Abstract
The development of dry powder inhalation (DPI) products of traditional Chinese medicine (TCM) remains to be a challenge due to chemical complexity and batch-to-batch variations in constituent composition. This study was to investigate the feasibility of using spray-dried corrugated particles to improve the aerodynamic performance of a TCM, Shuang-Huang-Lian (SHL), in carrier-based DPI. Particles with different surface roughness were spray-dried by the addition of leucine and concomitant manipulation of spray-drying parameters. The surface roughness was determined by atomic force microscopy, whilst the aerodynamic performance of drug particle–mannitol/lactose blends was evaluated using a next-generation pharmaceutical impactor through a Cyclohaler. Although the emission efficiency for corrugated particle-based DPI was ∼10% lower than that for smooth SHL, the fine particle fractions (FPF<4.4 μm) of 32.4–36.8% for the former were significantly higher than those of 14.7–16.2% for the latter. In particular, the FPF and fraction of drug detached from the carrier appeared not to be significantly affected by the variation in constituent composition of SHL. This study demonstrates that the use of corrugated particles in carrier-based DPI improved aerosol performance by facilitating drug detachment from the carrier, independent of variation in constituent composition, and such particles were potentially applicable to the development of SHL DPI products.
KEY WORDS: dry powder inhaler, Shuang-Huang-Lian, spray-drying, surface roughness, traditional Chinese medicine
INTRODUCTION
Shuang-Huang-Lian (SHL) is a traditional Chinese medicine (TCM) prepared from honeysuckle flower, radix Scutellariae and fructus Forsythiae (Chinese Pharmacopoeia 2010, vol II). SHL was initially approved in an injectable formulation in the early 1980s for the treatment of influenza and respiratory infections in China, and later, the peroral and inhalation products were also developed. As one of the top-selling TCM products in the last decade, SHL injections has been documented to cause adverse drug reactions (ADR), including serious adverse reactions, and a great number of cases have been reported to the state authority for ADR Monitoring Center and in the literature (1). Relative to SHL injectables, numerous clinical papers have documented the therapeutic efficacy of inhaled SHL without ADR cases being reported (2,3). However, the chlorofluorocarbon-based metered dose inhalers (MDI) of SHL are subjected to be phased out as a result of the Montreal Protocol, and no alternative inhalation products are available. The development of TCM MDI or dry powder inhalers (DPI) remains to be a challenge, mostly due to the chemical complexity and the batch-to-batch variation in constituent composition.
SHL preparations contain various chemical ingredients, including phenylethanoid glycosides, lignans, quinic acids, saponins and flavonoids (4,5), and the chemical complexity results in partial solubility of certain drug substances in the hydrofluoralkanes, leading to the difficulty in the development of stable suspended SHL particles in the propellants. In addition, the development of SHL DPI is not trivial. First, traditional milling means may not be suitable to produce dispersible micronised SHL particles since SHL is highly hygroscopic and it may lead to partial or complete liquefaction during processing. Second, the batch-to-batch variations of plant materials can lead to variations in SHL constituent composition, which, in turn, could influence the batch-to-batch cohesive and adhesive interactions of SHL particles, resulting in different batch-to-batch aerosol performance. Therefore, novel particles prepared by advanced particle engineering techniques are highly desirable, and ideally, the preparation and aerosol performance of such particles within DPI formulations should be independent of batch-to-batch variations in constituent composition.
In the literature, various novel particle engineering technologies, including spray-drying (6–8), spray freeze-drying (9), supercritical fluids (10), controlled crystallisation (11,12), etc., have been well documented for the preparation of respirable particles. Based upon the mechanisms by which the engineered particles provided the improved aerosol performance of DPI, Chow and colleagues (6) classified the engineered particles into three types, namely large porous particles, and particles with surface chemical modification and with irregular surface, respectively. However, these novel particle engineering technologies are designed for pure active pharmaceutical ingredients, and the applicability to TCM drugs with chemical complexity has not been investigated. Indeed, large porous particles confer to a relatively small payload, not applicable to DPI formulation of SHL, whereas particles with surface chemical modification, which increase dispersibility by decreasing the surface energy and depend upon the chemical characteristics of particles, have the limitations due to batch-to-batch variations in SHL constituent composition. In contrast, irregular surface particles improve dispersibility by decreasing cohesive and adhesive interaction strength arising from reducing contact surface area. Typical examples of irregular surface particles include corrugated (also called raisin-like or wrinkled) particles and the use of corrugated particles in drug alone or drug–carrier DPI system has been successfully demonstrated to improve aerosol performance of spray-dried bovine serum albumin (BSA) and gentamicin (13–17). Relative to regular particles, irregular surface particles have increased surface roughness. The existing literature has shown that the properties of surface roughness of both carrier and drug particles greatly affect the drug dispersion in carrier-based DPI (15,18–21). Most of these works have concentrated on the effect of carrier surface morphology on the drug dispersion (19–21), whereas only one study has focussed on the influence of the drug surface roughness on the aerosolisation properties (15). The latter study suggested that the role of the surface roughness of drug particles in improving aerosol performance was mainly attributable to surface geometric characteristics in nature rather than the chemical properties of the particles. Therefore, it is hypothesised that drug–carrier particle interactions for corrugated particles are indifferent to batch-to-batch variations in SHL constituent composition and suitable for the development of SHL carrier-based DPI. The main objective of the present study was to produce spray-dried SHL particles with varying degrees of surface roughness and to investigate the effect of SHL particle surface roughness on the aerosol performance in carrier-based DPI, with a view to demonstrating that the preparation and enhanced drug dispersion of corrugated particles are indifferent to the batch-to-batch content fluctuation of SHL.
MATERIALS AND METHOD
Materials
Inhalac® 230 lactose and Cyclohaler® devices were kindly gifted by MEGGLE Group Wasserburg BG Excipients & Technology (Wasserburg, Germany) and PCH Pharmachemie (The Netherlands), respectively. Baicalin was supplied by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). d-Mannitol, l-leucine, lecithin, methanol (high-performance liquid chromatography (HPLC)-grade) and formic acid (HPLC-grade) were obtained from Sinopharm Chemical Reagents Co., Ltd. (Shanghai, China), Alfa Aescar (Heysham, Lancs), Aoboxing Biotechnology Co. (Beijing, China), Merck KgaA (Darmstadt, Germany) and Dikma Technologies Inc. (USA), respectively. Water was purified by Reverse Osmosis Systems (Millipore, Ireland). All other reagents and solvents were supplied by Beijing Chemical Works (Beijing, China).
Preparation of SHL Extracts and Spray-Dried SHL Particles
Two batches of SHL extracts containing different constituent profiles were prepared using different raw herbal materials according to the preparation protocol required in Ministerial Standard-WS3-B-2104-96 (22). The variation in the constituent profiles of the extracts was indicated by the content of the chemical marker, baicalin. Batch-1 and batch-2 extracts were found to contain 267 and 175 mg of baicalin per gram of powders using an HPLC assay, and the batch-1 extract was used in the present study unless otherwise stated.
SHL particles were prepared by spray-drying using a Buchi B-290 mini spray dryer consisting of a high-performance cyclone and a two-fluid atomiser with a nozzle diameter of 1.5 mm (Flawil, Switzerland) after the preparation of drug and excipients solution, and spray-dried particles were obtained in the collection jar of the spray dryer. Drug and excipients solutions were prepared by dissolving in purified water. For the preliminary study, which tested the effect of the content of leucine and spray-drying parameters, including inlet temperature, feed concentration, feed rate and atomising pressure, on particle size and surface morphology of SHL particles, the total solid (SHL and leucine) amount in the feed was maintained at 350 mg, and the spray-drying experimental conditions were listed in Table I, sample nos. 1–15. For the spray-drying of SHL particles for further formulation study, the total solid amount in the feed was maintained at 2.50 g, and in order to obtain particles with varying degree of surface roughness but similar particle size, the processing parameters were customised as shown in Table I, sample nos. 16–18.
Table I.
Effects of Spray-Drying Conditions and Leucine Content on the Yields, Volume Median Diameter (D0.5) and Surface Morphology of Shuang-Huang-Lian Particles (Mean ± SD, n = 3)
| Sample no. | Leucine (%) | Spray-drying parameters | Yielda | D 0.5 (μm) | Surface morphology | |||
|---|---|---|---|---|---|---|---|---|
| T in (°C) | FC (mg/ml) | AP (L/h) | FR (ml/min) | |||||
| 1 | 0 | 100 | 10 | 540 | 3.75 | 36.8% | 1.96 ± 0.01 | S or dimpled |
| 2 | 1 | 100 | 10 | 540 | 3.75 | 52.5% | 1.98 ± 0.02 | MC |
| 3 | 5 | 100 | 10 | 540 | 3.75 | 59.2% | 1.99 ± 0.02 | C |
| 4 | 10 | 100 | 10 | 540 | 3.75 | 59.6% | 1.98 ± 0.02 | C |
| 5 | 20 | 100 | 10 | 540 | 3.75 | 58.7% | 2.01 ± 0.02 | C |
| 6 | 10 | 60 | 10 | 540 | 3.75 | 46.1% | 2.10 ± 0.01 | S or MC |
| 7 | 10 | 80 | 10 | 540 | 3.75 | 50.4% | 2.05 ± 0.02 | MC |
| 8 | 10 | 120 | 10 | 540 | 3.75 | 60.6% | 1.98 ± 0.02 | C |
| 9 | 10 | 140 | 10 | 540 | 3.75 | 59.2% | 1.96 ± 0.01 | C |
| 10 | 10 | 100 | 1 | 540 | 3.75 | 23.1% | 1.33 ± 0.01 | MC |
| 11 | 10 | 100 | 50 | 540 | 3.75 | 60.2% | 2.59 ± 0.01 | C |
| 12 | 10 | 100 | 10 | 360 | 3.75 | 55.4% | 2.63 ± 0.01 | C |
| 13 | 10 | 100 | 10 | 740 | 3.75 | 42.6% | 1.84 ± 0.01 | C or MC |
| 14 | 10 | 100 | 10 | 540 | 1.13 | 62.3% | 1.83 ± 0.01 | C |
| 15 | 10 | 100 | 10 | 540 | 1.88 | 57.8% | 1.90 ± 0.02 | C |
| 16 | 10 | 100 | 13 | 470 | 3.75 | 61.5% | 2.04 ± 0.02 | C |
| 17 | 10 | 60 | 10 | 540 | 3.13 | 55.4% | 2.12 ± 0.01 | MC |
| 18 | 10 | 60 | 13 | 600 | 3.75 | 47.9% | 1.93 ± 0.01 | S |
T in inlet temperature, FC feed concentration, AP atomisation pressure, FR feed rate, C corrugated surface, MC moderately corrugated surface, S smooth surface
aThe solid amount in the feed was maintained at 350 mg
Particle Morphology and Size Analysis
The particle morphology was investigated using scanning electron microscopy (SEM) (JSM-6510LV, JEOL, Japan) at 5 or 15 kV and atomic force microscopy (AFM) (Multimode AFM, Nanoscope controller, Veeco Inc., Cambridge, UK). Prior to SEM imaging, samples were mounted onto metal sample plates and gold-coated using Auto Fine Coater (JFC-1600, JEOL, Japan). For AFM analysis, samples were mounted on carbon sticky tabs and imaged at a scan rate of 0.799 Hz using tapping mode tips over 5 × 5 μm. The variation in surface corrugation was determined by analysing 1.2 × 1.2 μm2 areas of individual particles (n ≥ 5) and calculated the root mean square roughness (RRMS) using Eq. 1.
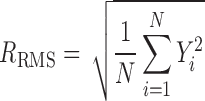 |
1 |
Where N is the number of data points in a topographical profile and Yi is the distance of asperities (i) from the centre line. The mean surface roughness was determined based on the RRMS of at least five different individual particles.
The particle size distribution was determined by laser diffraction (Malvern Mastersizer 2000, Malvern, UK). Approximately 5 mg of samples were dispersed in 0.1% w/v lecithin cyclohexane solution and sonicated in a water bath for 5 min to disperse any possible agglomerates before being added to the sample cell. All samples were analysed in triplicate. Span was defined as (D0.9 − D0.1)/D0.5, where D0.9,D0.5 and D0.1 were the diameters at 90%, 50% and 10% cumulative volumes, respectively.
Density Measurements
A 5-ml cylinder was used in the bulk density determination of spray-dried particles. The container was filled with accurately weighed particles, and the top was levelled. The bulk density was calculated as the ratio of the mass to the volume of the sample without any external force or taping. The tap density was determined using the same sample for the bulk density, but the volume was taken for calculations after taping. The taping is performed manually, in a vertical manner from a distance of approximately 5 cm onto a level bench top surface, for up to 500 strokes until the volume no longer changed. Measurements were performed in triplicate.
Water Content and Thermal Analysis
Water content was determined using thermo gravimetric analyser (TGA Q50, TA, USA). Approximately 5-mg samples were placed in aluminium pans, and data were collected between 20°C and 105°C at a heating rate of 10°C/min.
Thermal analysis was analysed using differential scanning calorimetry (DSC Q200, TA, USA). Approximately 5 mg of sample was placed in an aluminium pan, and data were collected between 20°C and 300°C at a heating rate of 10°C/min.
HPLC Analysis of the Samples
The amount of SHL samples was quantified using a HPLC assay. Baicalin was the major constituent in SHL, and hence, it was used as a chemical marker for quantitative analysis. The assay was carried out using a Waters HPLC system, which included a Waters e2695 system, Waters 2489 Detector and an Empower software system. Each sample was injected onto a phenomenex-C18 column (4.6 × 250 mm, 5 μm) equipped with a Phenomenex guard column (4.0 × 3.0 mm) (Phenomenex Inc.) with an aliquot of 10 μL. Subsequently, the column was eluted with methanol–water–formic acid (60:40:0.3) at a flow rate 1.0 ml/min and detected at a wavelength of 280 nm.
Preparation of SHL Particles–Mannitol/Lactose Carrier Blends
Mannitol and lactose were sieved through 90 μm and 125 μm sieves to obtain 90 to 125 μm size carrier crystals. Laser diffraction results indicated that the particle size of both carriers varied from 45.7 to 182.0 μm with mean volume diameters of 100.6 and 98.6 μm for lactose and mannitol, respectively. After sieving, spray-dried powders were blended with the coarse mannitol/lactose (90–125 μm) in drug-to-carrier mass ratios of 1:3 and 1:60 by hand-mixing with a batch size of 1.0 g at ∼30% RH and room temperature (24 ± 3°C). Briefly, 250 mg (drug/carrier 1:3) or 16.4 mg (drug/carrier 1:60) of SHL was weighed into a glass mortar, to which had been added 250 or 16.4 mg of carrier crystals, followed by mixing with a spatula. Then, more carrier (equal to the amount of the blend in the glass mortar) was added to the glass mortar and mixed with a spatula. This process was repeated until all the carrier (750 mg for drug/carrier 1:3 and 983.6 mg for drug/carrier 1:60 blends, respectively) had been incorporated into the blends. Homogeneity of each blend was measured by analysing the amount of baicalin at aliquots of 40 ± 0.1-mg blends (n = 10). Subsequently, aliquots of 40 mg drug–carrier blends were filled in Size 3 hard gelatin capsules and stored at ∼30% RH and room temperature for 24 h prior to testing.
In Vitro Aerosol Deposition Studies
An in vitro determination of the aerodynamic characteristics of SHL following the aerosolisation of formulations via a Cyclohaler was carried out using a next-generation pharmaceutical impactor (NGI, Apparatus E; British Pharmacopoeia, 2010) (MSP, USA) at ∼30% RH and room temperature. The Cyclohaler is a commercially available, breath-actuated, single-dose inhalation device that contains the drug/carrier blend as a dry powder formulation in gelatin capsules. The Cyclohaler was attached to the NGI via a rubber mouthpiece, and a filled capsule was placed into the Cyclohaler. Subsequently, the device was activated to disperse SHL into NGI at a flow rate of 60 L min−1 for 4-s intervals. All samples were run at least in triplicate. After each actuation, the drug particles in the device, capsule, throat, pre-separator and each stage of NGI were washed with deionised water into 20-ml volumetric flasks and made up to volume, followed by HPLC analysis. Subsequently, the data were analysed to give total drug dose (TD) (drug collected in the device, capsule, mouthpiece and throat, pre-separator, stages 1–7 and the micro-orifice collector, MOC), ED (drug collected in the mouthpiece and throat, pre-separator, stages 1-MOC), FPD (drug collected in stages 3-MOC) and FPF<4.4 μm (ratio of drug collected in stages 3-MOC to the TD). The mass median aerodynamic diameter (MMAD) was the diameter at the 50% cumulative percentage whilst the geometric standard deviation was defined as the ratio of the diameter at the 84.1% cumulative percentage to the 50%.
To further test the contents of drug adhering to the carrier surface during inhalation, drug–carrier blends (1:60) were dispersed via a Cyclohaler at different flow rates of 30, 45, 60, 90 and 120 L min−1 using the above-mentioned NGI testing protocol. The carriers in the throat and pre-separator were collected, and the contents of drug adhering to the carriers were determined using a HPLC assay.
Data Analysis
All values were expressed as mean ± SD. Data were analysed using Student’s t test or factorial analysis of variance (ANOVA). The latter was followed by post hoc comparisons using the Dunnett’s or Student–Newman–Keuls (SNK) tests if the ANOVA manifested a significant difference. A two-tailed P value of 0.05 or less was taken to indicate statistical significance. All tests were performed using SPSS for windows 17.0.
RESULTS AND DISCUSSION
Preparation of Spray-Dried SHL Particles
Spray-drying has been utilised for the preparation of microparticles with controlled particle morphology and suitable for pulmonary drug delivery (7,8). Previous studies showed that varying the feed solvent (23) and adjusting the outlet drying temperature (24) affected particle morphology, and concomitantly manipulating feed concentration and atomisation rate might produce particles with different degrees of surface corrugation (13,14).
In the present study, a preliminary study had tested the effect of excipients and spray-drying parameters such as inlet temperature, feed concentration, feed rate and atomising pressure on particle size and surface morphology of SHL particles. The results (Table I) suggested that inlet temperature and the presence of leucine played a crucial role in affecting corrugated degrees of particle surface, whilst particle size distribution was dictated by controlling the atomising pressure and feed concentration. In the absence of leucine, spray-dried SHL particles always presented smooth surfaces or smooth surfaces containing dimples, and no wrinkled particles could be produced, whilst the presence of leucine at the contents between 1.0% and 20.0% w/w affected the corrugated degrees of particle surface in a dose-dependent manner (Table I). When SHL was spray-dried in the presence of 10% leucine and the inlet temperature was 100°C or higher, corrugated particles were always obtained except one sample prepared with high atomisation pressure. Decreasing the inlet temperature to 60°C might lead to the production of particles with round and smooth surfaces, and at this low inlet temperature, decreasing atomising pressure and feed concentration tended to increase the corrugated degrees of particle surface. As a result, upon concomitantly manipulating the spray-drying parameters as listed in Table I, sample nos. 16–18, particles with similar particle size but different surface roughness were produced.
The mechanism by which inlet temperature and the presence of leucine affected the particle surface morphology was not entirely clear. Previous studies suggested that the surface morphology of spray-dried particles might be related to Peclet numbers, and compositions with higher Peclet numbers tended to form particles with irregular surface (8). Peclet numbers are dimensionless numbers to define the characteristics of solute transport in the fluids. High Peclet numbers represent solute transport dominated by advection, whereas low Peclet numbers correspond to transport dictated by molecular diffusion. In other words, for a feed solute with a high Peclet number, the solute transport is dominated by advection, and the molecular diffusion is limited, as a result, the solutes may not have sufficient time to diffuse to the inner core during spray-drying and tend to accumulate in the shell of droplets, favouring the formation of corrugated surface (8). The Peclet number increases with increasing the evaporation rate, and the latter is dictated by the combination of feed composition and spray-drying parameters (8). Therefore, it is conceivable that an increase in inlet temperature favours the formation of wrinkled particles since the Peclet number may increase with an increase in evaporation rate of sprayed droplets as a result of high inlet temperature. In addition, the feed solubility was also reported to affect the particle morphology previously (8,25,26). Vehring (8) suggested that the Peclet number of feed solute with low solubility increases dramatically during spray-drying, favouring the formation of a very early shell on the droplet surface, and the corrugated particles may be produced when the shell was shrivelled. In the present study, SHL has a high solubility of more than 200 mg/ml, and hence, it was difficult to be spray-dried to produce particles with corrugated surface. Leucine is a weak surfactant, and it tends to accumulate in the surface of spray-dried droplets (27). With the drying process progressing, leucine is assumed to reach supersaturation in the outside layer due to its low solubility of 22 mg/ml in water, leading to the formation of an early shell and the subsequent corrugated surface.
Apart from the particle size and surface morphology, spray-drying parameters also appeared to affect the yields of particles, and corrugated particles were generally produced at higher yields than smooth particles (Table I).
Physical Characteristics of Spray-Dried Particles
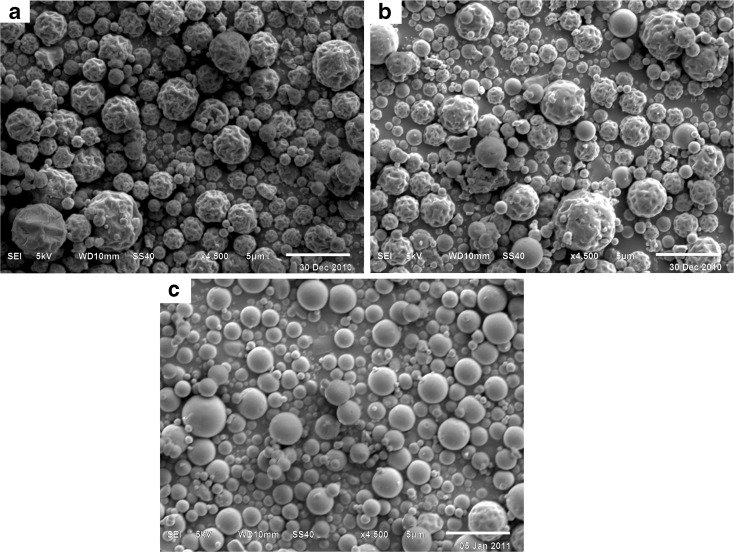
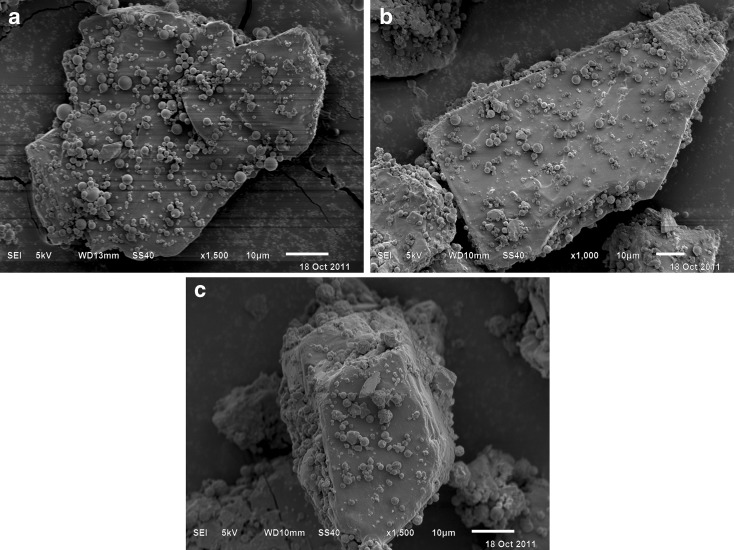
The surface morphology of spray-dried particles was characterised by SEM (Fig. 1a–c), which showed that the particle surfaces varied from smooth to moderately corrugated, and to raisin-like. The degree of surface corrugation of each sample was further quantified using AFM. The AFM topographical images showed RRMS values (Table II) of 115.6 ± 5.3, 76.9 ± 1.6 and 57.6 ± 5.1 nm (n = 5) for the corrugated, moderately corrugated and smooth particles, respectively. The AFM results suggested that there existed significantly (ANOVA, p < 0.01) different degrees of surface corrugation amongst the three types of particles in terms of RRMS. The SEM showed that the surface of either mannitol (data not shown) or lactose (Fig. 2) appeared to be smooth as compared with the corrugated or moderately corrugated particles. The RRMS values of mannitol and lactose were determined to be 44.3 ± 6.7 and 37.5 ± 7.8 nm (n = 5). When blended with carrier crystals, the spray-dried particles were generally attached to the carrier surface individually rather than as agglomerates (Fig. 2).
Fig. 1.
Scanning electron micrographs of spray-dried a corrugated, b moderately corrugated and c smooth Shuang-Huang-Lian particles
Table II.
Physicochemical Characteristics of Spray-Dried Shuang-Huang-Lian Particles (Mean ± SD, n = 3 or 5)
| Particle samples | Corrugated | Moderately corrugated | Smooth |
|---|---|---|---|
| R RMS roughness (nm) | 115.6 ± 5.3 | 76.9 ± 1.6 | 57.6 ± 5.1 |
| D 0.5 (μm) | 2.04 ± 0.02 | 2.12 ± 0.01 | 1.93 ± 0.01 |
| Span | 0.85 ± 0.01 | 0.83 ± 0.01 | 0.79 ± 0.01 |
| Bulk density (g ml−1) | 0.35 ± 0.02 | 0.28 ± 0.01 | 0.24 ± 0.02 |
| Tap density (g ml−1) | 0.51 ± 0.03 | 0.47 ± 0.02 | 0.46 ± 0.04 |
| Tap-to-bulk density ratio | 1.43 ± 0.02 | 1.67 ± 0.02 | 1.92 ± 0.03 |
| Water content (%) | 2.36 ± 0.47 | 2.53 ± 0.21 | 2.34 ± 0.11 |
| Melting point (°C) | 165.8 ± 3.8 | 166.4 ± 7.0 | 162.7 ± 8.3 |
D 0.5 volume median diameter, span defined as (D 0.9 − D 0.1)/D 0.5
Fig. 2.
Scanning electron microscopy images of spray-dried Shuang-Huang-Lian a smooth particles, b moderately corrugated particles and c corrugated particles blended with lactose crystals at a particle-to-carrier mass ratio of 60:1
The particle size and size distribution of the spray-dried particles are shown in Table II. The spray-dried SHL particles had similar particle size distributions with median volume diameters being between 1.93 and 2.12 μm. The particle size span for these batches were between 0.79 and 0.85, which indicated that all the particles exhibited a moderate degree of monodispersity. The DSC thermograms of the three batch particles were similar and exhibited a characteristic endothermic peak at ∼165°C, attributable to the melting point of baicalin mixture (data not shown). The TGA and DSC data suggested no significant differences (ANOVA, p > 0.05) in the water content and melting point amongst the spray-dried particles as shown in Table II. The bulk and tap density and the ratio of tap to bulk density (Table II) were found to relate to the surface morphology in general with the corrugated particles having the highest bulk and tap density as well as the lowest ratio of tap to bulk density. The ratio of tap to bulk density, also known as Hausner ratio, reflects the flowability of particles, which is closely related to the interparticle interactions, and the lower the Hausner ratio values, the less cohesive the particles are (28,29). Therefore, it could be deduced that corrugated particles had lower the interparticulate interactions than moderately corrugated or smooth particles, and such findings were in well agreement with previous results (13–16,30).
In Vitro Aerosol Deposition Studies
After the spray-dried particles were blended with mannitol/lactose carriers, the homogeneity of the blends was determined. When the samples were sampled at aliquots of 40-mg blends, the degree of homogeneity was acceptable with the mean drug content for all blends being within 100.0 ± 3.0% of the nominal value and each blend exhibiting a coefficient of variance <4.0% (Table III).
Table III.
In Vitro Deposition Results of Spray-Dried Shuang-Huang-Lian Particles Blended with Mannitol or Lactose Carriers after Dispersed at 60 L/min Using the Cyclohaler (Mean ± SD, n = 3, Unless Stated Otherwise)
| Particles | Corrugated | Moderately corrugated | Smooth | |||
|---|---|---|---|---|---|---|
| Carriers | Mannitol | Lactose | Mannitol | Lactose | Mannitol | Lactose |
| Homogeneity (%)a | 98.0 ± 2.0 | 98.7 ± 2.3 | 99.5 ± 3.4 | 100.8 ± 1.4 | 98.6 ± 2.2 | 97.6 ± 2.1 |
| TDb (mg) | 8.22 ± 0.41 | 8.02 ± 0.54 | 8.21 ± 0.36 | 8.41 ± 0.15 | 8.16 ± 0.60 | 8.36 ± 0.27 |
| EDc (mg) | 6.16 ± 0.38 | 6.01 ± 0.45 | 6.89 ± 0.18 | 7.16 ± 0.20 | 6.89 ± 0.49 | 7.25 ± 0.42 |
| MMADd (μm) | 2.71 ± 0.25 | 3.41 ± 0.55 | 3.11 ± 0.28 | 3.53 ± 0.39 | 3.61 ± 0.35 | 3.67 ± 0.06 |
| Geometric standard deviation | 3.09 ± 0.90 | 2.50 ± 0.88 | 2.55 ± 0.56 | 2.76 ± 0.80 | 2.22 ± 0.23 | 2.33 ± 0.10 |
| EDe (%) | 75.2 ± 2.8 | 74.9 ± 2.3 | 83.4 ± 1.6 | 84.8 ± 0.8 | 84.5 ± 5.1 | 87.1 ± 3.9 |
| FPFTD f (%) | 36.8 ± 1.9 | 32.4 ± 4.2 | 29.6 ± 2.2 | 25.4 ± 3.0 | 16.0 ± 2.6 | 14.7 ± 2.1 |
| Dispersibilityg (%) | 49.8 ± 5.7 | 43.2 ± 4.4 | 35.5 ± 2.9 | 29.9 ± 3.6 | 19.1 ± 3.9 | 18.2 ± 1.3 |
| Pre-separator (%) | 18.2 ± 3.6 | 14.1 ± 3.5 | 33.2 ± 1.7 | 37.6 ± 4.6 | 52.2 ± 9.0 | 56.5 ± 3.5 |
| Removal efficiencyh (%) | 91.0 ± 0.8 | 92.4 ± 1.5 | 94.2 ± 1.0 | 95.9 ± 0.8 | 93.9 ± 0.7 | 95.3 ± 0.9 |
TD total recovered dose, ED emitted dose, MMAD mass median aerodynamic diameter, FPF fine particle fractions
aThe relative mean drug content to the nominal value by analysing 40.0 ± 0.10 mg blends (n = 10)
bCumulative mass of drug collected from all parts of NGI, capsule and device
cCumulative mass of drug collected from all parts of NGI
dMass median aerodynamic diameter
eED/TD × 100
fFPD/TD × 100, FPD referred to the cumulative mass of drug depositing on stages 3 to the micro-orifice collector
gFPD/ED × 100
hRatio of drug mass exiting the capsule to TD
In vitro deposition results of spray-dried SHL particle–mannitol/lactose blends after dispersal at 60 L/min using the Cyclohaler are shown in Table III. The total recovered dose of SHL varied from 8.02 to 8.41 mg for different drug–carrier blends, corresponding to a percent recovery between 89.1% and 93.5%. No significant differences (t test, p > 0.05) were observed between depositing profiles of SHL aerosolised from formulations blended with either mannitol or lactose when the surface morphology of drug particles were the same (Table III). FPF<4.4 μm of drug from SHL–mannitol formulation was slightly, but not significantly (t test, p > 0.05) higher than that of the SHL–lactose blends, whereas the percentage of emitted dose (ED%) appeared to be almost the same between the two types of blends.
The deposition data showed that the ED%, fine particle fractions (FPF) and dispersibility depended upon the surface roughness of spray-dried particles (Table III). The ED% of SHL from corrugated particle–carrier mixtures were ∼75%, which was about 8–12% less than those of moderately corrugated or smooth particle–carrier blends. Although the ED% of the former blends were significantly (SNK, p < 0.05) lower than those the latter two kinds of blends, the corrugated SHL particles gave a significantly higher FPF than moderately corrugated (SNK, p < 0.05) or smooth (SNK, p < 0.01) particles. The FPF for corrugated, moderately corrugated and smooth particle based formulations were found to be about 32.4–36.8%, 25.4–29.6% and 14.7–16.0%, respectively. When compared with the smooth SHL particles, corrugated particles increased the FPF by over twofold. In terms of dispersibility, the corrugated SHL particles were also significantly better than either moderately corrugated (SNK, p < 0.01) or smooth (SNK, p < 0.01) particles.
Further analysis of the deposition profiles of SHL blends indicated that there were significant differences (ANOVA, p < 0.01) in the fractions of SHL drug recovered in the pre-separator. The pre-separator was intended for the removal of oversized particles, particularly the carrier. The fractions of SHL drug recovered in the pre-separator for corrugated particles were found to be between 14.1% and 18.2%, which were about one third of those for smooth particles (between 52.2% and 56.5%).
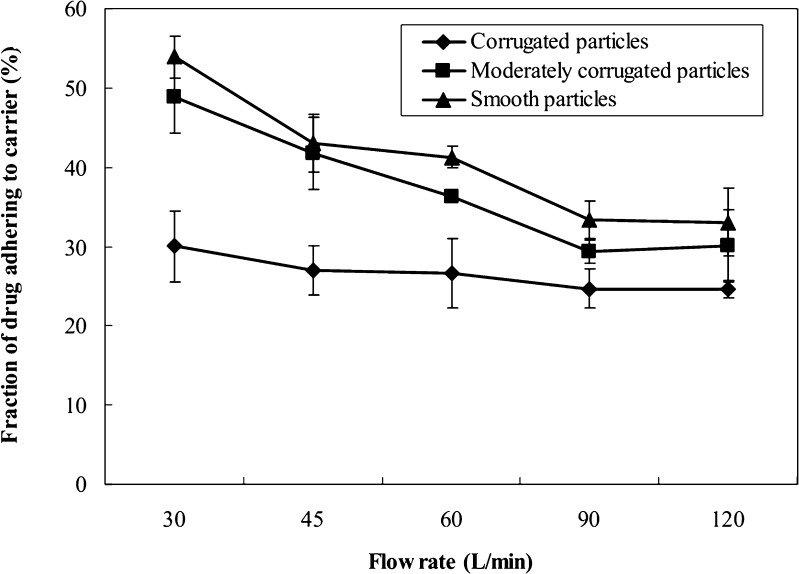
Since the amount of SHL recovered in the pre-separator was mainly attributable to the content of drug particles adhering to the carriers, the effects of airflow rates on the drug particle detachment from lactose carrier were investigated, and the data are shown in Fig. 3. Following dispersion, drug detachment from carrier crystals appeared to increase with not only the flow rate but also the degree of surface corrugation. Corrugated particles always provided a significantly higher detached fraction of drug during inhalation compared with moderately corrugated (SNK, p < 0.01) or smooth (SNK, p < 0.01) particles when the flow rate was maintained the same. In addition, the decreasing profiles of the adhered fraction of drug with increasing the flow rate were found to be different. With increasing the flow rate from 30 to 120 L/min, the adhered fraction of drug appeared to significantly (ANOVA, p < 0.01) decrease for moderately corrugated or smooth particles, whereas corrugated particles only resulted in slight, but not significant (ANOVA, p > 0.05) decrease (Fig. 3).
Fig. 3.
The fraction of corrugated, moderately corrugated and smooth particles adhering to carrier crystals after dispersed from a Cyclohaler at different flow rates (mean ± SD, n = 3)
Liberation of particles from the carrier surfaces is dictated by the magnitude of drug–carrier adhesive forces, and excessive adhesive forces could prevent detachment of drug from the carrier surfaces, leading to poor detachment (18,31–36), therefore an increase in the surface roughness of SHL particles could be assumed to result in the reduction in the particle–carrier adhesive forces and subsequently leading to the improved detachment efficiency. In fact, such an assumption was supported by the findings from a previous study (15). In the earlier study, Adi and colleagues had utilised corrugated particles to improve the aerosol performance of BSA in a drug–carrier DPI system for the first time. They have found that adhesive forces between the corrugated drug and carrier were significantly less than smooth/carrier interaction forces as measured using colloid probe microscopy and have attributed the increased aerosolisation efficiency to the lowered drug–carrier adhesive forces conferred by corrugated particles (15). Such an explanation could also explain similar enhanced aerosolisation properties conferred by SHL corrugated particles. In other words, the increased aerosolisation efficiency of SHL with increasing surface roughness was attributable to the reduced adhesive forces between drug and carrier. However, there existed discrepancy between the deposition profiles of the present SHL and previous BSA particles, in terms of the ED%, detachment efficiency and increased extent of FPF. The previous study showed that the corrugated BSA particles aerosolised from BSA/carrier blends gave a similar ED%, 26% increase in FPF and a marginal increase in the detachment efficiency, relative to the smooth particles (15). In contrast, the present study suggested that corrugated particles led to significantly lower ED% but dramatically increased detachment efficiency and FPF (by over twofold), when compared with the smooth SHL particles. The reason for such a discrepancy was unclear. One possible explanation might be due to the fact that the contribution of the carrier surface roughness to reduction in the drug–carrier adhesive forces was not negligible in the earlier study.
In addition, the lower adhesive forces between corrugated particle and carrier surfaces might also account for the lower ED% of corrugated particle–carrier blends. The ED% of the carrier-based formulation was previously found to relate to the affinity of drug particle to the carrier (34,35). Salbutamol sulphate–lactose formulations were found to exhibit high ED% of 81.9% ± 3.3% due to the strong drug–excipient interactions, whereas the low adhesion forces of budesonide particles in a carrier-based formulation led to low ED% of 47.7% ± 1.2% (34).
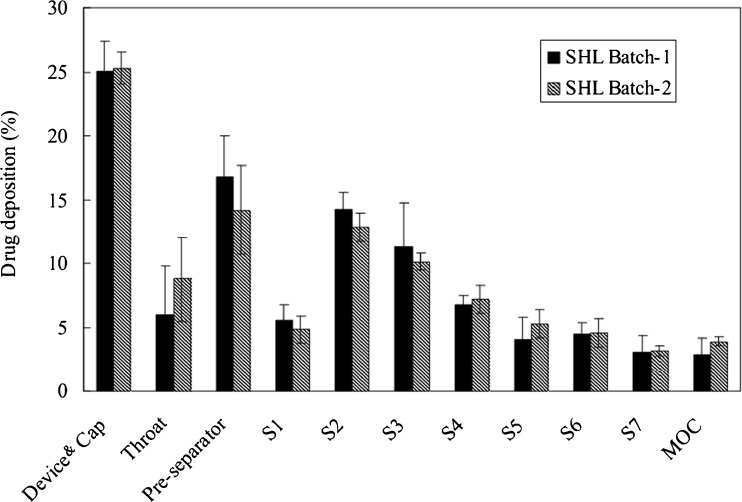
Two batches of SHL extracts, batch-1 and batch-2, were used to prepare spray-dried particles, and there were substantial variations in the chemical composition between the extracts. For example, the content of baicalin in the batch-2 extract was only ∼66% of that in the batch-1. However, the variation in constituent composition appeared not to affect the production of corrugated particles, and most importantly, the aerodynamic properties of the resultant particles. The deposition profiles of corrugated particles prepared from either extracts were similar, demonstrating a similar aerodynamic behaviour for batches 1 and 2 (Fig. 4). The reason for the finding that the variation in constituent composition was of insignificant relevance to aerosol performance seemed to be that adhesion forces between corrugated particle and carrier surfaces were weak enough to allow efficient drug detachment from the carrier. As a result, the variation in adhesion forces resulting from batch-to-batch variation in constituent composition did not affect the deposition profiles of corrugated particles. Such an assumption was supported by the data in Fig. 3, which showed that the detachment efficiency for corrugated particles increased insignificantly with increasing airflow rate.
Fig. 4.
In vitro NGI deposition of spray-dried Shuang-Huang-Lian corrugated particles containing different constituent composition blended with lactose carrier after dispersed at 60 L/min using a Cyclohaler (mean ± SD, n = 3)
The unique challenge for the development TCM DPI as compared with conventional medicine is due to the chemical complexity and batch-to-batch variations in constituent composition. Ideally, TCM DPI should be allowed the preparation and aerosol performance to tolerate the batch-to-batch variations in constituent composition. In this study, the surface roughness of spay-dried SHL particles was dictated by the presence of leucine, and the spray-drying parameters and the preparation of corrugated particles appeared to be indifferent to the composition of SHL. In addition, aerosol performance of corrugated particles in a carrier-based DPI was independent of the batch-to-batch variations in constituent composition. Therefore, the corrugated particles were considered potentially applicable to the development of SHL DPI. Nevertheless, it should be noted that the optimal surface roughness level and inter-batch variability of the whole corrugated particles were not determined. The issues concerning to the quality control of particle surface roughness need to be considered for the manufacture of corrugated SHL particles and their DPI products.
CONCLUSION
The present study has produced spray-dried SHL particles with a different degree of surface roughness, and the corrugated particles could be prepared independent of variations in SHL composition constituent by the presence of leucine at a high inlet temperature. The FPF of SHL in carrier-based DPI were found as a function of particle surface roughness, and the improved aerosol performance by corrugated particles was mainly attributed to the high detachment efficiency of SHL from the carrier upon aerosolisation as a result of decreases in drug–carrier adhesive forces. Considering that the production of corrugated particles and the aerosol performance of the particles in drug–carrier DPI systems were independent of the batch-to-batch variation in constituent composition, such particles might be utilised for the development of SHL DPI products.
ACKNOWLEDGEMENT
This work was supported by the National S&T Major Project (2012ZX09301-002-030) from the Ministry of Science and Technology of the People’s Republic of China and the National Natural Science foundation of China (grant no: 81172997).
REFERENCES
- 1.Lin F, Yin LF, Jin SH. Reaction and analysis on adverse drug reactions caused by Shuanghuanglian injections. Chin Pharm Affairs. 2009;23:499–502. [Google Scholar]
- 2.Lu YX. Effection of inhaled SHL to treatment influenza. Modern J Integr Tradit Chin Western Med. 2007;18:2523. [Google Scholar]
- 3.Wang C, Liu XB, Quan LH, Liu CY, Liao YH. Pulmonary delivery of traditional Chinese medicines. World Sci Tech-Mod Tradit Chin Med. 2007;9:59–64. [Google Scholar]
- 4.Ye JX, Wang W, Quan LH, Liu CY, Chang Q, Liao YH. An LC-MS/MS method for the simultaneous determination of chlorogenic acid, forsythiaside A and baicalin in rat plasma and its application to pharmacokinetic study of Shuang-Huang-Lian in rats. J Pharm Biomed Anal. 2010;52:625–30. doi: 10.1016/j.jpba.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Han R, Ye JX, Quan LH, Liu CY, Yang M, Liao YH. Evaluating pulmonary toxicity of Shuang-Huang-Lian in vitro and in vivo. J Ethnopharm. 2011;135:522–9. doi: 10.1016/j.jep.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 6.Chow AHL, Tong HHY, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24:411–37. doi: 10.1007/s11095-006-9174-3. [DOI] [PubMed] [Google Scholar]
- 7.Seville PC, Li HY, Learoyd TP. Spray-dried powders for pulmonary drug delivery. Crit Rev Ther Drug Carrier Sys. 2007;24:307–60. doi: 10.1615/CritRevTherDrugCarrierSyst.v24.i4.10. [DOI] [PubMed] [Google Scholar]
- 8.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25:999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maa YF, Nguyen PA, Sweeney T, Shire SJ, Hsu CC. Protein inhalation powders: spray drying vs spray freeze drying. Pharm Res. 1999;16:249–54. doi: 10.1023/A:1018828425184. [DOI] [PubMed] [Google Scholar]
- 10.Palakodaty S, York P. Phase behavioral effects on particle formation processes using supercritical fluids. Pharm Res. 1999;16:976–85. doi: 10.1023/A:1011957512347. [DOI] [PubMed] [Google Scholar]
- 11.Rasenack N, Steckel H, Muller BW. Micronization of anti-inflammatory drugs for pulmonary delivery by a controlled crystallization process. J Pharm Sci. 2003;92:35–44. doi: 10.1002/jps.10274. [DOI] [PubMed] [Google Scholar]
- 12.Kaerger JS, Price R. Processing of spherical crystalline particles via a novel solution atomization and crystallization by sonication (SAXS) technique. Pharm Res. 2004;21:372–81. doi: 10.1023/B:PHAM.0000016252.97296.f1. [DOI] [PubMed] [Google Scholar]
- 13.Chew NYK, Chan HK. Use of solid corrugated particles to enhance powder aerosol performance. Pharm Res. 2001;18:1570–7. doi: 10.1023/A:1013082531394. [DOI] [PubMed] [Google Scholar]
- 14.Chew NYK, Tang P, Chan HK, Raper JA. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res. 2005;22:148–52. doi: 10.1007/s11095-004-9020-4. [DOI] [PubMed] [Google Scholar]
- 15.Adi H, Traini D, Chan HK, Young PM. The influence of drug morphology on the aerosolisation efficiency of dry powder inhaler formulations. J Pharm Sci. 2008;97:2780–8. doi: 10.1002/jps.21195. [DOI] [PubMed] [Google Scholar]
- 16.Adi S, Adi H, Tang P, Traini D, Chan HK, Young PM. Micro-particle corrugation, adhesion and inhalation aerosol efficiency. Eur J Pharm Sci. 2008;35:12–8. doi: 10.1016/j.ejps.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Aquino RP, Prota L, Auriemma G, Santoro A, Mencherini T, Colombo G, Russo P. Dry powder inhalers of gentamicin and leucine: formulation parameters, aerosol performance and in vitro toxicity on CuFi1 cells. Int J Pharm. 2012;426:100–7. doi: 10.1016/j.ijpharm.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Chan HK. What is the role of particle morphology in pharmaceutical powder aerosols? Expert Opin Drug Deliv. 2008;5:909–14. doi: 10.1517/17425247.5.8.909. [DOI] [PubMed] [Google Scholar]
- 19.Zeng XM, Martin GP, Marriott C, Pritchard J. The effects of carrier size and morphology on the dispersion of salbutamol sulphate after aerosolization at different flow rates. J Pharm Pharmacol. 2000;52:1211–21. doi: 10.1211/0022357001777342. [DOI] [PubMed] [Google Scholar]
- 20.Flament MP, Leterme P, Gayot A. The influence of carrier roughness on adhesion, content uniformity and the in vitro deposition of terbutaline sulphate from dry powder inhalers. Int J Pharm. 2004;275:201–9. doi: 10.1016/j.ijpharm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Donovan MJ, Smyth HD. Influence of size and surface roughness of large lactose carrier particles in dry powder inhaler formulations. Int J Pharm. 2010;402:1–9. doi: 10.1016/j.ijpharm.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health of the PR China . Drug standard of Ministry of Health of the PR China, vol. 11. Beijing: People’s Medical Publishing House; 1996. pp. 11–38. [Google Scholar]
- 23.Gilani K, Najafabadi AR, Barghi M, Tehrani MR. The effect of water to ethanol feed ratio on physical properties and aerosolization behaviour of spray dried cromolyn sodium particles. J Pharm Sci. 2005;94:1048–59. doi: 10.1002/jps.20315. [DOI] [PubMed] [Google Scholar]
- 24.Maa YF, Costantino HR, Nguyen PA, Hsu CC. The effect of operating and formulation variables on the morphology of spray-dried protein particles. Pharm Dev Technol. 1997;2:213–23. doi: 10.3109/10837459709031441. [DOI] [PubMed] [Google Scholar]
- 25.Langrish TAG, Marquez N, Kota K. An investigation and quantitative assessment of particle shape in milk powders from a laboratory-scale spray dryer. Dry Technol. 2006;24:1619–30. doi: 10.1080/07373930601031133. [DOI] [Google Scholar]
- 26.Elversson JE, Millqvist-Fureby A. Particle size and density in spray drying—effects of carbohydrate properties. J Pharm Sci. 2005;94:2049–60. doi: 10.1002/jps.20418. [DOI] [PubMed] [Google Scholar]
- 27.Gliński J, Chavepeyer G, Platten JK. Surface properties of aqueous solutions of l-leucine. Biophys Chem. 2000;84:99–103. doi: 10.1016/S0301-4622(99)00150-7. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Rudolph V, Weigl B, Earl A. Interparticle van der Waals force in powder flowability and compactibility. Int J Pharm. 2004;280:77–93. doi: 10.1016/j.ijpharm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Castellanos A. The relationship between attractive interparticle forces and bulk behaviour in dry and uncharged fine powders. Adv Phys. 2005;54:263–376. doi: 10.1080/17461390500402657. [DOI] [Google Scholar]
- 30.Weiler C, Egen M, Trunk M, Langguth P. Force control and powder dispersibility of spray dried particles for inhalation. J Pharm Sci. 2010;99:303–16. doi: 10.1002/jps.21849. [DOI] [PubMed] [Google Scholar]
- 31.Dickhoff BHJ, de Boer AH, Lambregts D, Frijlink HW. The effect of carrier surface and bulk properties on drug particle detachment from crystalline lactose carrier particles during inhalation, as function of carrier payload and mixing time. Eur J Pharm Biopharm. 2003;56:291–302. doi: 10.1016/S0939-6411(03)00109-7. [DOI] [PubMed] [Google Scholar]
- 32.Hassan MS, Lau R. Inhalation performance of pollen-shape carrier in dry powder formulation: effect of size and surface morphology. Int J Pharm. 2011;413:93–102. doi: 10.1016/j.ijpharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Kaialy W, Martin GP, Larhrib H, Ticehurste MD, Kolosioneka E, Nokhodchi A. The influence of physical properties and morphology of crystallised lactose on delivery of salbutamol sulphate from dry powder inhalers. Colloid Surface B. 2012;89:29–39. doi: 10.1016/j.colsurfb.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Begat P, Morton DAV, Staniforth JN, Price R. The cohesive-adhesive balances in dry powder inhaler formulations II: influence on fine particle delivery characteristics. Pharm Res. 2004;21:1827–33. doi: 10.1023/b:pham.0000045236.60029.cb. [DOI] [PubMed] [Google Scholar]
- 35.Begat P, Price R, Harris H, Morton DAV, Staniforth JN. The influence of force control agents on the cohesive–adhesive balance in dry powder inhaler formulations. KONA. 2005;23:109–21. [Google Scholar]
- 36.Boshhiha AM, Urbanetz NA. Influence of carrier surface fines on dry powder inhalation formulations. Drug Dev Ind Pharm. 2009;35:904–16. doi: 10.1080/03639040802698794. [DOI] [PubMed] [Google Scholar]