 (1.23, 1.75) | |
| Hi | 1.99 (1.35, 2.93) | 1.82 (1.49, 2.23) | 1.38 (0.89, 2.15) | 1.61 (1.36, 1.91) | |
| Low HDL | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.24 (0.85, 1.81) | 1.19 (0.92, 1.56) | 0.67 (0.49, 0.92) | 0.89 (0.74, 1.06) | |
| Hi | 1.34 (0.92, 1.95) | 2.29 (1.80, 2.92) | 0.68 (0.49, 0.93) | 1.17 (0.99, 1.39) | |
| High TC | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.63 (1.12, 2.36) | 2.05 (1.69, 2.48) | 1.40 (0.90, 2.18) | 1.38 (1.17, 1.64) | |
| Hi | 1.86 (1.29, 2.68) | 1.79 (1.48, 2.16) | 1.41 (0.91, 2.19) | 1.45 (1.23, 1.72) | |
| Hypertension | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.78 (0.55, 1.10) | 1.20 (1.01, 1.44) | 1.02 (0.64, 1.65) | 1.14 (0.96, 1.35) | |
| Hi | 0.89 (0.63, 1.26) | 1.28 (1.07, 1.52) | 0.54 (0.31, 0.94) | 1.42 (1.21, 1.68) | |
| Pre-HTN | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.76 (0.50, 1.15) | 1.07 (0.89, 1.28) | 1.02 (0.54, 1.91) | 1.11 (0.92, 1.32) | |
| Hi | 0.63 (0.41, 0.98)* | 1.13 (0.94, 1.36) | 0.32 (0.13, 0.77) | 1.32 (1.11, 1.57) | |
| High CRP | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.72 (1.14, 2.59) | 1.05 (0.86, 1.28) | 1.03 (0.68, 1.56) | 1.13 (0.93, 1.37) | |
| Hi | 1.66 (1.10, 2.51) | 0.99 (0.81, 1.21) | 0.86 (0.56, 1.32) | 1.33 (1.11, 1.61) | |
| Income | Males | Females | |||
|---|---|---|---|---|---|
| Age 18–40 years | Age ≥40 years | Age 18–40 years | Age ≥40 years | ||
| Overweight | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.00 (0.69, 1.44) | 1.19 (0.97, 1.45) | 0.68 (0.45, 1.02) | 1.12 (0.94, 1.34) | |
| Hi | 1.07 (0.75, 1.52) | 1.59 (1.31, 1.93) | 0.76 (0.51, 1.12) | 1.29 (1.08, 1.54) | |
| IFG | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.98 (0.58, 1.66) | 1.16 (0.93, 1.44) | 0.88 (0.58, 1.35) | 0.88 (0.74, 1.06) | |
| Hi | 1.13 (0.69, 1.87) | 1.20 (0.96, 1.49) | 0.97 (0.65, 1.46) | 1.08 (0.91, 1.30) | |
| Diabetic | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.59 (0.24, 1.45) | 1.06 (0.77, 1.46) | 4.05 (0.47, 34.84) | 0.67 (0.49, 0.93) | |
| Hi | 0.86 (0.39, 1.91) | 1.51 (1.12, 2.04) | 2.16 (0.22, 20.84) | 0.88 (0.65, 1.17) | |
| Impaired A1c | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.75 (0.49, 1.12) | 0.95 (0.78, 1.14) | 0.85 (0.41, 1.18) | 1.08 (0.84, 1.36) | |
| Hi | 0.95 (0.65, 1.41) | 1.04 (0.86, 1.26) | 0.86 (0.42, 1.78) | 0.93 (0.73, 1.20) | |
| High A1c | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.48 (0.17, 1.37) | 0.94 (0.69, 1.29) | 1.34 (0.32, 5.65) | 0.73 (0.55, 0.97) | |
| Hi | 0.92 (0.39, 2.12) | 1.45 (1.08, 1.93) | 0.71 (0.14, 3.56) | 1.04 (0.80, 1.36) | |
| High TG | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.27 (0.91, 1.79) | 1.23 (1.02, 1.49) | 0.77 (0.51, 1.16) | 1.10 (0.92, 1.31) | |
| Hi | 1.04 (0.75, 1.46) | 1.33 (1.11, 1.61) | 0.64 (0.42, 0.98) | 1.16 (0.97, 1.38) | |
| High LDL | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.28 (0.85, 1.92) | 1.08 (0.89, 1.31) | 0.93 (0.59, 1.45) | 1.10 (0.93, 1.30) | |
| Hi | 1.34 (0.90, 1.98) | 1.13 (0.93, 1.37) | 0.76 (0.48, 1.19) | 0.88 (0.75, 1.05) | |
| Low HDL | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.14 (0.76, 1.71) | 1.21 (0.95, 1.53) | 0.77 (0.55, 1.07) | 1.01 (0.85, 1.20) | |
| Hi | 1.12 (0.75, 1.65) | 1.17 (0.92, 1.49) | 0.85 (0.62, 1.17) | 1.08 (0.91, 1.29) | |
| High TC | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 1.33 (0.90, 1.96) | 0.99 (0.82, 1.19) | 1.00 (0.65, 1.53) | 1.13 (0.96, 1.33) | |
| Hi | 1.28 (0.88, 1.87) | 1.11 (0.92, 1.33) | 0.65 (0.41, 1.01) | 0.92 (0.77, 1.08) | |
| Hypertension | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.81 (0.57, 1.16) | 0.92 (0.77, 1.10) | 1.07 (0.64, 1.79) | 0.85 (0.73, 1.01) | |
| Hi | 0.62 (0.44, 0.89) | 0.89 (0.74, 1.06) | 0.80 (0.47, 1.37) | 0.79 (0.67, 0.93) | |
| Pre-HTN | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.83 (0.53, 1.28) | 0.95 (0.79, 1.14) | 0.97 (0.47, 2.01) | 0.87 (0.73, 1.03) | |
| Hi | 0.65 (0.42, 1.02) | 0.99 (0.82, 1.19) | 0.81 (0.39, 1.69) | 0.77 (0.64, 0.91) | |
| High CRP | Low | 1.00 | 1.00 | 1.00 | 1.00 |
| Med | 0.92 (0.60, 1.41) | 0.91 (0.74, 1.11) | 0.79 (0.51, 1.20) | 0.96 (0.80, 1.15) | |
| Hi | 1.11 (0.74, 1.67) | 0.85 (0.69, 1.03) | 0.62 (0.40, 0.95) | 1.03 (0.86, 1.24) | |
NOTE: urbanization and income categorized by tertiles, with approximately 300 per tertile in the 18–40 year age group and approximately 1,000 per tertile in the ≥40 year age group
Unstable estimates due to n<10 in the reference category
Sex- and age (18–40 years; Age ≥40 years)-stratified crude logistic regression models including 3 levels each for urbanicity and income
Acknowledgments
We thank Ms. Frances Dancy, BS, UNC Carolina Population Center for her helpful administrative assistance, Jennifer Poti, BS for helpful research assistance, Jim Terry, AB, Phil Bardsley, PhD, Donna Miles, PhD, and Dan Blanchette, BA for programming and technical support. None of the individuals acknowledged received compensation for any role in the study.
Funding
NIH (R01-HD30880, DK056350, R24 HD050924, R01-HD38700, R01-HL108427, and R21DK089306) with added financial support from the Chinese Center for Disease Control and Prevention National Institute of Nutrition and Food Safety and the China-Japan Friendship Hospital, Ministry of Health of China.
Footnotes
Conflicts of Interest: No author has a conflict of interest
P.G.L. and L.S.A. contributed to study design, P.G.L., B.M.P. and L.S.A. contributed to data analysis, and all authors contributed to writing of the manuscript. P.G.L. and L.S.A. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References Cited
- 1.Popkin BM. Recent dynamics suggest selected countries catching up to US obesity. Am J Clin Nutr. 2010;91:284S–88S. doi: 10.3945/ajcn.2009.28473C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CM, Huxley RR, Lam TH, et al. Prevalence of diabetes mellitus and population attributable fractions for coronary heart disease and stroke mortality in the WHO South-East Asia and Western Pacific regions. Asia Pac J Clin Nutr. 2007;16:187–92. [PubMed] [Google Scholar]
- 4.Gu D, Gupta A, Muntner P, et al. Prevalence of cardiovascular disease risk factor clustering among the adult population of China: results from the International Collaborative Study of Cardiovascular Disease in Asia (InterAsia) Circulation. 2005;112:658–65. doi: 10.1161/CIRCULATIONAHA.104.515072. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 6.Popkin BM. Will China’s nutrition transition overwhelm its health care system and slow economic growth? Health Aff (Millwood) 2008;27:1064–76. doi: 10.1377/hlthaff.27.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dearth-Wesley T, Wang H, Popkin BM. Obesity dynamics in China: The poor are catching up. Eur J Clin Nutr. 2007;18:1–6. [Google Scholar]
- 8.Jones-Smith JC, Gordon-Larsen P, Siddiqi A, Popkin BM. Emerging disparities in overweight by educational attainment in Chinese adults (1989–2006) Int J Obes. 2011 doi: 10.1038/ijo.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 10.Chan JCN, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 11.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 12.Popkin BM, Du S, Zhai F, Zhang B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol. 2009;39:1435–1440. doi: 10.1093/ije/dyp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Diabetes Federation. 2006 The IDF consensus worldwide definition of the metabolic syndrome http://www.idf.org/webdata/docs/MetS_def_update2006.pdf.
- 14.National High Blood Pressure Education Program. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 15.World Health Organisation International Association for the Study of Obesity International Obesity TaskForce. The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications; Sydney: 2000. pp. 1–56. [Google Scholar]
- 16.Cole TJ, Bellizz MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Br Med J. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koenker R, Hallock, Kevin F. Quantile Regression. J Econ Perspect. 2001;15:143–156. [Google Scholar]
- 18.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 19.Jones-Smith JC, Popkin BM. Understanding community context and adult health changes in China: development of an urbanicity scale. Soc Sci Med. 2011;71:1436–1446. doi: 10.1016/j.socscimed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinson ML, Teitler JO, Reichman NE. Health across the life span in the United States and England. Am J Epidemiol. 2011;173:858–865. doi: 10.1093/aje/kwq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in U.S. children aged 3–16 years. Am J Prev Med. 2010;39:314–320. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch Pediatr Adolesc Med. 2009;163:371–377. doi: 10.1001/archpediatrics.2009.3. [DOI] [PubMed] [Google Scholar]
- 23.SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among us youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 24.Kitagawa T, Owada M, Urakami T, Tajima N. Epidemiology of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in Japanese children. Diabetes Res Clin Practice Suppl. 1994;24:S7-13.R. doi: 10.1016/0168-8227(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 25.Wei JN, Chuang LM, Lin CC, Chiang CC, Lin RS, Sung FC. Childhood diabetes identified in mass urine screening program in Taiwan, 1993–1999. Diabetes Res Clin Practice. 2003;59:201–206. doi: 10.1016/s0168-8227(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Wang K, Li T, et al. Childhood diabetes in China: enormous variation by place and ethnic group. Diabetes Care. 1998;21:525–529. doi: 10.2337/diacare.21.4.525. [DOI] [PubMed] [Google Scholar]
- 27.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Bureau of Statistics of China. Beijing: China Statistics Press. 2010. China Statistical Yearbook 2010. [Google Scholar]
- 29.Nguyen TT, Adair LS, Suchindran CM, He K, Popkin BM. The association between body mass index and hypertension is different between East and Southeast Asians. Am J Clin Nutr. 2009;89:1905–1912. doi: 10.3945/ajcn.2008.26809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen T, Adair LS, Stevens J, Popkin BM. Prediction of hypertension by different anthropometric indices in adults: the change in estimate approach. Public Health Nutr. 2010;13:639–646. doi: 10.1017/S1368980009991479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care Suppl. 2010;33:62–69. [Google Scholar]
- 33.The DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabetic Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 34.Saaddine JB, Fagot-Campagna A, Rolka D, et al. Distribution of HbA(1c) levels for children and young adults in the U.S: Third National Health and Nutrition Examination Survey. Diabetes Care. 2002;25:1326–1330. doi: 10.2337/diacare.25.8.1326. [DOI] [PubMed] [Google Scholar]
- 35.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 36.Eldeirawi K, RBL Predictors of hemoglobin A1c in a national sample of nondiabetic children: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2003;157:624–632. doi: 10.1093/aje/kwg023. [DOI] [PubMed] [Google Scholar]
- 37.Shultis WA, Leary SD, Ness AR, et al. Haemoglobin A1c is not a surrogate for glucose and insulin measures for investigating the early life and childhood determinants of insulin resistance and Type 2 diabetes in healthy children. An analysis from the Avon Longitudinal Study of Parents and Children (ALSPAC) Diabetic Med. 2006;23:1357–1363. doi: 10.1111/j.1464-5491.2006.01990.x. [DOI] [PubMed] [Google Scholar]
- 38.Wei J-N, Sung F-C, Lin C-C, Lin R-S, Chiang C-C, Chuang L-M. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA. 2003;290:1345–1350. doi: 10.1001/jama.290.10.1345. [DOI] [PubMed] [Google Scholar]
- 39.Chan JCN, Malik V, Jia W, et al. Diabetes in Asia. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 40.Ministry of Health. WHO Global Infobase: National Health Survey 2004. 2005. Singapore: World Health Organization; 2005. Web site. http://www.who.int/infobase/ [Google Scholar]
- 41.Mustafa N, Kamarudin NA, Ismail AA, et al. Prevalence of abnormal glucose tolerance and risk factors in urban and rural Malaysia. Diabetes Care. 2011;34:1362–1364. doi: 10.2337/dc11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]