Abstract
Sickle cell anemia (SCA) associated cerebrovascular disease includes vascular remodeling, abnormal cerebral blood flow (CBF) and infarction. We studied the relationships between plasma brain derived neurotropic factor (BDNF), platelet derived growth factors (PDGF-AA and -AB/BB) and high trans-cranial Doppler (TCD) velocity, an indication of CBF velocity. Baseline plasma samples from 39 children (19 SCA with abnormal/high TCD [SATCD], 13 SCA with normal TCD [SNTCD] and 7 healthy non-SCA), were assayed for BDNF, PDGF-AA and – AB/BB plus 11 other cytokines. The sensitivity, specificity and usefulness of these biomarkers for prediction of stroke incidence was investigated. All subject groups were of similar age and gender distribution. Mean BDNF was significantly higher among SATCD than SNTCD (p=0.004) as was mean PDGF-AA (p=0.001). Similarly, mean PDGF-AA was higher among SCA subjects who developed stroke than for those who did not (p=0.012). Elevated BDNF and PDGF-AA were both associated with severity of anemia. Elevated BDNF and PDGF-AA were good predictors of the presence of abnormally high CBF velocity, and PDGF-AA predicted stroke development. Stroke incidence and high TCD velocity were associated with elevated BDNF and PDGF-AA. These findings suggest a role for BDNF and PDGF-AA in the patho-physiological mechanism of cerebrovascular disease in SCA.
Keywords: Sickle cell anemia, Cerebral ischemia, Stroke, Biomarkers, Trans-cranial Doppler
1. Introduction
Sickle cell anemia (SCA) is a genetic disease resulting from a point mutation in the DNA, leading to the substitution of valine for glutamic acid at the 6th position of the β-globin chain and consequent polymerization of the hemoglobin molecule (during periods of hypoxia, dehydration or acidosis), forming hemoglobin polymers which distort the shape of the red blood cell [1]. The most common complication of SCA is repeated vaso-occlusion resulting in acute pain and end organ ischemic injury such as stroke [2]. The incidence of ischemic stroke in individuals with SCA is four times higher in children 2 – 15 years old, than in any other age group [3,4,7]. Evidence from angiography and autopsy has documented several pathological changes in the cerebral vasculature including stenosis and fibrosis [3,4], exuberant intimal growth or endothelial proliferation [5], and formation of sickle red cell sludge in small blood vessels [5,6]. Children with SCA may also develop silent cerebral infarcts, without overt stroke [8], which have been associated with cognitive decline and poor school performance [9,10].
The progressive nature of the pathologic mechanisms leading to disturbed cerebral blood flow (CBF) and infarction among children with SCA makes it possible to develop screening tests [11] for predicting the risk of developing stroke. A recent study suggested the use of a combination of magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) to predict stroke risk, because the vascular changes that could lead to the development of stroke were detected in the cohort of children that was studied [12]. However, the nature and cost of these procedures lessen their usefulness for predictive screening. Adams et al documented that a high TCD velocity (a non-invasive marker of cerebral blood flow velocity) is predictive of stroke risk in children with SCA and that chronic red cell transfusion is effective in preventing stroke onset in high risk subjects [13]. Despite the ability to identify children with SCA at risk for stroke and offer effective preventive therapy, our understanding of the patho-physiological mechanisms leading to cerebrovascular disease in SCA is limited. We proposed to study the relationship between plasma levels of biomarkers related to vascular remodeling (platelet derived growth factor) and cerebral ischemia/neuronal survival adaptation (brain derived neurotropic factor) with high cerebral blood flow velocity measured by TCD and the risk of developing stroke in children with SCA. Our group has previously shown that moderately elevated interleukin-1β (IL-1β) level is associated with decreased risk of developing stroke in children with SCA [14].
Brain derived neurotropic factor (BDNF) and platelet derived growth factor (PDGF) types AA and AB/BB are biomarkers with anti-apoptotic activity and involved in the cell survival pathway [15,16]. Brain derived neurotropic factor is associated with neuronal survival adaptation and response to ischemia [17,18] and PDGF modulates endothelial and smooth muscle cell proliferation [15]. Brain derived neurotropic factor is a member of the nerve growth factor family of proteins and a ligand for the neurotropic tyrosine kinase type 2 (NTRK2 or TRKB) receptor [19]. It has a role in the differentiation of several mammalian neuronal cell types [18,19] and has been localized in several regions of the brain, especially the hippocampus. In addition, BDNF messenger ribonucleic acid (mRNA) expression is increased during seizure [20], and it causes attenuation of induced seizure [21] and a decrease in cerebral infarct size when administered intravenously or directly into the ventricles [17,22]. To our knowledge, there has not been any study reporting changes in circulating BDNF or PDGF levels during periods of prolonged or chronic cerebral hypoxia and/or ischemia, as is observed in SCA subjects.
Platelet derived growth factor (PDGF) is an angiogenic factor first isolated from platelets [23], and later vascular and inflammatory cells [24,25]. It is an endothelial and smooth muscle cell mitogen [15] and various combinations of the PDGF-α and -β chains give rise to the isoforms AA, AB and BB [26]. Messenger ribonucleic acid expression for PDGF-AA and BB is elevated four-fold by reduction in arteriolar and capillary blood flow [27]. Children with sickle cell disease and elevated tricuspid regurgitant velocity (TRV), a non-invasive measure suggestive of pulmonary hypertension, had higher concentrations of PDGF-BB than those without elevated TRV or healthy controls [28]. Furthermore, PDGF-AA, AB and BB play vital roles in the regulation of vascular tone and attraction and maintenance of capillary mural cells [29].
Thus we posit that intimal proliferation will be associated with elevated plasma levels of PDGF, and that cerebral artery stenosis leading to reduction in cerebral blood flow, will increase BDNF due to cerebral ischemia. Our hypothesis is that, plasma BDNF and PDGF levels will be elevated in children with SCA and high TCD compared with those who have normal TCD or healthy controls. Furthermore, SCA subjects at risk for developing stroke will have higher plasma levels of these biomarkers compared with those having reduced risk for developing stroke.
2. Subjects, materials and methods
2.1. Study subjects and sample
This is a cross-sectional, nested prospective study design. Stored, frozen plasma samples were obtained from the Stroke Prevention in sickle cell anemia (STOP) study and from a study at Morehouse School of Medicine (MSM) investigating the effect of nutrition on inflammation in children with SCA (NUTSCD). The STOP study ran from 1995 – 1997. Subjects were age 2 – 16 years, with HbSS (SCA) or HbSβ°thalasssemia and no previous history of stroke, with mean velocities of cerebral blood flow of ≥200cm/s on at least two separate occasions, or a single measurement of ≥220cm/s classified as having abnormal or high trans-cranial Doppler (TCD) measurement. STOP study subjects were randomized to receive either standard care (n=67) or transfusion (n=63) and were followed with annual TCD measurements. The standard care arm showed a significantly higher incidence of stroke, enough to warrant the early termination of the trial. The full details of the STOP study has been described elsewhere [13]. The present study is ancillary to the STOP study and enabled us to use anonymized, stored plasma samples collected at study entry. The Institutional Review Boards of MSM, Georgia Health Science University and New England Research Institutes, granted approval for the use of the anonymized samples for this purpose.
The NUTSCD study is a pilot clinical trial that commenced at MSM in December of 2009 to study the benefits of providing additional calories to children with SCA. Institutional review boards of Children Healthcare of Atlanta (CHOA) and MSM approved the study. The subjects were children with SCA, aged 6 – 12 years who were not receiving hydroxyurea therapy or blood transfusion in the last 4 months, chronic oral corticosteroids or non-steroidal anti-inflammatory drug (NSAID) therapy and had no serious medical illness 2 weeks prior to enrollment. Healthy African American children ages 6 – 12 years without SCA were recruited as controls. The SCA subjects all had normal TCD measurements and were enrolled from CHOA's Hughes Spalding and Egleston campuses after signed informed consent. In total, 19 SCA baseline samples were obtained from the STOP study subjects who all had abnormal TCD velocity (SATCD) and from 20 subjects in the NUTSCD, including 13 SCA subjects with Normal TCD (SNTCD) and 7 healthy controls.
2.2. Sample processing and analysis
All samples were initially stored at −80°C and allowed to thaw slowly at room temperature. Before analysis samples and reagents were brought to room temperature. The samples were analyzed in duplicate using 25μl of plasma per well. Plasma levels of a combination of 11 pro- or anti-inflammatory cytokines and angiogenic markers (IL-1β, IL-1ra, IL-4, IL-6, IL-10, IL-13, IFN-γ TGF-α, TNF-α, VEGF and GMCSF) were measured undiluted, the same was done for BDNF, PDGF-AA, and PDGF-AB/BB, with overnight incubation. Assay was done with commercially available antibody immobilized beads (Millipore, Billerica, MA) and Bio-Rad Bioplex system powered by Luminex (Bio-Rad, Hercules, CA). Manufacturer's protocols for these analyses were closely followed with results obtained from interpolated 5-PL logistic curves generated using the kit manufacturer's standards.
2.3. Data analysis and presentation
Data analysis was done with input from all authors, using SPSS/PASW version 18 for Windows (IBM Corp. Amonk, NY), Microsoft office Excel (Microsoft Corp, Seattle, WA) and STATA 12 (STATA Corps, College Station, TX). Plasma levels of analytes are presented as mean±SD and also graphically in bar graphs. Bi-variate analysis was done using t-test to compare means for two groups while an analysis of variance (ANOVA) test with post-hoc analysis (Tamhane's T2 test) was used to compare means among the three groups. This was then followed by plotting receiver operator characteristic (ROC) curves for BDNF, PDGF-AA and PDGF-AB/BB, which were the analytes of interest and showed association with abnormal TCD velocity and/or incidence of stroke. The mean age, gender and some physical and hematological characteristics of the subjects are presented using tables. Because the plasma levels of the other 11 cytokines measured were similar in all groups and showed no significant relationship with high TCD or incidence of stroke, they were not reported in the results.
3. Results
3.1. Physical and hematological characteristics of subjects
Age, gender and other physical and hematological characteristics of the subjects are presented in tables 1 – 2. There was no significant difference in age and gender distribution among the groups, but as expected, the SCA subjects had a significantly lower mean body mass index (BMI) compared with the healthy controls (p<0.001). Similarly, SCA subjects had significantly higher mean white cell count, platelet count, reticulocytes percent and a significantly lower mean hemoglobin level than the healthy controls (p<0.001). Also, SATCD had significantly higher mean reticulocytes percent (p=0.009) and lower mean hemoglobin level than SNTCD. Subjects with SCA who developed stroke had significantly higher mean white blood cell counts compared with those who did not develop stroke (p=0.004).
Table 1.
Characteristic of subjects and their distribution based on the defined groups. There was no significant difference in age and gender distribution among groups. Except in height, SCA subjects were significantly different from controls (p<0.001). There was no significant difference between the SNTCD and SATCD except in reticulocytes percent (p=0.009) and hemoglobin level (p=0.004).
| Characteristics | Controls | SNTCD | SATCD |
|---|---|---|---|
| Number | 7 | 13 | 19 |
| Gender (m/f) a | 4/3 | 3/10 | 9/10 |
| Age (y) a | 9.6 ± 1.7 | 8.8 ± 2.3 | 8.1 ± 3.1 |
| Height (m) a | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.2 |
| Weight (kg) a | 40.4 ± 7.9 | 24.5 ± 4.5 | 24.5 ± 7.0 |
| BMI (kg/m2) a | 22.9 ± 6.8 | 14.8 ± 0.9 | 15.7 ± 1.5 |
| Platelet count (×1000/μL) a | 250 ± 39 | 452 ± 169 | 385 ± 67 |
| Reticulocytes percent (%) a | 1.0 ± 0.5 | 9.2 ± 3.0 | 12.9 ± 3.5 |
| White Blood Cells count (×1000/μL) a | 5.1 ± 1.8 | 10.8 ± 2.6 | 11.0 ± 2.6 |
| Hemoglobin level (g/dL) a | 12.4 ± 0.6 | 8.6 ± 0.8 | 7.5 ± 0.8 |
Values are means±SD. SNTCD = sickle cell anemia subject with normal TCD, SATCD = Sickle cell anemia subject with abnormal or high TCD, TCD = Transcranial Doppler
Table 2.
Shows physical and hematologic characteristics of SCA subjects who developed stroke and those did not. Except in levels of white blood cells count (p= 0.040), there was no significant difference between the groups based on all other characteristics.
| Characteristics | SCA with Stroke | SCA without Stroke |
|---|---|---|
| Number | 7 | 25 |
| Gender (m/f) | 4/3 | 8/17 |
| Age (y) a | 7.9 ± 3.2 | 8.5 ± 2.7 |
| Height (m) a | 1.2 ± 0.2 | 1.3 ± 0.1 |
| Weight (kg) a | 24.6 ± 6.5 | 24.5 ± 6.1 |
| BMI (kg/m2) a | 15.9 ± 1.8 | 15.1 ± 1.2 |
| Platelet count (×1000/μL) a | 396 ± 49 | 417 ± 135 |
| Reticulocytes count (%) a | 11.5 ± 2.2 | 11.3 ± 4.1 |
| White Blood Cells count (×1000/μL) a | 12.7 ± 2.9 | 10.4 ± 2.3 |
| Hemoglobin level (g/dL) a | 7.6 ± 0.7 | 8.0 ± 1.0 |
Values are means±SD.
3.2. All SCA subjects vs. controls
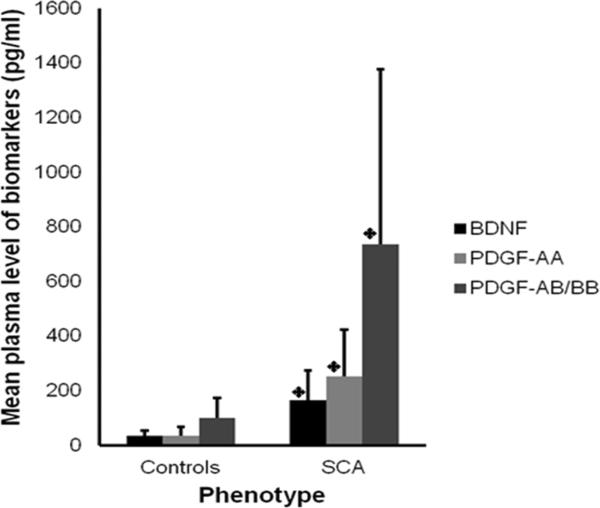
The mean concentrations of BDNF (163.9±110.8 vs. 34.1±20.3 pg/ml, p<0.001), PDGF-AA (251.2±174.6 vs. 33.8±32.4 pg/ml, p<0.001) and PDGF-AB/BB (736.8±649.3 vs. 101.0±72.2 pg/ml, p<0.001) were significantly higher among the subjects with SCA, compared with controls (Figure 1). There was no significant difference in the levels of the other cytokines and angiogenic factors between the groups.
Figure 1. Mean level of each biomarker was higher among all children with sickle cell anemia than healthy controls.
A t-test showed that mean levels of BDNF, PDGF-AA and PDGF-AB/BB were significantly higher among children with SCA than controls p < 0.001. The error bars indicate the standard deviation (SD).
3.3. SCA with abnormal TCD vs. SCA with normal TCD vs. controls
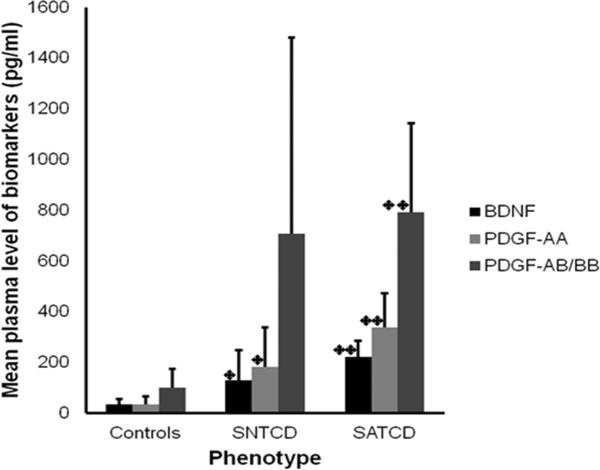
Mean plasma level of BDNF (213.6±91.4pg/ml) for the SATCD group was significantly higher than for the SNTCD group (91.2±97.7pg/ml, p=0.004) or controls (34.1±20.3 pg/ml, p<0.001).The SNTCD group also had significantly higher plasma BDNF than the controls (p=0.006). Similarly, plasma PDGF-AA was significantly higher among SATCD (346.7±152.3pg/ml, p<0.001) and SNTCD (111.5±91.6pg/ml, p=0.041) compared with controls (33.7±32.4pg/ml) and was also significantly higher for the SATCD group compared with SNTCD (346.7±152.3 vs. 111.5±91.6 pg/ml, p<0.001). Plasma PDGF-AB/BB was significantly higher among SATCD (790.6±350.9pg/ml, p<0.001) compared with controls (101.0±72.3pg/ml), but there were no significant differences in the levels of this biomarker between SATCD and SNTCD, or SNTCD and controls (Figure 2).
Figure 2. Levels of BDNF, PDGF AA and AB/BB are higher in children with sickle cell anemia (SCA) and with SCA and high TCD velocity.
The figure presents mean plasma brain derive neurotropic factor (BDNF), platelet derived growth factor-AA (PDGF-AA) and platelet derived growth factor-AB/BB (PDGF-AB/BB) levels in children who are healthy controls (Controls), have SCA with normal TCD (SNTCD) and SCA with abnormal TCD (SATCD). TCD = trans-cranial Doppler velocity. Values are mean ± SD and an ANOVA test with post-hoc analysis shows that the mean plasma levels of the biomarkers were significantly higher among SATCDs than either SNTCD (**p<0.01) or Controls (**p<0.01). Similarly, except for PDGF-AB/BB, the mean plasma levels of the biomarkers were significantly higher among SNTCD than controls (*p<0.001)
3.4. Bi-variate correlation analysis
Bi-variate correlation analysis showed positive correlations between plasma PDGF-AA and high TCD velocity (r=0.5, p=0.032) as well as transforming growth factor alpha (TGF-α) levels (r=0.7, p<0.001), but a negative correlation with hemoglobin (Hb) levels (r=−0.4, p=0.028) among SCA subjects. Similarly, plasma BDNF levels correlates positively with PDGF-AA (r=0.8, p<0.001), interleukin-10 (r=0.4, p=0.032) and TGF-α (r=0.6, p=0.001), but showed a trend towards negative correlation with hemoglobin (r =−0.3, p=0.077). Only TGF-α levels were positively correlated with plasma PDGF-AB/BB (r=0.4, p=0.012) among all the SCA subjects. Among only SCA subjects with high TCD velocity, plasma BDNF correlated positively with PDGF-AA (r=0.5, p=0.038) and interleukin-10 (r=0.5, p=0.019). Similarly, plasma PDGF-AA levels were positively correlated with high TCD velocity (r=0.5, p=0.035), but negatively with reticulocyte percentage (r=−0.6, p=0.014). There was no significant relationship between plasma levels of these biomarkers and white blood cell count, platelet count or hemoglobin concentration in this group.
3.5. SCA with stroke vs. SCA without stroke
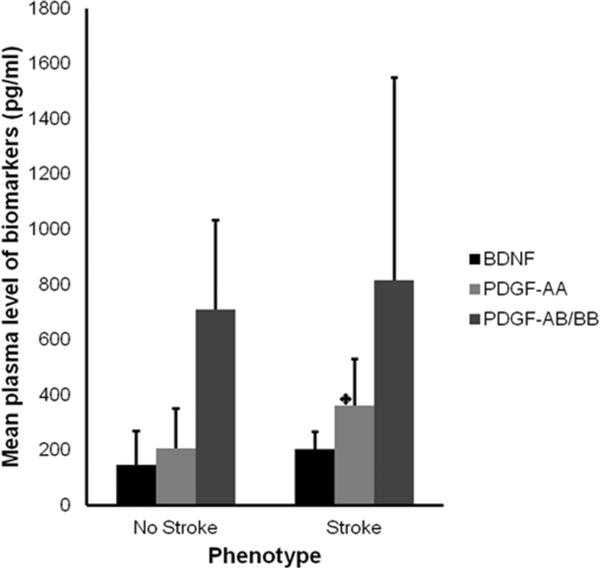
Seven of the SCA subjects with high TCD developed stroke and we compared mean levels of these biomarkers in SCA subjects who developed stroke with those who did not (Figure 3). Only mean PDGF-AA level was significantly higher among those who developed stroke compared with those who did not (399.5±143.4pg/ml vs. 209.6±161.3pg/ml, p=0.012).
Figure 3. Comparison of mean plasma levels of BDNF, PDGF-AA and -AB/BB in SCA subjects who developed stroke with those who did not.
Independent sample t-test shows a statistically significant difference in PDGF-AA levels among SCA subjects who developed stroke compared with those who did not (*p=0.012). Difference in the levels of the other two biomarkers were not significant. The SCA subjects with stroke all had abnormal TCD, while those who did not develop stroke included those with normal and abnormal TCD velocities. No Stroke = sickle cell anemia subjects who did not develop stroke, Stroke = sickle cell anemia subjects who subsequently developed stroke
3.6. Predictive value of biomarkers
Using logistic regression modeling corrected for age and gender to obtain odds ratios (OR), we investigated the usefulness of the 3 biomarkers for predicting abnormally high TCD velocity in the SCA subjects. Elevated BDNF (OR=1.022/unit rise, p=0.004) and PDGF-AA (OR=1.023/unit rise, p=0.014) levels were significantly associated with increased odds of having abnormally high CBF velocity. The model also showed that elevated PDGF-AA levels were associated with a significant risk of developing stroke (OR= 1.014/unit rise, p=0.044). Elevated BDNF levels were associated with a slight but statistically non-significant risk of developing stroke (OR= 1.006/unit rise).
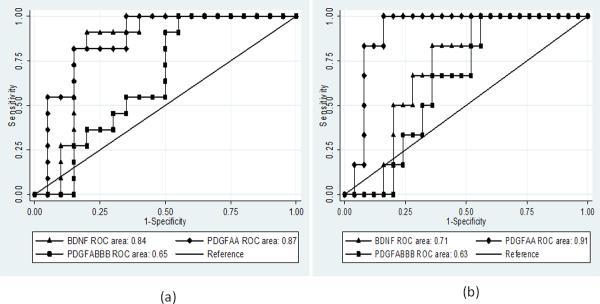
Receiver Operator Characteristic (ROC) curves were plotted to explore the usefulness of BDNF, PDGF-AA and PDGF-AB/BB as potential biomarkers for predicting the presence of high TCD velocity and risk of subsequent stroke. Figure 4a – b provides graphical representation of the ROC curve analyses. The usefulness of a test or biomarker is determined by the Area Under the Curve (AUC), which is statistically significant when it is between 0.8 and 1.0, with 1.0 signifying an ideal test or biomarker. The ROC plot indicated that both BDNF and PDGF-AA were good biomarkers of abnormally high cerebral blood flow (high TCD) velocity, with AUCs of approximately 0.84 and 0.87 respectively. Furthermore, sensitivity and specificity co-ordinates plots of the ROC curves indicates that a plasma BDNF level of ≥135.3pg/ml was 83% sensitive and specific in detecting abnormally high TCD velocity, whereas a PDGF-AA level of ≥196.9pg/ml was 94% sensitive and 77% specific in detecting abnormally high TCD velocity. The PDGF-AB/BB level was neither reliably sensitive or specific (at 61% and 69% respectively).
Figure 4. Receiver Operator Characteristic (ROC) curves for (a) high TCD velocity and (b) stroke development on plasma levels BDNF, PDGF-AA and -AB/BB.
The Area Under the Curve (AUC) was 0.84, and 0.87 for BDNF and PDGF-AA respectively in Fig. 4a. While the AUC was 0.71, and 0.91 respectively for BDNF and PDGF-AA in Fig. 4b. It shows that although BDNF and PDGF AA are sensitive in detecting presence of abnormally high cerebral blood flow velocity, only PDGF AA was a useful biomarker of risk for stroke development in SCA subjects (AUC = 0.91). The AUC for PDGF-AB/BB was not significant in both situation. TCD = trans-cranial Doppler.
In considering stroke risk, only PDGF-AA met the minimum AUC threshold for a useful biomarker, with an AUC of 0.91, while the AUC for BDNF was only 0.71. In addition, we were able to determine that a plasma PDGF-AA level of ≥306.8pg/ml was 100% sensitive and 84% specific for predicting stroke development among the SCA subjects. However, neither BDNF (67 and 64%) or PDGF-AB/BB (67 and 64%) levels were reliably sensitive or specific for predicting risk for developing stroke among SCA subjects.
4. Discussion
The STOP study demonstrated the efficacy of chronic blood transfusions in stroke prevention for children with SCA. However, a lifetime of transfusions is recommended and this therapy requires multiple hospital visits and iron overload is a major side effect [30]. Our results demonstrate a relationship between elevated plasma levels of BDNF, PDGF-AA and PDGF-AB/BB and abnormally high TCD velocity and/or stroke development in children with SCA. Because elevated TCD velocity is a marker of abnormally high cerebral blood flow [13,31], these biomarker levels might be a reflection of dysregulation in cerebral perfusion.
Based on evidence from the literature, we believe that elevation in BDNF level seen in children with SCA is an adaptive/protective response to reduce cell death during periods of cerebral ischemia caused by abnormal CBF [17,32–33], with a goal of restoring cerebral perfusion for instance via stimulating compensatory mechanisms such as increasing prostacyclin [34]. The strong correlation between BDNF and interleukin-10 (IL-10) levels demonstrated in this study is consistent with reports of an association between elevated levels of BDNF and elevation in the plasma levels of anti-inflammatory biomarkers [35,36]. Our data was not consistent with those from other studies that have reported an inverse relationship between plasma BDNF levels and those of pro-inflammatory cytokines, but it supported reports of a direct relationship between BDNF and anti-inflammatory cytokine [21,35,37,38]. This possibly indicates that the higher mean BDNF levels observed among SATCD and those SCA subjects who developed stroke are an indication that the reported cerebral ischemia associated with abnormally high CBF velocity has an even greater role in up-regulating BDNF than inflammation which in children with SCA is a subclinical and chronic process [39]. This might also explain why there were no significant difference in the plasma levels of inflammatory markers in our study despite the marked difference in the levels of BDNF between the groups, especially between SATCD and SNTCD.
Our study is the first to report elevated PDGF levels among SCA subjects with elevated TCD and/or stroke. Increased angiogenesis, endothelial activation and proliferation along with global activation of angiogenic factors are well documented for SCA subjects [40] and PDGF reportedly has a role in these processes [26,41], which are associated with arterial remodeling. Sickle cell sludge formation leading to decreased effective cerebral blood flow could partly be responsible for the elevated PDGF levels observed among SCA subjects with high TCD and/or stroke in our study, since other studies show that reduced carotid arteriolar and capillary blood flow induce PDGF ligand expression [27]. Taken together with the results of Niu et al [28] which demonstrate an association between PDGF-BB and elevated tricuspid regurgitant velocity, our data suggest that elevated PDGF is a biomarker of arterial remodeling and could play an important role in cerebrovascular pathology associated with development of stroke in children with SCA.
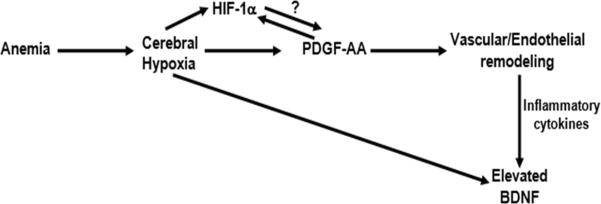
Our data shows a negative correlation between anemia and plasma PDGF-AA level in SCA patients. This is in support of reports that anemia in SCA patients may trigger PDGF-mediated cerebral arterial remodeling by causing disturbed and hyperemic cerebral blood flow [42], reduced cerebral oxygenation [43] and cerebral hypoxia, in addition to fluid shear stress via turbulent blood flow [44]. Cerebral hypoxia has been linked to elevated PDGF possibly via hypoxia inducible factor- 1alpha (HIF-1α) signaling [45]. This mechanism might partly be responsible for the elevated PDGF and specifically PDGF-AA level observed among SCA subjects with high TCD velocity and/or developed stroke. The strong correlation between BDNF and the PDGF sub-types is a possible evidence of their collective regulation by reduced effective cerebral perfusion from abnormally high or dysregulated cerebral blood flow velocity, anemia and hypoxia. Thus we propose the model in figure 5.
Figure 5.
Proposed model for vascular remodeling leading to cerebrovascular disease in sickle cell anemia.
The results from the ROC curve plots and analyses suggest that early estimation of BDNF and PDGF-AA could be useful for detecting the presence abnormally high cerebral blood flow velocity, cerebral ischemia and cerebral vascular dysfunction in children with SCA. Furthermore, PDGF-AA levels could be useful for predicting stroke risk in these children. Therefore, in combination with TCD screening, these biomarkers of arterial remodeling and cerebral ischemia may strengthen stroke risk prediction in SCA, or they may prove to be useful for stroke risk screening in resource-limited areas where TCD screening is not feasible. The clinical implication of our study is supported by our finding of a higher pre-transfusion PDGF-AA level (312pg/ml) for one of the three SCA subjects with high TCD and receiving transfusion who developed stroke. This level was almost as high as the mean levels for the other six SCA subjects with high TCD velocity who developed stroke on standard care (358±197pg/ml) and was only five units above the threshold of 306.8pg/ml established based on the ROC curves sensitivity and specificity coordinate plots. The two subjects who did not develop stroke while receiving transfusion had lower individual and mean pre-transfusion PDGF-AA levels (233±0.5pg/ml) than this one subject. Consequently, BDNF and/or PDGF-AA may be helpful in determining future risk for cerebrovascular events in children with silent cerebral infarcts and normal TCD. However, these relationships will need to be evaluated in future studies. These results show no significant relationship between platelet count and levels of either PDGF-AA or PDGF-AB/BB, indicating that platelets are unlikely to be the source of the observed elevated levels.
5. Summary/Conclusions
The conclusions of this study are limited by the small sample size and although preliminary, the strength of the associations between BDNF and PDGF-AA levels and abnormally high TCD velocity and stroke risk in children with SCA is note worthy. This study provides new important findings that BDNF, PDGF-AA and PDGF-AB/BB levels are elevated in children with SCA. BDNF and PDGF-AA levels are associated with abnormally high TCD, hence CBF velocity and development of stroke in SCA subjects. BDNF and PDGF-AA levels were associated with the severity of anemia. Brain derived neurotropic factor is likely to indicate the presence of cerebral ischemia and PDGF-AA may be a biomarker of cerebral artery remodeling. With further studies, these biomarkers may provide insights into the mechanisms leading to cerebral arterial changes and stroke in children with SCA.
Sickle cell anemia (SCA) subjects show higher plasma BDNF and PDGF levels than controls
Mean plasma BDNF and PDGF-AA levels were higher among SCA subjects with high TCD than other subjects
BDNF and PDGF-AA levels were associated with abnormally high cerebral blood flow velocity
Plasma PDGF-AA level was sensitive and specific for predicting stroke incidence
Acknowledgements
This work was support in part by grants from the National Institutes of Health NIH-RCMIRR033062, ACTSI NIH-NHLBI-R21HL92358, NIH-FIC-1R90-HG004151-01, NIH-ACTSI-UL-1RR025008 and the Cooperative Agreements with the National Heart, Lung, and Blood Institute (U10 HL 52193 and U10 HL 52016) supported this work.
Definition of abbreviations
- SNTCD
Sickle cell anemia subject with Normal TCD velocity
- SATCD
Sickle cell anemia subject with Abnormal/high TCD velocity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship contribution statement H.I.H., performed sample and data analysis and writing of draft manuscript; R.J.A and A.K., supplied the STOP samples, subject clinical information and critical review of manuscript drafts; J.M.H., supplied the NUTSCD samples, critical review of manuscript and data analysis; B.E.G., T.V.A., and J.K.S., provided methodological guidance and review of concept, manuscript and data analysis.
The authors declare that there is no conflict of interest, financial or otherwise.
References
- [1].Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- [2].Aster JC. Disease of organs systems: Red blood cells and bleeding disorders – sickle cell disease. In: Vinay K, Fausto N, Abbas AK, editors. Robins and Cotran's Pathologic basis of disease. 7 Ed Elselvier Saunders; Philadelphia: 2005. pp. 628–632. [Google Scholar]
- [3].Woods DH. Cerebrovascular complications of sickle cell anemia. Stroke. 1978;9:73–75. doi: 10.1161/01.str.9.1.73. [DOI] [PubMed] [Google Scholar]
- [4].Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- [5].Merkel K, Ginsberg P, Parker J, Post M. Cerebrovascular disease in sickle cell anemia: a clinical, pathological and radiological correlation. Stroke. 1978;9:45–52. doi: 10.1161/01.str.9.1.45. [DOI] [PubMed] [Google Scholar]
- [6].Bridgers WH. Cerebral vascular disease accompanying sickle cell anemia. Am J Pathol. 1939;15:353–365. [PMC free article] [PubMed] [Google Scholar]
- [7].Boros L, Thomas C, Weiner WJ. Large cerebral vessel disease in sickle cell anaemia. J Neurol, Neurosurg Psychiatry. 1976;39:1236–1239. doi: 10.1136/jnnp.39.12.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yalçin H, Melek I, Okuyucu E, et al. Sickle cell disease with regional silent cerebral infarction detected by SPECT. Clin Nucl Med. 2007;32:842–843. doi: 10.1097/RLU.0b013e318156bb11. [DOI] [PubMed] [Google Scholar]
- [9].DeBaun MR, Schatz J, Siegel MJ, et al. Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology. 1998;50:1678–1682. doi: 10.1212/wnl.50.6.1678. [DOI] [PubMed] [Google Scholar]
- [10].Schatz J, Brown RT, Pascual JM, Hsu L, DeBaun MR. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56:1109–1111. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- [11].Bruce N, Pope D, Stanistreet DL. Prevention strategies and evaluation of screening. In: Bruce N, Pope D, Stanistreet DL, editors. Quantitative methods for health research: A practical interactive guide to epidemiology and statistics. John Wiley & Sons; Chichester: 2008. pp. 433–470. [Google Scholar]
- [12].Wiznitzer M, Ruggieri PM, Masaryk T, Ross J, Modic MT, Berman B. Diagnosis of cerebrovascular disease in sickle cell anemia by magnetic resonance angiography. J Pediatr. 1990;117:551–555. doi: 10.1016/s0022-3476(05)80687-0. [DOI] [PubMed] [Google Scholar]
- [13].Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;399:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- [14].Asare K, Gee BE, Stiles JK, et al. Plasma interleukin-1β concentration is associated with stroke in sickle cell disease. Cytokine. 2010;49:39–44. doi: 10.1016/j.cyto.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heldin CH. Structural and functional studies on Platelet derived growth factor. EMBO J. 1992;11:4251–4259. doi: 10.1002/j.1460-2075.1992.tb05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lindholm D, Dechant G, Heisenberg C-P, Thoenen H. Brain derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur J Neurosci. 1993;5:1455–1464. doi: 10.1111/j.1460-9568.1993.tb00213.x. [DOI] [PubMed] [Google Scholar]
- [17].Schabitz W-R, Sommer C, Zoder W, et al. Intravenous brain derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 2000;31:2212–2217. doi: 10.1161/01.str.31.9.2212. [DOI] [PubMed] [Google Scholar]
- [18].Ernfors P, Ibáñez CF, Ebendal T, Olson L, Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci. 1990;87:5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hohn A, Leibrock J, Bailey K, Barde Y-A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- [20].Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- [21].Bovolenta R, Zucchini S, Paradiso B, et al. Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J Neuroinflammation. 2010;7:81. doi: 10.1186/1742-2094-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schabitz W-R, Schwab S, Spranger M, Hacke W. Intraventricular brain derived neurotrophic factor size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1997;17:500–506. doi: 10.1097/00004647-199705000-00003. [DOI] [PubMed] [Google Scholar]
- [23].Hart CE, Bailey M, Curtis DA, et al. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry. 1990;29:166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- [24].Inaba T, Shimano H, Gotoda T, et al. Expression of Platelet derived growth factor β receptor on human monocyte-derived macrophages and effects of Platelet derived growth factor BB dimer on the cellular function. J Biol Chem. 1993;268:24353–24360. [PubMed] [Google Scholar]
- [25].Tanizawa S, Ueda M, Van Der Loos CM, Van Der Wal AC, Becker AE. Expression of platelet derived growth factor B chain and β receptor in human coronary arteries after percutaneous transluminal coronary angioplasty: an immunohistochemical study. Heart. 1996;75:549–556. doi: 10.1136/hrt.75.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elaine WR. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [27].Mondy JS, Lindner V, Miyashiro JK, Berk BC, Dean RH, Geary RL. Platelet derived growth factor ligand and receptor expression in response to altered blood flow in vivo. Circ Res. 1997;81:320–327. doi: 10.1161/01.res.81.3.320. [DOI] [PubMed] [Google Scholar]
- [28].Niu X, Nouraie M, Campbell A, et al. Angiogenic and inflammatory markers of cardiopulmonary changes in children and adolescents with sickle cell disease. PLoS One. 2009;11:e7956. doi: 10.1371/journal.pone.0007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sachinidis A, Locher R, Hoppe J, Vetter W. The platelet derived growth factor isomers, PDGF-AA, PDGF-AB and PDGF-BB, induce contraction of vascular smooth muscle cells by different intracellular mechanisms. FEBS Lett. 1990;275:95–98. doi: 10.1016/0014-5793(90)81447-v. [DOI] [PubMed] [Google Scholar]
- [30].Raghupathy R, Manwani D, Little JA. Iron overload in Sickle Cell Disease. Adv Hematol. 2010:1–9. doi: 10.1155/2010/272940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stroobant N, Vingerhoets G. Transcranial Doppler ultrasonography monitoring of cerebral hemodynamics during performance of cognitive tasks: a review. Neuropsychology Review. 2000;10:213–231. doi: 10.1023/a:1026412811036. [DOI] [PubMed] [Google Scholar]
- [32].Ferrer I, Krupinski J, Goutan E, Martí E, Ambrosio S, Arenas E. Brain derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 2001;101:229–238. doi: 10.1007/s004010000268. [DOI] [PubMed] [Google Scholar]
- [33].Yamashita K, Wiessner C, Lindholm D, Thoenen H, Hossmann KA. Post-occlusion treatment with BDNF reduces infarct size in a model of permanent occlusion of the middle cerebral artery in rat. Metab Brain Dis. 1997;12:271–280. doi: 10.1007/BF02674671. [DOI] [PubMed] [Google Scholar]
- [34].Santhanam AVR, Smith LA, Katusic ZS. Brain derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke. 2010;41:350–356. doi: 10.1161/STROKEAHA.109.564492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kauer-Sant'Anna M, Kapczinski F, Andreazza AC, et al. Brain-derived neurotrophic factor and inflammatory markers in subjects with early vs. late-stage bipolar disorder. Int J Neuropsychopharmacol. 2009;12:447–458. doi: 10.1017/S1461145708009310. [DOI] [PubMed] [Google Scholar]
- [36].Bilbo SD, Barrientos RM, Eads AS, et al. Early-life infection leads to altered BDNF and IL-1β mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- [37].Makar TK, Trisler D, Sura KT, Sultana S, Patel N, Bever CT. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci. 2008;270:70–76. doi: 10.1016/j.jns.2008.02.011. [DOI] [PubMed] [Google Scholar]
- [38].Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- [39].Hibbert JM, Hsu LL, Bhathena SJ, et al. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med. 2005;230:68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and chronic vasculopathy. Microcirculation. 2004;11:129–151. [PubMed] [Google Scholar]
- [41].Hannink M, Donoghue DJ. Structure and function of platelet derived growth factor and related proteins. Biochim Biophy Acta. 1989;989:1–10. doi: 10.1016/0304-419x(89)90031-0. [DOI] [PubMed] [Google Scholar]
- [42].Prohovnik I, Hurlet-Jensen A, Adams RJ, De Vivo D, Pavlakis SG. Hemodynamic etiology of elevated flow velocity and stroke in sickle-cell disease. J Cereb Blood Flow Metab. 2009;29:803–810. doi: 10.1038/jcbfm.2009.6. [DOI] [PubMed] [Google Scholar]
- [43].Nahavandi M, Tavakkoli F, Hasan SP, Wyche MQ, Castro O. Cerebral oximetry in subjects with sickle cell disease. Eur J Clin Invest. 2004;34:143–148. doi: 10.1111/j.1365-2362.2004.01307.x. [DOI] [PubMed] [Google Scholar]
- [44].Khachigian LM, Anderson KR, Halnon NJ, Gimbrone MA, Resnick N, Collins T. Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A-Chain promoter. Arterioscler Thromb Vasc Biol. 1997;17:2280–2286. doi: 10.1161/01.atv.17.10.2280. [DOI] [PubMed] [Google Scholar]
- [45].Bos R, Van Diest PJ, De Jong JS, Van Der Groep P, Van Der Valk P, Van Der Wall E. Hypoxia-inducible factor-1α is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology. 2005;46:31–36. doi: 10.1111/j.1365-2559.2005.02045.x. [DOI] [PubMed] [Google Scholar]