Abstract
Background
Evidence suggests that the protease-activated receptor-1 (PAR-1), a thrombin receptor, mediates neuronal injury in experimental cerebral ischemia. The present study investigated whether PAR-1 plays a role in brain injury after global cerebral ischemia.
Methods
Adult male wild type (WT) or PAR-1 knockout mice underwent a 20-minute bilateral common carotid artery occlusion (BCCAO) or a sham operation. Behavior tests were performed before ischemia, and 1, 2 and 3 days after BCCAO. Mice were euthanized at different time points for thrombin activity, brain edema, Western blot analysis and brain histology.
Results
Thrombin activity and PAR-1 expression were increased in the brain after BCCAO. Compared with WT mice, PAR-1 knockout mice had less brain edema formation, neuronal death and behavior impairment after BCCAO. In addition, BCCAO-induced activation of mitogen-activated protein kinases was absent in PAR-1 knockout mice.
Conclusion
PAR-1 contributes to the brain injury induced by global cerebral ischemia, which may be related to activation of mitogen-activated protein kinases.
Keywords: brain edema, global cerebral ischemia, mitogen-activated protein kinases, protease activated receptor-1(PAR-1), thrombin
Introduction
Global cerebral ischemia is caused by events such as cardiac arrest, cardiovascular surgery and neurosurgical procedures. Severe brain damage occurs after global cerebral ischemia 1, and clinical outcomes remain poor. Therefore, it is critical to understand the mechanisms of neuronal death after global cerebral ischemia.
Evidence shows that thrombin activation plays a role in ischemic injury2–5. This is mediated, at least in part, by protease-activated receptors (PARs)6, 7. PARs are a superfamily that includes four G-protein coupled receptors (PAR-1 to PAR-4) and PAR-1 is considered as the main subtype. PAR-1 can be activated by thrombin, and is known as a thrombin receptor 8. PAR-1 is expressed throughout the central nervous system6, 7 and participates in brain injury after hemorrhagic and focal ischemic stroke 2, 6, 9–12. However, the role of PAR-1 in brain injury after global cerebral ischemia remains unclear.
The hippocampus shows vulnerability to global ischemia and it has been the focus of much research11. However, the basal ganglia are also susceptible to damage following global ischemia13, 14, which has not been as well studied. Recent studies have described a transient global cerebral ischemia model with consistent basal ganglia injury in mice15, 16.
In this study, we investigated the role of PAR-1 in brain injury and activation of mitogen-activated protein kinases (MAPK) after global cerebral ischemia. PAR-1 knockout mice displayed markedly reduced brain injury and an absence of the MAPK activation found in wild-type mice.
Materials and Methods
Animal preparation, transient global cerebral ischemia and intracerebral injection of thrombin
The University of Michigan Committee on the Use and Care of Animals approved the protocols for these animal studies. Male PAR1 knockout (PAR-1 −/−) mice with C57BL/6J background and their wild-type littermates (8–12 weeks, University of Michigan Breeding Core) were used. Mice were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). Blood glucose levels were measured in tail blood samples taken just before occlusion of bilateral common carotid arteries. Rectal temperature was maintained at 37.0 ± 0.5 °C by a feedback-controlled heating pad. Regional cerebral blood flow (rCBF) was monitored by a laser Doppler flow-meter. The probe was fixed on the skull, 4-mm lateral to the bregma. Changes in rCBF after bilateral common carotid artery occlusion (BCCAO) were expressed as a percentage of the pre-ischemic baseline value. Only animals whose rCBF decreased to <15% of pre-ischemic value were included in this study. BCCAO model was induced by using non-traumatic micro-aneurysm clips to occlude both common carotid arteries for 20 minutes. After clip removal, the skin incision was closed with suture, and animals were kept in a warm chamber (33–34°C) for 4 hours before returned to their home cages at room temperature. Sham-operated animals underwent the same procedures described above but without BCCAO. Normal saline (0.5ml) was administered subcutaneously in all animals 30 minutes and 24 hour after reperfusion.
In some mice, thrombin was injected intracerebrally. The mice received an injection of rat thrombin (1 U, Sigma) in 10-μl saline into the right basal ganglia (coordinates: 0.2 mm anterior, 3.5 mm ventral, and 2.5 mm lateral to the bregma).
Experiments groups
This study was divided into five sets. In the first set, male wild type mice underwent BCCAO or sham operation. They were euthanized at 4, 24, 72 hours (n=3 each group) for thrombin activity determination. PAR-1 knockout mice (n=3) also had BCCAO and were euthanized at 24 hours for thrombin activity measurement. In the second set, male wild type or PAR-1 −/− mice underwent BCCAO or sham operation and they were euthanized at 4, 24 and 72 hours (n=4 each group) for Western blot analysis. In the third set, male wild type or PAR-1 −/− mice underwent BCCAO or sham operation and they were euthanized 24 hours later for brain water and ion content determination (n=5~6 each group). In the fourth set, male wild type or PAR-1 −/− mice underwent BCCAO or sham operation and they were euthanized at 24 and 72 hours (n=5~6 each group) for histology. In the last set, male wild type or PAR-1 −/− mice had an intracaudate injection of thrombin (1 U) and were euthanized at 24 hours for Western blot analysis. All mice were subjected to behavior tests.
Thrombin activity determination
Thrombin activity was determined according to the method described by Chapman and coworkers 17 with some modification. Briefly, animals were reanesthetized, perfused with saline and the brain removed. The brain was cut into 6 to 8 coronal sections (at 1mm thickness) using a mouse brain slicer matrix after weighing. Each brain section was then transferred to a well in a 96-well plate which was prefilled with 50μl buffer in each well. Another 100μl buffer containing substrate Boc-Asp (OBzl)-Pro-Arg-AMC · HCl (final concentration 13 μM, I-1560, Bachem) and protease inhibitor prolyl endopeptidase inhibitor II (final concentration 20 μM, 537011, Calbiochem) was added to each well before measurement. The fluorescence was measured continuously for 40 min at 25°C using a fluorescence detection system (Glomax ®-Multi+ Detection System E8032, Promega, Madison, WI; Excitation 365nm, emission 410–460nm). The hydrolysis of Boc-Asp (OBzl)-Pro-Arg-AMC · HCl was determined by the increase of fluorescence. Purified rat thrombin (T5772, Sigma) was used as a calibration standard. Total thrombin activity was then calculated. Thrombin activity was expressed as the ratio of BCCAO/sham.
Western blot analysis
Western blot analysis was performed as described earlier18. The primary antibodies were: rabbit polyclonal anti-PAR1 (1:1000 dilution, Abcam), rabbit polyclonal anti-phospho-p38 and -p38 MAPKs, anti-phospho-JNK and -JNK, and anti-phospho-p44/42 and -p44/42 MAPKs (1:1000, Cell Signaling Technology). The secondary antibody was goat anti-rabbit IgG (1:4,000 dilution; Bio-Rad).
Immunohistochemisty, double staining and brain histology
Immunohistochemistry and double staining were performed as previously described18. The primary antibodies were: rabbit polyclonal anti-PAR1 (1:400 dilution, Abcam), sheep anti-human thrombin (1:500, Affinity Biologicals), and rabbit anti-phospho-p38 MAPK, -phospho-p44/42 MAPKs (1:400, Cell Signaling Technology), chicken anti-MAP2 (1:800 dilution, Abcam), goat anti-GFAP (1:200 dilution, Santa Cruz) and goat anti-Iba1 (1:200 dilution, Santa Cruz). Normal chicken, goat, rabbit or sheep IgG was used as negative controls.
For immunofluorescent labeling, brain sections were reacted with rabbit anti-DARPP-32 monoclonal antibody (1:400, Cell Signaling Technology) and then incubated with Alexa 488-conjugated donkey anti-rabbit antibody (Invitrogen). DARPP-32 is a cytosolic protein highly enriched in medium-sized spiny neurons of the striatum and is used to reflect neuron viability in the striatum. Brain sections were also stained with Fluoro-Jade C, a marker of neurodegeneration 19.
Brain water and ion content determination
Animals were reanesthetized at 24 hours after BCCAO. The brains were removed and quickly divided into 3 parts: right hemisphere, left hemisphere and cerebellum. Tissue samples were immediately weighed to obtain the wet weight. Samples were dried in a gravity oven at 95–100°C for more than 24 hours to determine dry weight. Tissue water content (%) was calculated as ([wet weight-dry weight]/wet weight)*100. Dehydrated brain samples were digested in 1 mL of 1 N nitric acid for 2 weeks. The sodium ion content of this solution was measured by flame photometry and expressed in milli-equivalents per kilogram of dehydrated brain tissue (mEq/kg dry tissue).
Behavior tests and body weight
We performed three behavioral tests in this study. All the animals were pretested one day prior to surgery to assess for baseline or abnormalities. Animals were re-tested at 24, 48 and 72 hours after BCCAO or sham surgery. All tests were done at a fixed time in the early evening in a quiet behavior tests special use room with a dim red light. All behavioral testing equipment and surfaces were cleaned before and after test. All animals were housed in a temperature and humidity controlled room with an automatic 12h dark/light cycles.
(1) Open field test
Mice were placed in the open field chamber (90 × 90cm) for 5 minutes in each test. Locomotive behavior was monitored and analyzed by an auto-tracking system (Smart1.1) which quantified the total distance travelled. All mice were brought to the testing room 10 min before the beginning of the test and the activity was recorded immediately after the mice were placed in the open field apparatus.
(2) Hanging wire
The hanging wire tests both limb strength and balance after ischemia. A standard wire cage lid with its edges taped off was used for this experiment. The mouse was placed on the center of the wire lid and the lid was then slowly turned upside down and held at a height approximately 30–40cm above a protective device. Latency to fall from the wire was recorded. The time out period was 60 seconds. All animals with latency less than 60 seconds in the pretest were ruled out in this study.
(3) Neurological disability status scale
This part of experiment was performed as described by R. Rodriguez et al.20. It is a method developed to access the neurological deficits after forebrain ischemia in mice. The neurological disability status scale has 10 progressive steps from 0 (normal) to 10 (death); the higher score means the greater neurological dysfunction. Animals with abnormalities found in pretest were all ruled out in this study.
The body weight of each mouse was measured before anesthesia and each day after behavior tests post surgery.
Cell counting
Cell counting was performed according to the method described by Yoshioka H et al.15 Five sub-regions of the caudate (central, dorsomedial, dorsolateral, ventromedial, and ventrolateral) were assigned for quantification of Fluoro-Jade C and DARPP-32 staining, each consisting of a rectangle of 250×174 μm.
Statistical Analysis
All the data in this study are presented as mean ± SD. Data were analyzed by Student t test and one-way analysis of variance (ANOVA). A level of P<0.05 was considered statistically significant.
Results
Regional cerebral blood flow, blood glucose and mortality after BCCAO
Regional cerebral blood flow reduction was no different in WT mice (% of the baseline: 9.8 ± 3.3%) and PAR-1 −/− mice (10.7 ± 2.2%) after BCCAO. Similarly, after reperfusion, blood flow returned to 86.5± 12.4 and 85.2± 20.9% of baseline in WT and PAR-1 −/− mice, respectively. These values were not significantly different. Hyperglycemia was found in both wild-type (281 ± 41mg/dl) and PAR-1 −/− mice (295 ± 43mg/dl) before BCCAO (p>0.05) because xylazine can cause an increase of blood glucose levels. The death rate was 4.1% (2/49) at 24 and 9.1% (2/22) at 72 hours in the WT mice, but no death was found in PAR-1 −/− mice after BCCAO.
Thrombin activity and PAR-1 expression was upregulated after BCCAO
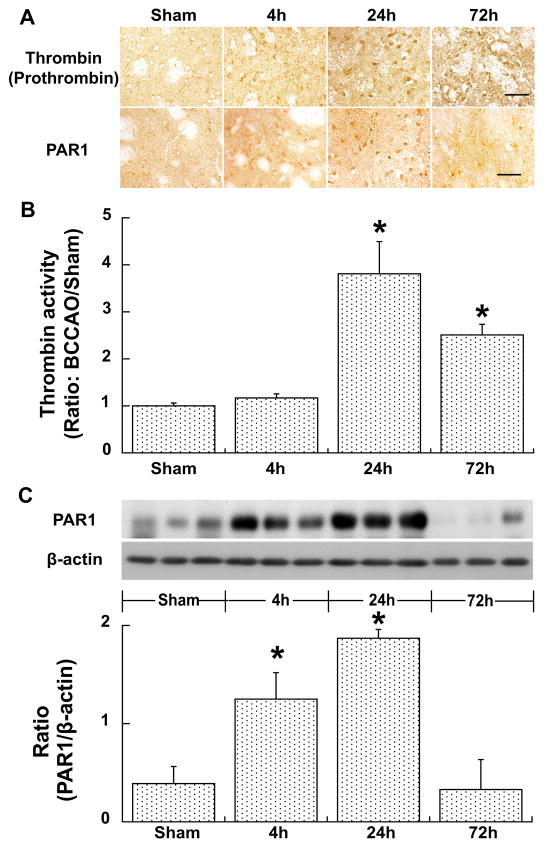
Thrombin positive cells were found in the basal ganglia after BCCAO at 4 hours, reached the peak at 24 hours and returned to a lower level at 72 hours. There were no thrombin positive cells in sham-operated mice. Brain thrombin activity expressed as a ratio to that in sham was slightly increased at 4 hours (1.17±0.09, p>0.05), peaked at 24 hours (3.81±0.23, p<0.01) and declined at 72 hours (2.51±0.06, p<0.01) after BCCAO (Fig. 1). Similarly, BCCAO induced PAR-1 expression in the basal ganglia. Basal ganglia PAR-1 protein levels were upregulated significantly at 4 and 24 hours (ratio to β-actin: 1.87±0.09 vs. 0.39±0.17 in sham, p<0.01), but were back to normal at 72 hours after BCCAO (Fig. 1).
Figure 1.
Immunoreactivity of thrombin (prothrombin) and PAR-1 in the basal ganglia (A) and thrombin activity in the cerebrum (B), and protein levels of PAR-1 in the basal ganglia (C) at 4, 24 or 72 hours after BCCAO, or at 24 hours after sham operation. Scale bar=50μm. Values are mean±SD, n=3–4, *p<0.05 vs. the other groups.
We also measured thrombin activity after BCCAO in PAR-1 knockout mice. We found that brain thrombin activity is same 24 hours after BCCAO in wild type and PAR-1 knockout mice (ratio: BCCAO/sham, 3.8±0.7 vs. 3.3±1.4, p>0.05).
BCCAO caused less brain edema in PAR-1 −/− mice
BCCAO induced brain edema in both hemispheres. At 24 hours after BCCAO, brain water content in PAR-1 −/− mice (78.4±0.2%) was significant lower than in WT mice (80.2±1.4%, p<0.01, Fig. 2). Also, there was less BCCAO-induced brain sodium accumulation in the PAR-1 −/− mice (214±9 vs. 313±109 mEq/kg dry wt in WT mice, p<0.01, Fig. 2).
Figure 2.
Brain water (A) and sodium (B) contents in the cerebrum and cerebellum of WT and PAR-1 −/− mice 24 hours after BCCAO or sham operation. Values are mean±SD, n=5~6, #p<0.01 vs. the other groups and *p<0.05 vs. sham group.
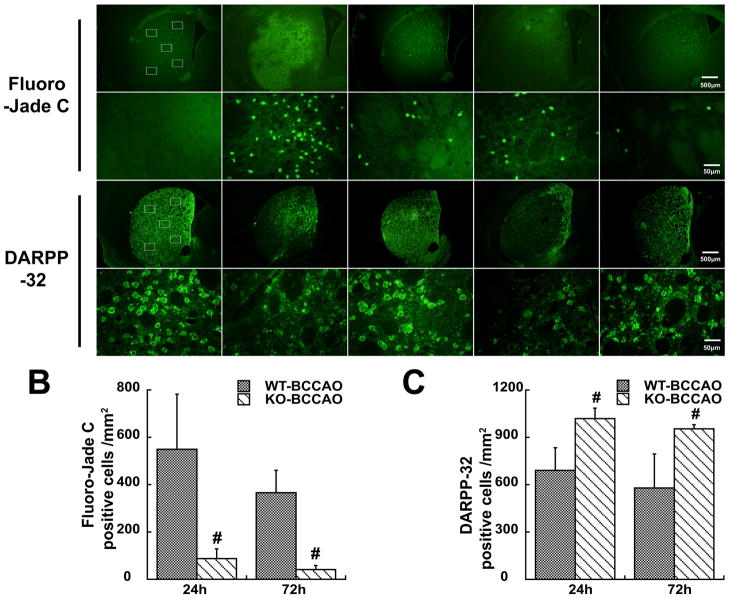
Neuronal death was less in the basal ganglia of PAR-1 −/− mice after BCCAO
Fluoro-Jade C staining and DARPP-32 immunofluorescence staining were used to examine neuronal death after BCCAO. DARPP-32 was used as a marker of medium spiny neurons 15 that represent 95% of striatal neurons. Five sub-regions showed in Figure 3 were used for the cells counting. There were less Fluoro-Jade C positive cells in the caudate of PAR-1 −/− mice at 24 (87±41 vs. 549±232 cells/mm2 in the WT mice, p<0.01) and 72 hours (41±17 vs. 365±94 cells/mm2 in WT mice, p<0.01) after BCCAO(Fig. 3). More DARPP-32 positive cells were found in the caudate of PAR1 −/− mice at 24 (1018±67 vs. 690±104 cells/mm2 in the WT, p<0.01) and 72 hours (954±26 vs. 579±215 cells/mm2 in WT mice, p<0.01) following BCCAO(Fig. 3).
Figure 3.
Fluoro-Jade C and DARPP-32 staining showing neuronal death after BCCAO (A). Rectangles represent the counting areas in the lower magnification, scale bar=500 μm. Cell counting was made at higher magnification, scale bar=50 μm. Fluoro-Jade C and DARPP positive cells were counted (B & C). Values are mean±SD, n=5~6, #p<0.01 vs. WT mice.
Neurological deficits and body weight loss were less in PAR-1 −/− mice
For behavior tests, all animals were tested prior to global ischemia. We found no differences in behavior between WT and PAR-1 −/− mice prior to ischemia(Fig 4). Compared with the sham group, BCCAO resulted in shorter total travelled distance, reduced wire hang time and a worse neurological disability score. Behavioral outcomes were better in PAR-1 −/− mice compared with those in WT mice(Fig. 4). Body weight loss (% of initial) after BCCAO was also less in PAR-1 −/− mice(Fig. 4).
Figure 4.
(A) Total travelled distance, (B) Latency to fall time, (C) Neurological disability scores and (D) Body weight deduction in WT or PAR-1 −/− mice at 24, 48 and 72 hours after BCCAO or sham operation. Values are mean±SD, n=10~34, #p<0.01 vs. other groups.
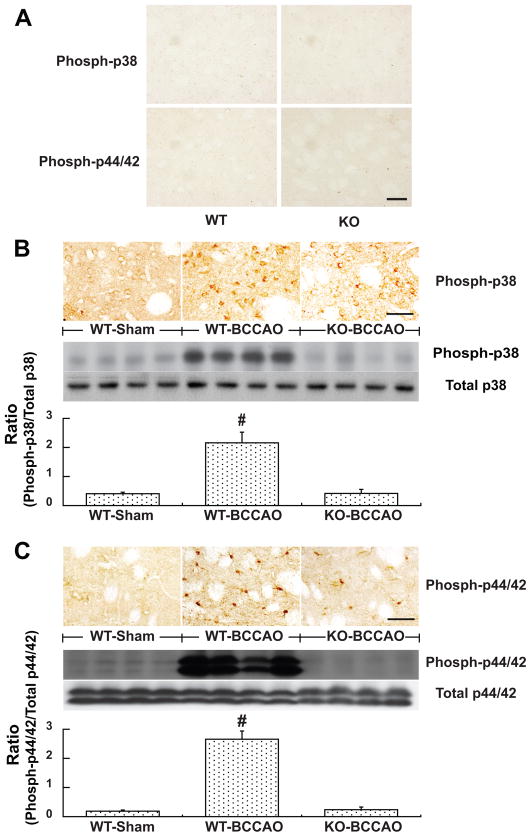
Activation of mitogen-activated protein kinases (MAPKs) after BCCAO was lower in PAR-1 −/− mice
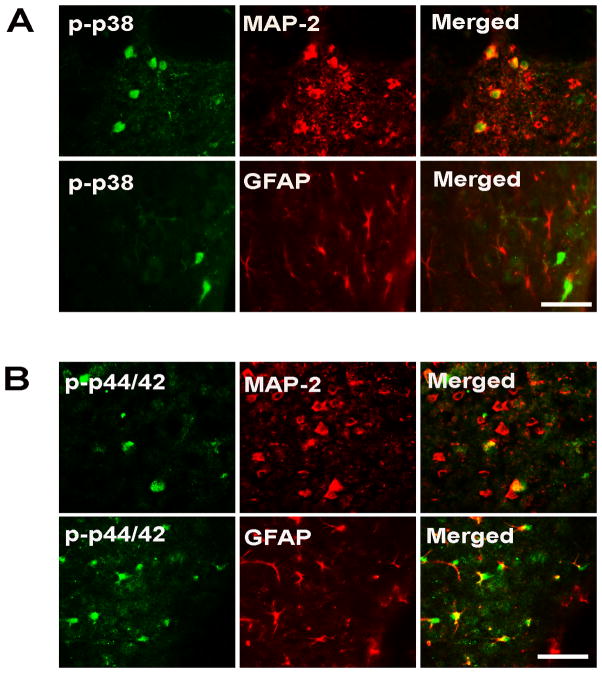
Many phospho-p38 MAPK and phospho-p44/42 MAPK positive cells were found in the caudate after BCCAO. Only a few of these positive cells were found in the caudate after sham operation (Fig. 5). Western blots showed that protein levels of phosphorylated MAPKs were upregulated in the caudate 24 hours after BCCAO compared with those after sham operation (Fig. 5). Levels of phospho- p38 and phospho-p44/42 MAPKs were less in PAR-1 −/− mice compared to that in WT mice at 24 hours after BCCAO (Fig. 5). It should be noted that PAR-1 gene knockout does not affect MAPK expression in non-ischemic mice. For example, brain total (including non-phosphorylated) p44/42 MAPK levels were the same in normal WT mice and PAR-1 −/− mice (16440±1597 vs. 18761±1633 pixels, p>0.05). Both immunostaining and Western blotting did not detect phospho-p38 and phospho-p44/42 MAPKs in non-ischemic mice. Double staining showed that most phospho-p38-MAPK positive cells were neurons while phospho-p44/42 MAPK positive cells were either astrocytes or neurons (Fig. 6). No microglia were activated p38- or p44/42 MAPK positive (data not shown).
Figure 5.
(A) Immunoreactivity of the phospho-p38 MAPK and phospho-p44/42 MAPK in the basal ganglia of normal WT or PAR-1 −/− mice. (B & C) The phospho-p38 MAPK and phospho-p44/42 MAPK levels in the basal ganglia of WT or PAR-1 −/− mice at 24 hours after BCCAO of sham operation. Scale bar= 50μm. Values are mean ± SD, n=4, #p<0.01 vs. other groups a *p<0.05 vs. sham group.
Figure 6.
Double staining examining colocalization of phospho-p38 MAPK (A) and phospho-p44/42 MAPK (B) staining with MAP-2 positive neurons and GFAP positive astrocytes in WT mice 24 hours after BCCAO. Scale bar=50μm. Phospho-p38 MAPK was found in neurons and phospho-p44/42 MAPK was found in neurons and astrocytes.
We hypothesized that increases of brain thrombin levels contribute to brain injury after global cerebral ischemia. We then injected thrombin intracerebrally and found that thrombin-induced increases of brain phospho-MAPK were much less in PAR-1 knockout mice (phospho-p44/42: 158±121 vs. 6531±3710 pixels in the wild type mice, p<0.01; phospho-p38: 47±54 vs. 3772±3133 pixels, p<0.01).
Discussion
The major findings of the current study are: 1) thrombin activity was increased and PAR-1 expression was upregulated in the caudate after BCCAO; 2) BCCAO caused less brain edema, neuronal death, neurological deficits in PAR-1 −/− mice; 3) BCCAO- and thrombin-induced p38-and p44/42 MAPK activation was less in PAR-1 −/− mice.
Thrombin plays crucial roles in brain injury after cerebral ischemia3, 21. Low concentrations of thrombin are neuroprotective, while high concentrations are detrimental to neurons or astrocytes21, 22. In our previous study, low concentrations of thrombin may also cause cell death after stroke. For example, a small dose of exogenous thrombin exacerbates ischemic brain injury3, 23, 24. It has been shown that systemic thrombin inhibition attenuated neurodegeneration and brain edema formation after transient cerebral ischemia25, 26. In the current study, we found that thrombin activity after BCCAO increased four-fold compared to sham-operated mice at 24 hours, which suggests that thrombin could be an important factor that causes brain injury after global ischemia. Future study should measure exact thrombin activity using thrombin inhibition method.
PAR-1 is involved in thrombin-induced brain damage after cerebral ischemia. Brain PAR-1 expression was upregulated by BCCAO in the basal ganglia areas. Our previous study showed PAR-1 upregulation in a rat model of focal cerebral ischemia 3. In addition, increased brain PAR-1 levels were reported in hippocampal slice cultures after oxygen-glucose deprivation 6. In the present study, BCCAO induced less brain edema formation, neuronal death, and neurological deficits in PAR-1 knock out mice. In addition, BCCAO-caused animal death was only found in wild type mice but not in PAR-1 KO mice. Other studies also found that PAR-1 is associated with brain injury after focal cerebral ischemia. For example, brain infarct volume is reduced in PAR-1 KO mice and intracerebroventricular injection of BMS-200261, a PAR-1 antagonist, reduces infarct volume in a mouse focal cerebral ischemia model12. PAR-1 blockade using PAR-1 antagonists might be a promising therapy for global cerebral ischemia.
The results in the current study indicate that PAR-1 plays an important role in MAPKs activation after BCCAO. The MAPK family regulates a diverse array of functions, such as neuronal death and survival, proliferation and apoptosis in response to extracellular stimuli, including cerebral ischemia27, 28. MAPKs are associated with brain injury after cerebral ischemia 29–31. In our current study, the phosphorylation levels of p38 MAPK and p44/42 MAPK were enhanced markedly 24 h after BCCAO or thrombin stimulation. The upregulation for phospho-p38 occurred in neurons, while phospho-p44/42 upregulation occurred in neurons and astrocytes. This upregulation of both was almost absent in the PAR-1 −/− mice.
Effects of PAR-1 deletion on MAPK upregulation after BCCAO and thrombin stimulation were significant. It is known that thrombin and PAR-1 agonist can markedly upregulate brain p44/42 MAPK4, 32. Intracerebral hemorrhage also causes an upregulation in phosphorylated p44/42 MAPK and there is some evidence that this is blocked by argatroban, a thrombin antagonist 33. This suggests that PAR-1 may also be involved in MAPK upregulation in hemorrhagic stroke. However, brain thrombin levels are higher in hemorrhagic stroke compared to cerebral ischemia and it is still uncertain whether thrombin is the sole cause of the MAPK upregulation after BCCAO. Although we did find four-fold increases in the level of this serine protease it should be noted that there are other naturally occurring PAR-1 agonists 2. This merits further investigation, as does the potential role of PAR-1 in MAPK upregulation after focal cerebral ischemia.
In conclusion, PAR-1 activation has a major role in brain injury after transient global cerebral ischemia. That activation is probably due to thrombin production in the brain and it is responsible for the marked activation of MAPKs after global ischemia.
Acknowledgments
Sources of Funding
Supported by grants NS-039866 and NS-057539 from NIH and 0840016N from AHA.
Footnotes
Disclosures
None.
References
- 1.Khot S, Tirschwell DL. Long-term neurological complications after hypoxic-ischemic encephalopathy. Seminars in Neurology. 2006;26:422–431. doi: 10.1055/s-2006-948323. [DOI] [PubMed] [Google Scholar]
- 2.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? J Neurochem. 2003;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 3.Karabiyikoglu M, Hua Y, Keep RF, Ennis SR, Xi G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:159–166. doi: 10.1097/01.WCB.0000100062.36077.84. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, Yamashita S, Hua Y, Keep RF, Liu W, Xi G. Thrombin-induced neuronal protection: Role of the mitogen activated protein kinase/ribosomal protein s6 kinase pathway. Brain Res. 2010;1361:93–101. doi: 10.1016/j.brainres.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Cheng Q, Yang K, Lyden PD. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010;41:2348–2352. doi: 10.1161/STROKEAHA.110.584920. [DOI] [PubMed] [Google Scholar]
- 6.Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, et al. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Ubl JJ, Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia. 2002;37:53–63. doi: 10.1002/glia.10012. [DOI] [PubMed] [Google Scholar]
- 8.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 9.Hamill CE, Mannaioni G, Lyuboslavsky P, Sastre AA, Traynelis SF. Protease-activated receptor 1-dependent neuronal damage involves nmda receptor function. Exp Neurol. 2009;217:136–146. doi: 10.1016/j.expneurol.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henrich-Noack P, Riek-Burchardt M, Baldauf K, Reiser G, Reymann KG. Focal ischemia induces expression of protease-activated receptor1(PAR1) and par3 on microglia and enhances par4 labeling in the penumbra. Brain Res. 2006;1070:232–241. doi: 10.1016/j.brainres.2005.10.100. [DOI] [PubMed] [Google Scholar]
- 11.Olson EE, Lyuboslavsky P, Traynelis SF, McKeon RJ. PAR-1 deficiency protects against neuronal damage and neurologic deficits after unilateral cerebral hypoxia/ischemia. J Cereb Blood Flow Metab. 2004;24:964–971. doi: 10.1097/01.WCB.0000128266.87474.BF. [DOI] [PubMed] [Google Scholar]
- 12.Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, et al. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci USA. 2003;100:13019–13024. doi: 10.1073/pnas.2235594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 14.Olsson T, Wieloch T, Smith ML. Brain damage in a mouse model of global cerebral ischemia. Effect of nmda receptor blockade. Brain Res. 2003;982:260–269. doi: 10.1016/s0006-8993(03)03014-2. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka H, Niizuma K, Katsu M, Okami N, Sakata H, Kim GS, et al. NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J Cereb Blood Flow Metab. 2011;31:868–880. doi: 10.1038/jcbfm.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshioka H, Niizuma K, Katsu M, Sakata H, Okami N, Chan PH. Consistent injury to medium spiny neurons and white matter in the mouse striatum after prolonged transient global cerebral ischemia. J Neurotrauma. 2011;28:649–660. doi: 10.1089/neu.2010.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shavit E, Michaelson DM, Chapman J. Anatomical localization of protease-activated receptor-1 and protease-mediated neuroglilal crosstalk on peri-synaptic astrocytic endfeet. J Neurochem. 2011;119:460–473. doi: 10.1111/j.1471-4159.2011.07436.x. [DOI] [PubMed] [Google Scholar]
- 18.Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez R, Santiago-Mejia J, Gomez C, San-Juan ER. A simplified procedure for the quantitative measurement of neurological deficits after forebrain ischemia in mice. J Neurosci Meth. 2005;147:22–28. doi: 10.1016/j.jneumeth.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci USA. 2000;97:2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua Y, Keep RF, Hoff JT, Xi G. Thrombin preconditioning attenuates brain edema induced by erythrocytes and iron. J Cereb Blood Flow Metab. 2003;23:1448–1454. doi: 10.1097/01.WCB.0000090621.86921.D5. [DOI] [PubMed] [Google Scholar]
- 23.Xi G, Hua Y, Wu J, Keep RF. Increase of brain thrombin concentration in cerebral ischemia. Stroke. 2002;33:399. [Google Scholar]
- 24.Weinstein JR, Lau AL, Brass LF, Cunningham DD. Injury-related factors and conditions down-regulate the thrombin receptor (par-1) in a human neuronal cell line. J Neurochem. 1998;71:1034–1050. doi: 10.1046/j.1471-4159.1998.71031034.x. [DOI] [PubMed] [Google Scholar]
- 25.Mima T, Jin YJ, Hirayama T, Mostafa MG, Mori K. Argatroban, a thrombin inhibitor, decreased mortality after 10 min of forebrain ischemia in the gerbil. Neurosci Lett. 2000;279:93–96. doi: 10.1016/s0304-3940(99)00959-3. [DOI] [PubMed] [Google Scholar]
- 26.Ohyama H, Hosomi N, Takahashi T, Mizushige K, Kohno M. Thrombin inhibition attenuates neurodegeneration and cerebral edema formation following transient forebrain ischemia. Brain Res. 2001;902:264–271. doi: 10.1016/s0006-8993(01)02354-x. [DOI] [PubMed] [Google Scholar]
- 27.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Roux PP, Blenis J. Erk and p38 mapk-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiology and molecular biology reviews : MMBR. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nito C, Kamada H, Endo H, Niizuma K, Myer DJ, Chan PH. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase a2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 2008;28:1686–1696. doi: 10.1038/jcbfm.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawe N, Steinberg G, Zhao H. Dual roles of the mapk/erk1/2 cell signaling pathway after stroke. Journal of Neuroscience Research. 2008;86:1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- 31.Narasimhan P, Liu J, Song YS, Massengale JL, Chan PH. Vegf stimulates the erk 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke. 2009;40:1467–1473. doi: 10.1161/STROKEAHA.108.534644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi G, Hua Y, Keep RF, Duong HK, Hoff JT. Activation of p44/42 mitogen activated protein kinases in thrombin-induced brain tolerance. Brain Res. 2001;895:153–159. doi: 10.1016/s0006-8993(01)02064-9. [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi M, Katsuki H, Fujimoto S, Takagi M, Kume T, Akaike A. Involvement of thrombin and mitogen-activated protein kinase pathways in hemorrhagic brain injury. Experimental neurology. 2007;206:43–52. doi: 10.1016/j.expneurol.2007.03.030. [DOI] [PubMed] [Google Scholar]