Abstract
Current pharmacopeial methods for testing dry powder inhalers (DPIs) require that 4.0 L be drawn through the inhaler to quantify aerodynamic particle size distribution of “inhaled” particles. This volume comfortably exceeds the internal dead volume of the Andersen eight-stage cascade impactor (ACI) and Next Generation pharmaceutical Impactor (NGI) as designated multistage cascade impactors. Two DPIs, the second (DPI-B) having similar resistance than the first (DPI-A) were used to evaluate ACI and NGI performance at 60 L/min following the methodology described in the European and United States Pharmacopeias. At sampling times ≥2 s (equivalent to volumes ≥2.0 L), both impactors provided consistent measures of therapeutically important fine particle mass (FPM) from both DPIs, independent of sample duration. At shorter sample times, FPM decreased substantially with the NGI, indicative of incomplete aerosol bolus transfer through the system whose dead space was 2.025 L. However, the ACI provided consistent measures of both variables across the range of sampled volumes evaluated, even when this volume was less than 50% of its internal dead space of 1.155 L. Such behavior may be indicative of maldistribution of the flow profile from the relatively narrow exit of the induction port to the uppermost stage of the impactor at start-up. An explanation of the ACI anomalous behavior from first principles requires resolution of the rapidly changing unsteady flow and pressure conditions at start up, and is the subject of ongoing research by the European Pharmaceutical Aerosol Group. Meanwhile, these experimental findings are provided to advocate a prudent approach by retaining the current pharmacopeial methodology.
KEY WORDS: cascade impactor, compendial method, dry powder inhaler, sample volume
INTRODUCTION
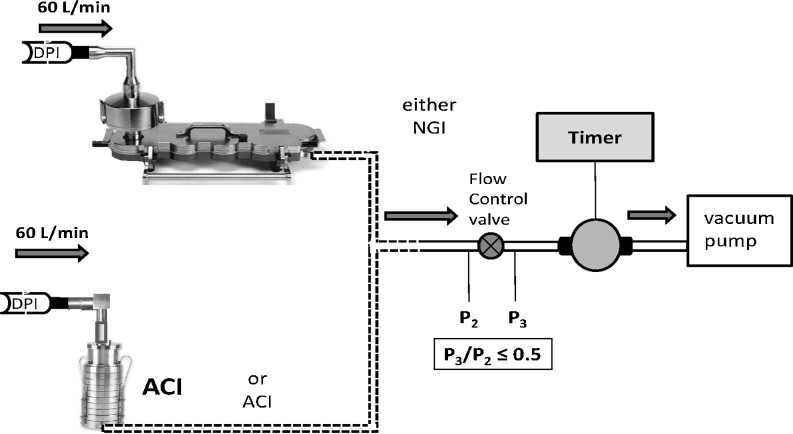
The current pharmacopeial procedure for assessing the aerosol aerodynamic particle size distribution (APSD) from a dry powder inhaler (DPI) includes the aerosolization and release of the powder bolus containing the active pharmaceutical ingredient(s) (API(s)) from the inhaler (1,2). The aerosol generation process and release of the aerosol bolus from the inhaler is accomplished by coupling the mouthpiece of the DPI containing the API(s) in either bulk powder (reservoir) or single-dose format to the entrance of an induction port whose purpose is to provide a basic simulation of an adult oropharynx (3). A preseparator is commonly inserted between the induction port and the pre-assembled impactor for the purpose of capturing carrier-bound API particles that collectively may be an order of magnitude in size larger than the carrier-free, deaggregated micronized API particles that are typically inhaled to provide therapeutic benefit. The preseparator also serves to capture any API-containing particles (agglomerates etc.) that may be released particularly from reservoir-based DPIs, particles whose size exceeds the effective cutoff diameter of the first stage of the cascade impactor (3). The complete system is connected to a vacuum pump via a flow controller containing a critical orifice that eliminates the impact of fluctuations caused by variations in pump performance (Fig. 1). The capacity of the vacuum pump is chosen such that the ratio of pressures downstream (P3) and upstream (P2) of the flow control valve is maintained at ≤0.5 to ensure critical flow after the initial rise to stable sampling conditions. This control valve is also used to adjust the flow rate to provide a 4-kPa pressure drop over the device. The word “critical” means that the flow has reached sonic velocity right at the controlling orifice. This condition therefore has nothing to do with the impactor, but it is merely a method of controlling the impactor flow rate in a reliable and reproducible manner. Other methods of controlling flow rely on human intervention (valves and flow meters) or on electronic feedback control. These more electronic control methods could be subject to wear, drift over time, and electronic failures.
Fig. 1.
Schematic of cascade impactor systems evaluated for DPI testing
Once the system has been assembled, actuation of the DPI takes place by initiation of flow propagating backwards from the vacuum pump, through the impactor, preseparator and induction port, and finally through the device itself. In the simplest methodology, the sampling duration can be set by a timer-operated solenoid valve that allows flow from the pump to enter the impactor system for a predetermined duration.
The cascade impactor should ideally operate at the final, fixed flow rate that is governed by the inhaler resistance for as long as is practical, as this type of particle size analysis equipment is designed to operate at a constant flow rate (3). In practice, a compromise is achieved by allowing the system to sample a total of 4.0 L from the DPI, which is intended to be a sufficient volume to permit the bolus to penetrate to the distal region of the impactor, thereby effecting a complete size fractionation from which the aerosol APSD can be determined. This volume compares with the internal volumes of 1.155 and 2.025 L for the Andersen eight-stage non-viable cascade impactor (ACI) and next-generation pharmaceutical impactor (NGI), respectively, including the pre-separator (4). In the case of the ACI which was used with a GlaxoSmithKline (GSK) throat, the assumption was made that it made negligible difference to the overall internal volume reported by Copley et al. (4) for the ACI used with the United Stated Pharmacopeia (USP) induction port. A 4-L sample volume is therefore considered large enough to allow an assessment of DPI performance without bias with either impactor system.
Recently, however, there has been an interest from those seeking greater clinical realism in the in vitro evaluation of inhalers, to attempt to match the volume inhaled by an adult in a single inspiratory maneuver (5). However, this is only one aspect of the process that is related to clinical relevance and cascade impactors (CIs) neither simulate the human inhalation action nor do they model the spatial particle deposition profile in the human respiratory tract (6). The present study was therefore undertaken to investigate how the NGI, which was developed to provide more accurate assessments of APSD than predecessor impactor systems, performed compared with the ACI when the total volume sampled was reduced from the recommended value.
MATERIALS AND METHODS
All measurements were undertaken under environmental conditions that were typically 21 ± 3°C, with RH from 35% to 70%, with precautions in place to mitigate bias from electrostatic charge (e.g., the risk of electrostatic charge from the operators to the equipment was minimized by limiting manual handling of equipment during inhaler actuations). The ACI was equipped with a GSK induction port (throat) and appropriate pre-separator, whereas the NGI was fitted with the USP throat and its appropriate pre-separator. The GSK throat is considered similar to the USP throat and dimensions are detailed in USP Pharm Forum 1996. The collection cups for the NGI and the collection plates for the ACI were each coated by a validated procedure, in order to avoid bias from particle bounce and re-entrainment within the impactor. One actuation of the DPI per determination was collected in the CI to ensure that there was no opportunity for material in the CI to become re-entrained. This approach provided adequate API for recovery and assay. The API was recovered from each component of the impactor system using a suitable solvent and subsequently assayed by means of a validated high-performance liquid chromatography -ultraviolet spectrophotometric procedure.
The principal metric was fine particle mass/actuation (FPM), which was expressed in terms of the mass % of the total recovered mass from the CI system (excluding wall losses) under evaluation. Wall losses were determined prior to any investigations and confirmed to be lower than 5% of the total recovered mass. The NGI was operated at 60 L/min, as there are archival calibration data from 30 to 100 L/min for this apparatus (7). The size range assigned to FPM for the NGI measurements was from 0.94 to 4.46 μm aerodynamic diameter (Table I). The ACI configuration normally used for measurements at 28.3 L/min (stages 0–7) was retained for these studies at 60 L/min, rather than optionally modifying the stage order as described by Nichols (8) for use at this higher flow rate. The API captured on stages 1–5 of the ACI in this hybrid configuration, consisted of particles nominally between 0.76 and 6.18 μm aerodynamic diameter (Table I). Large-particle mass (LPM), also expressed as a percentage of the total recovered mass, was a secondary metric. As a minimum, this term comprised the total mass collected by the appropriate pre-separator together with the mass deposited in the GSK induction port. In the case of the measurements made with the ACI system, the mass of API collected by stage 0 of this impactor (the first impaction stage) was added to the mass retained by the preseparator because the effective cutoff diameter for the preseparator and that for stage 0 had been shown by calibration with monodisperse particle standards to be nearly identical at 60 L/min (9).
Table I.
Cascade Impactor Configurations and Stage Effective Cutoff Diameters
| Impactor system | Component | ECD at 60 L/min (μm) | Stage groupings |
|---|---|---|---|
| NGI | Induction port | N.D. | LPM>4.46 μm |
| Preseparatora | 12.7 | ||
| Stage 1 | 8.06 | ||
| Stage 2 | 4.46 | ||
| Stage 3 | 2.82 | FPM0.94–4.46 μm | |
| Stage 4 | 1.66 | ||
| Stage 5 | 0.94 | ||
| Stage 6 | 0.55 | EPM<0.94 μm | |
| Stage 7 | 0.34 | ||
| ACI | Induction port | N.D. | LPM>6.18 μm |
| Preseparatorb | N.D. | ||
| Stage 0b | 6.18 | ||
| Stage 1 | 3.99 | FPM0.76–6.18 μm | |
| Stage 2 | 3.22 | ||
| Stage 3 | 2.77 | ||
| Stage 4 | 1.44 | ||
| Stage 5 | 0.76 | EPM<0.76 μm | |
| Stage 6 | 0.48 | ||
| Stage 7 | 0.27 |
N.D. effective cutoff diameters (ECD) not defined
aAPI assayed treating NGI preseparator as a separate stage
bMass on stage 0 added to mass in preseparator
In the first part of the investigation, all experiments were performed using a DPI (DPI-A, GSK plc, Ware, UK), having moderate flow resistance (1.5–3.5 kPa), in order to assess the effect of reduced sample volumes with an inhaler at a midpoint in the range of device resistance to air flow for currently marketed products. A high unit dose formulation of approximately 6.4% w/w of active drug (800 μg/actuation) was delivered from this DPI to either the ACI or NGI whose flow was initially controlled through a timer-operated solenoid valve set to achieve a stable inlet flow rate of 60 L/min after initial ramp up when the timer actuated the solenoid valve to initiate the sample. Measurements of API mass-per-stage were made with each CI, decreasing the sampling duration (directly proportional to sample volume) from 16 s (air flow of 16 and eight times the dead space (internal volume) of the ACI and NGI respectively) to 1 s (air flow of 1 and 0.5 times the dead space of the ACI and NGI, respectively). Three replicate measurements were made at each condition with each CI. A reference determination was also made following a standardized procedure (GSK plc, UK), in which the sampled volume was 4.0 L, but in this instance, seven successive actuations were delivered to the CI being evaluated before initiating API recovery. Each of the seven actuations was performed with 4.0 L of air drawn into the impactors. The reference was performed to enable comparison to the data that is generally generated for the product in routine analysis conducted at GSK plc, UK.
In the second part of the investigation, concerns about possible re-entrainment of the deposited particles in association with the timer operation in part 1 resulted in some of the APSD determinations with the ACI having to be repeated, this time replacing the basic timer unit (Fig. 1) with a TPK critical flow rate controller (Model TPK 2000, Copley Scientific Ltd., Nottingham, UK). The TPK controller had been previously evaluated by Beron et al., with a medium resistance DPI. In the Beron et al. study, each apparatus was equipped with a preseparator and Ph.Eur./USP induction port, and this group found rise times to the stable flow rate of 60 L/min in the region of 300 and 150 ms were possible for NGI and ACI configurations, respectively (10). In the present evaluation with the TPK controller, sample durations were chosen to be 0.5, 1, and 4 s (Table III), the shortest duration being intentionally selected with the expectation that reducing the sample volume substantially smaller than the internal volume of the ACI system would elicit detectable changes in FPM as the result of incomplete aerosol bolus transfer, as had been observed with the NGI in the first part of the study (Table II).
Table III.
Second Series of Assessments: Sequence of Measurements with Each Cascade Impactor System Using DPIs “A” and “B”
| Sample Time (s) | Use of TPK (for ACI) | DPI | Number of actuations into each CI | Number of replicate determinations for each measurement group | No of volume changes (approximate) | |
|---|---|---|---|---|---|---|
| NGI | ACI | |||||
| 0.5 | Y | A | 1 | 3 | 0.25 | 0.5 |
| 0.5 | N | A | 1 | 3 | Series 1 | 0.5 |
| 1 | Y | A | 1 | 3 | 0.5 | 1 |
| 1 | N | A | 1 | 3 | Series 1 | 1 |
| 4 | Y | A | 1 | 3 | 2 | 4 |
| 4 | N | A | 1 | 3 | Series 1 | 4 |
| 0.5 | N | B | 1 | 2 | 0.25 | 0.5 |
| 1 | N | B | 1 | 2 | 0.5 | 1 |
| 4 | N | B | 1 | 2 | 2 | 4 |
Table II.
First Series of Assessments: Sequence of Measurements with Each Cascade Impactor System Using DPI ‘A’
| Sample time (s) | Number of actuations into each impactor | Number of replicate determinations for each measurement group | No of volume changes (approximate) | |
|---|---|---|---|---|
| NGI | ACI | |||
| 1 | 1 | 3 | 0.5 | 1 |
| 2 | 1 | 3 | 1 | 2 |
| 4 | 1 | 3 | 2 | 4 |
| 8 | 1 | 3 | 4 | 8 |
| 16 | 1 | 3 | 8 | 16 |
| Reference | 7 | 1 | 2a | 4a |
a4-L sample volume as required in the pharmacopeial methodology
Finally, a further series of measurements, also with sample durations of 0.5, 1, and 4 s were undertaken with a second DPI (inhaler “B”) having a similar flow resistance to that of inhaler “A” (Table III). The purpose of changing the inhaler was to establish whether the trend seen in the results with DPI inhaler “A” was specific to the type of DPI used or a more generally applicable phenomenon. The ACI system was evaluated using the original timer controller, but measurements were also undertaken with the NGI/TPK controller (0.25 L internal volume) to provide benchmark data.
RESULTS AND DISCUSSION
Preliminary testing had shown that internal losses of particles to the walls of each CI were <5% of the total recovered mass, as required by the pharmacopeial procedure (1,2), confirming that DPI operation and API recovery from both impactor systems were within system suitability expectations. Thus, the total recovered mass of API (mean±SD) from the first series of measurements (742 ± 46 μg/actuation, NGI; 760 ± 16 μg/actuation, ACI) was in agreement with label claim (800 μg/actuation) and with unreported values obtained from delivered dose uniformity testing using a dosage uniformity sampling apparatus.
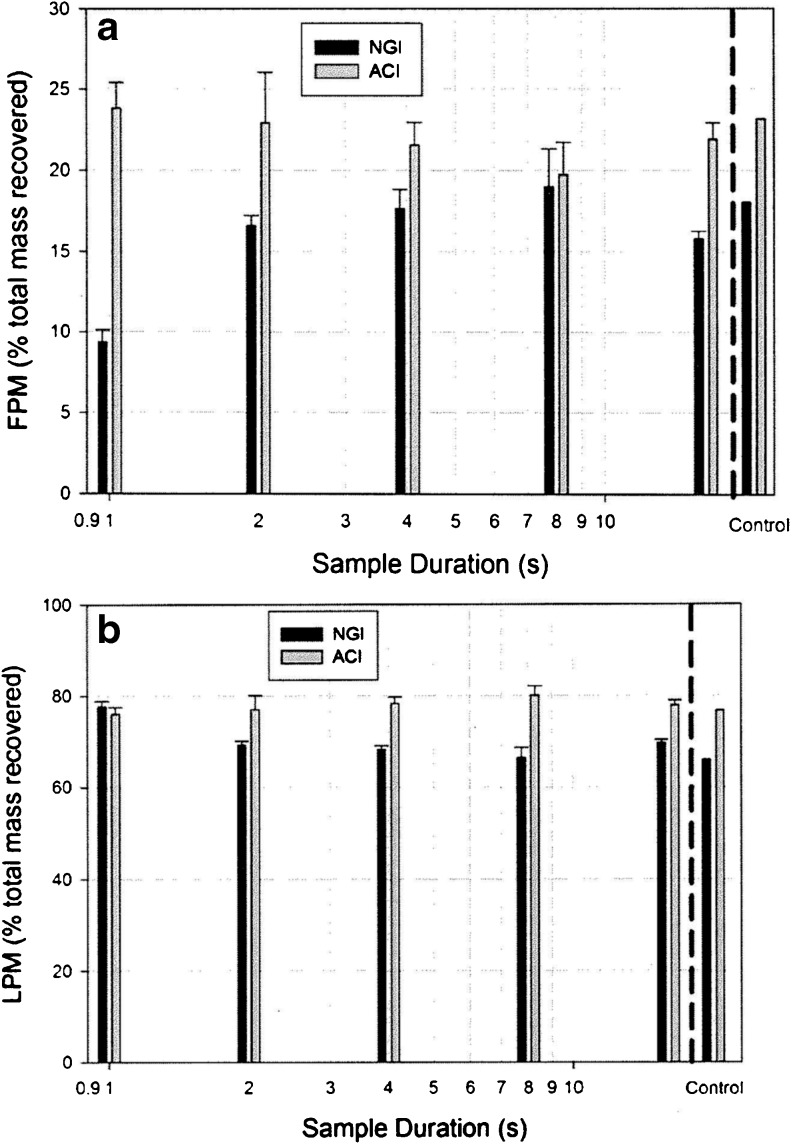
The effects of changing sampling time on both FPM and LPM are illustrated in Fig. 2a and b, respectively, for the first series of determinations with DPI “A”. In the case of the NGI system, FPM0.94–4.46 μm decreased markedly at sample times <2 s (Table IV). The total sample volume for a 2 s sample time was 2.0 L, assuming an instantaneous rise in flow rate from 0 to 60 L/min at the start of testing. This volume is comparable with the internal volume of this CI system (2.025 L). These observations are consistent with a basic model of aerosol transport in plug flow passing through the system. Shorter sampling times corresponding to smaller volumes than the magnitude of the internal dead space (2.025 L) would therefore not have allowed sufficient time for the aerosol bolus to pass entirely through the impactor system. This explanation is also supported by the corresponding increases in LPM when the sample duration was decreased to 1 s (1 L sample volume). Such behavior is indicative of premature deposition of the aerosol bolus emitted by the DPI within the induction port and preseparator of this system.
Fig. 2.
First series of measurements: cascade impactor measured metrics representing fine and large particle mass as a function of sample duration for a medium resistance DPI inhaler “A” tested in accordance with pharmacopeial procedures—a FPM captured on stages 1–5 of the ACI and 3–5 of the NGI; b LPM captured in the induction port and preseparator
Table IV.
Values of FPM and LPM from First Series of Assessments with Each Cascade Impactor System Using DPI “A”
| Impactor | Sample duration (s) | LPM (% total recovered mass) | |
|---|---|---|---|
| FPM0.94–4.46 μm (% total recovered mass) | |||
| NGI | 1 | 9.37 ± 0.75 | 77.63 ± 1.23 |
| 2 | 16.60 ± 0.63 | 69.12 ± 0.88 | |
| 4 | 17.66 ± 1.18 | 68.13 ± 0.88 | |
| 8 | 19.03 ± 2.29 | 66.41 ± 2.16 | |
| 16 | 15.78 ± 0.50 | 69.64 ± 0.62 | |
| Reference | 18.06 | 65.94 | |
| FPM0.76–6.2 μm (% total recovered mass) | |||
| ACI | 1 s | 23.84 ± 1.54 | 75.98 ± 1.61 |
| 2 s | 22.93 ± 3.11 | 76.93 ± 3.16 | |
| 4 s | 21.57 ± 1.38 | 78.30 ± 1.41 | |
| 8 s | 19.75 ± 1.98 | 80.12 ± 2.04 | |
| 16 s | 21.91 ± 1.01 | 77.94 ± 1.03 | |
| Reference | 23.17 | 76.74 | |
n = 3 replicates (except for reference); mean±SD
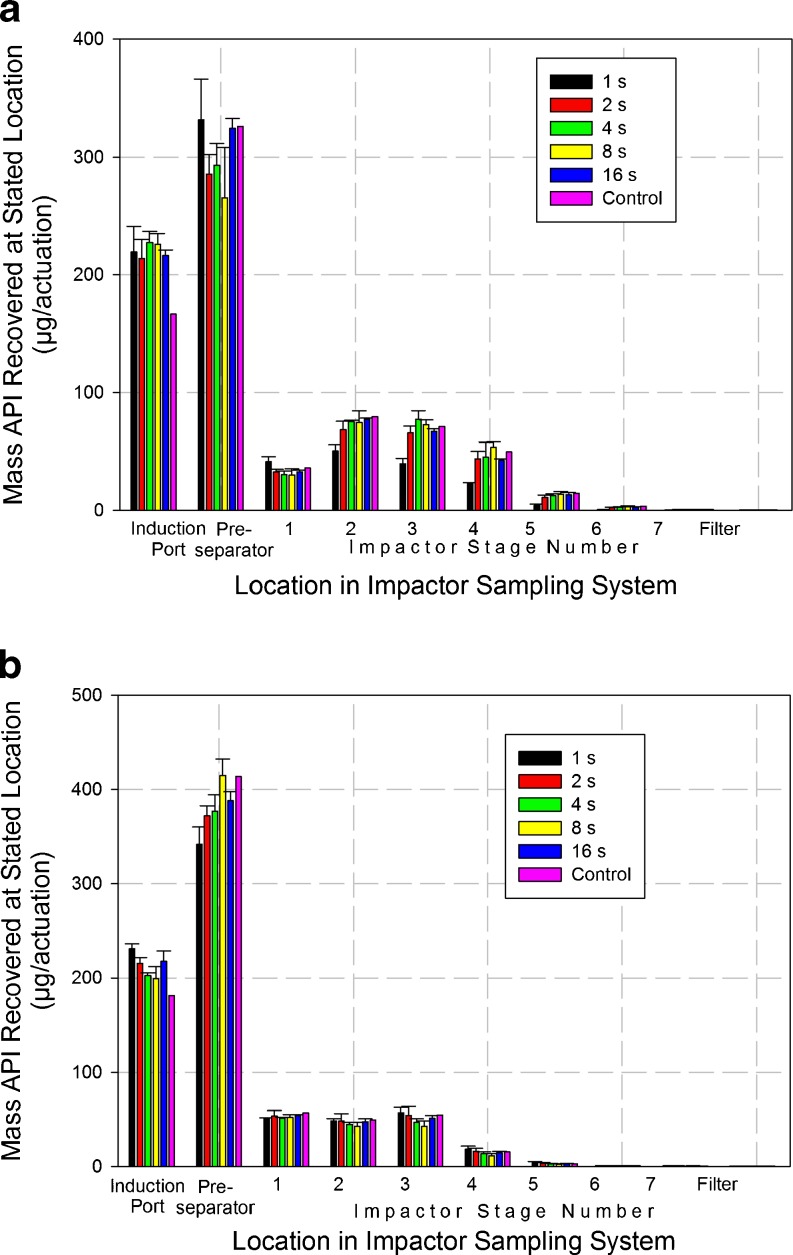
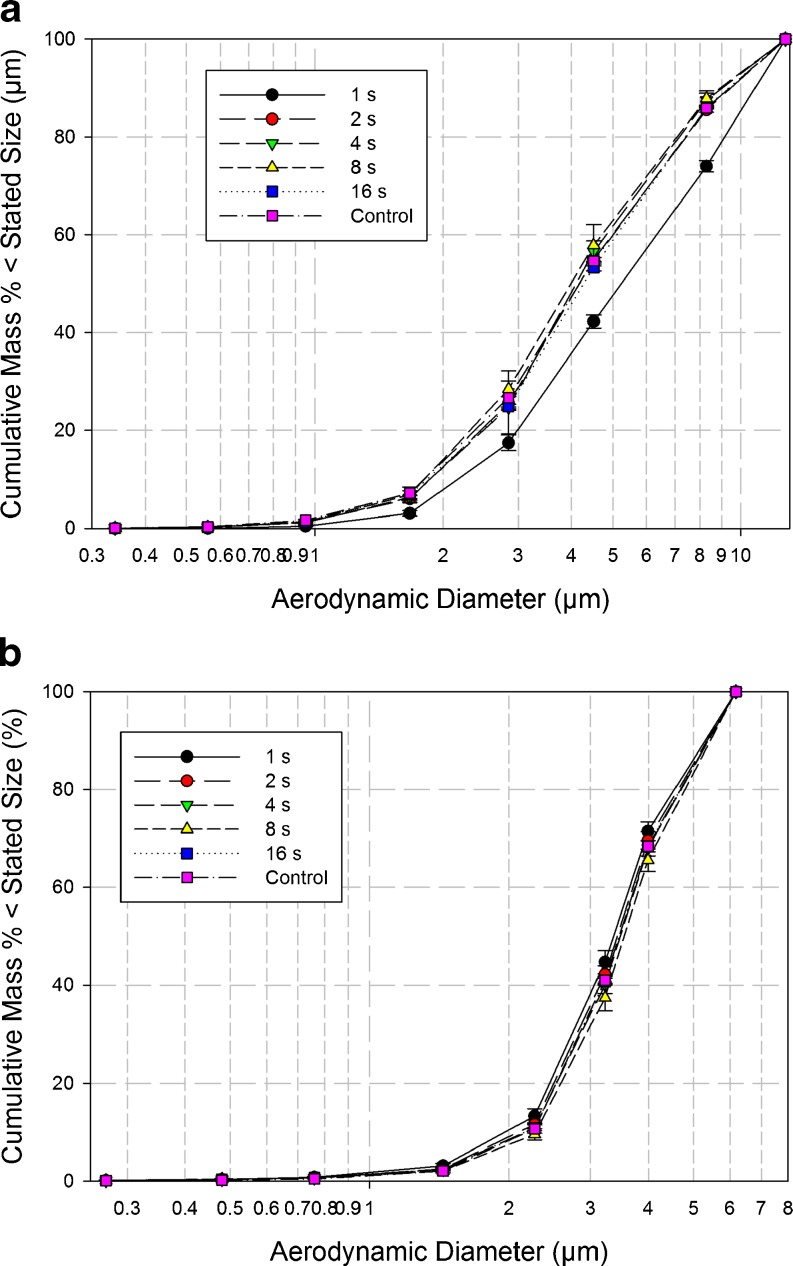
Detailed deposition profiles from the NGI system at each sample duration (Table V) exhibited a small but consistent shift in the direction of reduced particle deposition with decreasing time for all collection cups beyond stage 1 (Fig. 3a). This trend was considered substantial when sampling duration was reduced from 2 to 1 s. This behavior is fully consistent with the previously discussed measures of FPM0.94–4.46 μm, and is indicative that the more distal stages of this impactor were being starved of incoming aerosol particularly at the shortest duration. Interestingly, however, there was no obvious trend towards increased mass recovered from either the induction port or the pre-separator across all sample times, although a 16% increase in mass recovered from the preseparator was observed when the sample time was reduced from 2 to 1 s. It should be noted that the mass of API recovered from these nonsizing components was more than twice that which penetrated as far as the impactor for the longer sampling durations >4 s, when passage of the aerosol through the entire system would have been assured. The effect of shortening the sample time on the cumulative mass weighted APSDs for the product sized by the NGI (Fig. 4a) was reflected in an apparent increase in mass median aerodynamic diameter (MMAD) from close to 4.0 μm (sample times ≥2 s) to 5.3 μm when the duration was only 1 s (Table VI).
Table V.
First Series of Assessments: Deposition Profile Data (microgram per actuation) from DPI Inhaler “A” for the NGI at Different Sample Durations
| Duration | 1 s | 2 s | 4 s | 8 s | 16 s | Controla | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Mean | CofV | Mean | CofV | Mean | CofV | Mean | CofV | Mean | CofV | |
| I.P. | 219.5 | 9.8 | 213.8 | 7.4 | 227.0 | 4.2 | 225.7 | 4.0 | 216.3 | 2.3 | 166.5 |
| P.S. | 331.5 | 10.5 | 285.5 | 5.8 | 293.1 | 6.2 | 265.3 | 16.1 | 324.3 | 2.5 | 325.8 |
| Stage 1 | 41.3 | 10.4 | 32.4 | 7.1 | 30.4 | 10.0 | 30.2 | 16.4 | 32.8 | 3.6 | 36.0 |
| Stage 2 | 50.4 | 10.7 | 68.3 | 10.5 | 75.2 | 1.4 | 74.4 | 13.3 | 77.2 | 1.1 | 79.4 |
| Stage 3 | 39.4 | 12.2 | 65.6 | 8.9 | 77.3 | 9.3 | 72.7 | 5.5 | 67.0 | 3.4 | 71.0 |
| Stage 4 | 22.7 | 3.8 | 43.7 | 14.2 | 45.0 | 28.6 | 53.5 | 8.4 | 42.4 | 3.1 | 49.6 |
| Stage 5 | 4.2 | 25.1 | 10.8 | 20.4 | 12.4 | 11.0 | 13.5 | 16.7 | 13.1 | 15.3 | 14.2 |
| Stage 6 | 0.7 | 17.3 | 2.1 | 19.5 | 2.3 | 19.3 | 2.8 | 40.0 | 2.5 | 14.4 | 3.3 |
| Stage 7 | 0.0 | 173.2 | 0.5 | 12.4 | 0.4 | 25.0 | 0.4 | 25.0 | 0.5 | 20.0 | 0.6 |
| Filter | 0.0 | – | 0.1 | 0.0 | 0.1 | 43.3 | 0.1 | 0.0 | 0.1 | 43.3 | 0.2 |
IP GSK Induction Port, PS NGI preseparator, CofV coefficient of variation (%)
aSingle measurement for control
Fig. 3.
First series of measurements: deposition profiles from a medium resistance DPI inhaler “B” testing in accordance with pharmaceutical procedures as a function of sample duration—a NGI at 60 L/min, b ACI at 60 L/min
Fig. 4.
Cumulative mass-weighted APSDs for DPI inhaler “A”—the mass of API collected only by the size-fractionating components of the cascade impactor is considered in this analysis—a NGI at 60 L/min, b ACI at 60 L/min
Table VI.
First Series of Assessments: Variation in Impactor-Measured MMAD from Cumulative APSD Data for DPI Inhaler “A”
| Impactor | Sample duration (s) | MMAD (μm) |
|---|---|---|
| NGI | 1 | 5.3 |
| 2 | 4.1 | |
| 4 | 4.1 | |
| 8 | 4.0 | |
| 16 | 4.2 | |
| Referencea | 4.1 | |
| ACI | 1 | 3.5 |
| 2 | 3.6 | |
| 4 | 3.6 | |
| 8 | 3.7 | |
| 16 | 3.6 | |
| Referencea | 3.6 |
aSeven actuations from inhaler in accordance with standardized procedure in effect at GSK plc
In the case of the measurements with the ACI system, in contrast with the NGI behavior, there was no clear sign of a transition to incomplete sampling of the aerosol bolus at shorter sample times in the corresponding values of FPM0.76–6.2 μm obtained with the ACI (Fig. 2a). Instead, FPM0.76–6.2 μm remained in the range of 20–24%, irrespective of sample time (Table IV). The internal dead space of this impactor is 1.155 L (4), so that this volume could only have been sampled at 60 L/min after approximately 1.25 s, again assuming an instantaneous rise to the stable flow rate at start up. The measurement of 23.84 ± 1.54% for FPM0.76–6.2 μm after only 1 s duration was therefore regarded as an anomaly, requiring further investigation. It should also be noted that values of LPM also remained stable in the range from 76% to 80%, irrespective of the sampling duration (Fig. 2b). Such behavior with both FPM0.76–6.2 μm and LPM is at first sight suggestive that mass transfer of the aerosol bolus through the entire ACI system had somehow taken place normally, even though at 1 s duration, the sample volume of 1.0 L was slightly less than the internal volume for this impactor system.
In contrast to the more explicable performance of the NGI, the component-by-component deposition profiles from the ACI system (Table VII) indicate consistent mass recovery was achieved from each of the size-fractionating stages irrespective of sample volume (duration). MMAD values derived from the cumulative mass-weighted APSD data (Fig. 4b), were consistently in the range from 3.5 to 3.7 μm regardless of sample duration. However, in passing, it is notable that these values are slightly smaller than the corresponding stable range of MMADs from 4.0 to 4.2 μm achieved with the NGI. In explanation, it should be noted that the preseparator for the ACI was the version designed for use at 28.3 L/min; so at 60 L/min, its effective cutoff diameter would have been close to 7 μm aerodynamic diameter. Its relatively poor size selectivity (9) compared with that for the preseparator of the NGI (11) could have resulted in slightly less aerosol penetrating to the size-separating stages of the ACI irrespective of sample volume considerations.
Table VII.
First Series of Assessments: Deposition Profile Data (microgram per actuation) from DPI Inhaler “A” for the ACI at Different Sample Durations
| Duration | 1 s | 2 s | 4 s | 8 s | 16 s | Controla | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Mean | CofV | Mean | CofV | Mean | CofV | Mean | CofV | Mean | CofV | |
| I.P. | 231.0 | 2.3 | 215.6 | 2.9 | 202.3 | 1.5 | 199.1 | 6.5 | 217.7 | 5.0 | 181.2 |
| P.S. | 341.9 | 5.4 | 371.8 | 2.9 | 376.9 | 4.6 | 414.9 | 4.2 | 387.9 | 2.5 | 413.8 |
| Stage 1 | 51.6 | 0.4 | 53.4 | 10.8 | 51.3 | 1.5 | 52.3 | 5.6 | 54.1 | 2.0 | 57.0 |
| Stage 2 | 48.4 | 5.1 | 48.4 | 16.0 | 44.8 | 5.2 | 42.8 | 9.5 | 47.5 | 6.7 | 49.4 |
| Stage 3 | 57.0 | 9.5 | 54.2 | 17.6 | 47.0 | 7.9 | 42.6 | 13.5 | 51.2 | 5.7 | 54.7 |
| Stage 4 | 18.5 | 16.3 | 16.1 | 19.8 | 13.6 | 14.0 | 11.4 | 18.4 | 14.6 | 10.4 | 15.5 |
| Stage 5 | 4.3 | 21.3 | 3.3 | 20.4 | 2.8 | 17.9 | 2.2 | 23.7 | 2.8 | 12.4 | 3.0 |
| Stage 6 | 0.8 | 19.9 | 0.6 | 27.0 | 0.5 | 10.8 | 0.5 | 44.6 | 0.6 | 10.2 | 0.4 |
| Stage 7 | 0.4 | 53.3 | 0.4 | 50.0 | 0.3 | 45.8 | 0.4 | 43.3 | 0.4 | 13.3 | 0.2 |
| Filter | 0.2 | 69.3 | 0.1 | 43.3 | 0.1 | 43.3 | 0.1 | 43.3 | 0.2 | 34.6 | 0.1 |
aSeven actuations from inhaler in accordance with standardized procedure in effect at GSK plc
The relative independence of the ACI deposition profiles to changes in sample volume are consistent with the similar behavior of FPM0.76–6.2 μm previously discussed. However, neither outcome would be anticipated from the plug flow consideration that seems to explain the NGI data at sample volumes commensurate with internal dead space quite well. Instead, the observed behavior with the ACI system appears to hint at a cause related to maldistribution of flow through the system, given that even at the shortest time, when 1.0 L of air had entered the ACI (equal to 90% of the total internal volume encompassed within the induction port, preseparator, and impactor), that particles containing API still reached the lowermost stages of this impactor.
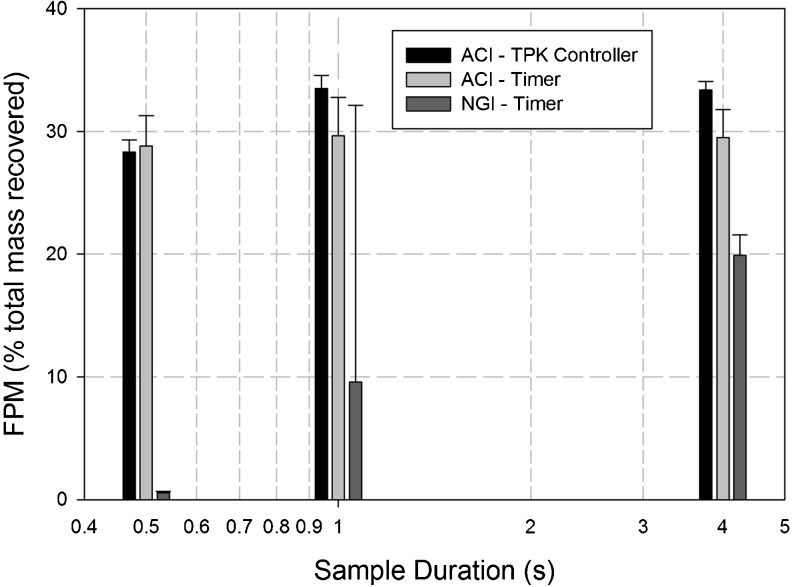
The simple timer-based sampling arrangement used in the first series of assessments with both CI systems involved a solenoid valve timer switch to supply power to the vacuum pump. After considering the unexpected findings from the ACI system, it was considered plausible, though unlikely that this arrangement for flow control might have caused a residual flow from the DPI following power-off at the end of each sampling period. Such a flow may have resuspended the powder that had been collected in the preseparator of this system during the sampling period, resulting in mass transfer and subsequent size separation in the impactor, resulting in the apparently normal behavior for this system even when the sample duration at 1 s was apparently insufficient for a complete volume exchange. In the second part of the investigation, the timer was therefore replaced by the TPK controller unit and the measurements with sample durations of 1 and 4 s repeated with both cascade impactors, and in addition also reducing the sample duration even further to 0.5 s (Table III).
FPM0.94–4.46 for the NGI system decreased almost to zero at this shorter duration (Table VIII), as would be expected, given that the sample volume was only 24.6% of the internal dead space. In contrast, FPM0.76–6.2 μm from the ACI remained close to the values achieved with the longer sampling durations, and the change of flow controller had no effect on this outcome. Given that at 500 ms duration, the sample volume was only 43.2% of the dead space within the ACI system; the insensitivity of FPM0.76–6.2 μm to sample volume at such a small size relative to its dead space reinforced the belief arising from the outcomes of the first part of the investigation that movement of the aerosol bolus through this system cannot be described in straightforward terms by plug flow.
Table VIII.
Second Series of Assessments: Values of FPM with Each Cascade Impactor System Using DPI “A” with Timer and TPK Controller Options
| Impactor | Controller | Sample Duration (s) | |
|---|---|---|---|
| FPM0.94–4.46 μm (% total recovered mass) | |||
| NGI | TPK controller | 0.5 | 0.6 ± 0.1 |
| 1.0 | 9.6 ± 0.6 | ||
| 4.0 | 19.9 ± 1.7 | ||
| FPM0.76–6.2 μm (% total recovered mass) | |||
| ACI | TPK controller | 0.5 s | 28.3 ± 1.0 |
| 1.0 s | 33.5 ± 1.1 | ||
| 4.0 s | 33.4 ± 0.7 | ||
| Timer | 0.5 s | 28.8 ± 2.5 | |
| 1.0 s | 29.6 ± 3.1 | ||
| 4.0 s | 29.5 ± 2.3 | ||
n = 3 replicates/condition; mean±SD
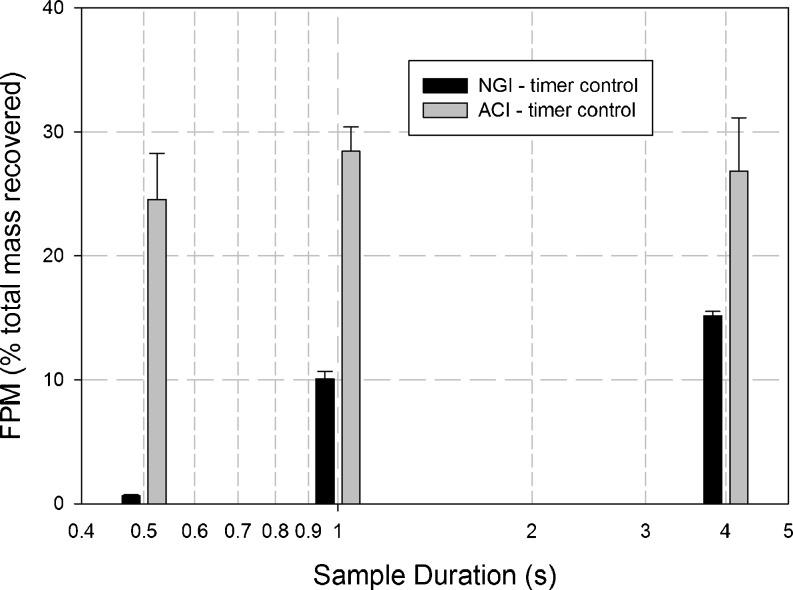
When the same series of tests was repeated with both impactor systems using the timer control system, but this time using DPI inhaler “B” (Fig. 5), the profiles of FPM with sample duration for each system were comparable with the corresponding profiles observed with inhaler “A” (Fig. 6). Thus for the NGI, FPM0.94–4.46 decreased from 15.2 ± 0.4% at 4 s to 10.1 ± 0.6% at 1 s and finally to 0.7 ± 0.1% at 500 ms, whereas the corresponding values of FPM0.76–6.2 μm obtained with the ACI system remained nearly constant at 26.8 ± 4.3%, 28.4 ± 2.0%, and 24.5 ± 3.7% for 4 s, 1 s, and 500 ms durations, respectively. This finding adds further strength to the hypothesis that the anomalous behavior of the ACI is not specific to a particular DPI, but instead is a more general property of this impactor system.
Fig. 5.
FPM captured on stages 1–5 of the ACI and stages 3–5 of the NGI for DPI Inhaler “B” with timer control system
Fig. 6.
FPM captured on stages 1–5 of the ACI and stages 3–5 of the NGI for DPI inhaler “A” with different flow control systems
Without undertaking a computational fluid dynamics analysis of the unsteady state flow through the ACI system, a task that is outside the scope of this experimental investigation, it is not possible to do more than conjecture where the flow maldistribution may be taking place at start-up, and whether it persists after the flow has reached a steady state. However, a strong contender for investigation is the flow development that takes place from the central axis to the periphery of the internal flow pathway into the impactor in response to the applied vacuum initiated at start-up. Such behavior may arise as a consequence of non-uniform expansion of the flow streamlines leaving the relatively narrow exit from the induction port into the uppermost impactor stage.
The rate at which the flow field within the CI reaches its designed, stable laminar flow condition is also pertinent in attempts to explain the observed phenomena from both parts of this investigation. Laminar flow start-up in a tube has been solved analytically (12), and the time to reach 90% of the steady-state velocity is 45% of the tube radius squared divided by the kinematic viscosity (approximately 0.15 cm2 s−1 for air at normal conditions). Hence, flow in the entry to the GSK induction port common to both impactor systems (13.4 mm diameter) requires approximately 1.3 s to nearly reach steady state. As a result, the flow field in the induction port and preseparator is essentially undeveloped during short sampling times. Since the direction of forward motion propagates from the EXIT of the CI upstream to the induction port (13), flow in these components will take the longest time to rise to its stable value (estimated at about 0.25 s (13), similar to the observations of Beron et al. for the NGI (10)). Unsteady pressure flow dynamics of CI start-up are therefore more complex than would be predicted from considerations of steady flow through each component. However, further development of a theoretical understanding of flow development within these cascade impactors is beyond the scope of this investigation, which was experimental in nature.
CONCLUSIONS
A reasonable expectation for the performance of a multistage cascade impactor under the unsteady flow conditions that exist during the interval between start-up of the vacuum pump and the achievement of a constant flow rate within the CI system in its entirety is that the APSD-related data should indicate incomplete transfer of the emitted dose from the DPI. Such behavior should be self-evident, particularly when the sampling duration decreases such that the ratio of sample volume to internal volume is smaller than 1.0. The NGI stage-by-stage data clearly demonstrated evidence of incomplete mass transfer through the system at sample volumes commensurate with internal dead space with both DPI products evaluated. In contrast, the consistent stage-by-stage behavior with the equivalent ACI data irrespective of whether or not the ratio of sample volume to internal volume was substantially less than unity was unexpected, suggesting that the flow profile across this CI from the central axis to the periphery of the internal flow pathway is more complex than that which exists within the NGI at start-up, and may be maldistributed. It is well-known that CIs are calibrated and meant for sampling at steady flow rates, which is not the way they are used in connection with the testing of DPIs in accordance with compendial procedures. Until a more comprehensive understanding of these experimentally observed phenomena is developed, based on computational fluid dynamics in unsteady-state conditions, it is recommended that for DPI testing, sample times be chosen such that the current compendial volume of 4 L is retained. Under these circumstances, APSD determinations can be made by either ACI or NGI systems without bias from transient behavior at start-up caused by withdrawing an insufficient sample volume from the DPI through the impactor system.
ACKNOWLEDGMENTS
The authors wish to acknowledge the support of GSK plc, UK for the supply of DPI products, and to other members of the Cascade Impactor Sub-Team of the European Pharmaceutical Aerosol Group (EPAG) for their advice and support during the experimental work and in the internal reviewing of this article.
REFERENCES
- 1.European Directorate for the Quality of Medicines and Healthcare (EDQM): European Pharmacopeia 6(8). Chapter 2.9.18. Preparations for inhalations: aerodynamic assessment of fine particles. Strasbourg: Council of Europe; 2010.
- 2.US Pharmacopeial Convention. United States Pharmacopeia; USP 33-NF 28; chapter 601—physical tests and determinations: aerosols. United States Pharmacopeia, Rockville, MD, USA; 2010.
- 3.Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med. 2003;16(4):341–377. doi: 10.1089/089426803772455622. [DOI] [PubMed] [Google Scholar]
- 4.Copley M, Smurthwaite M, Roberts DL, Mitchell JP. Revised internal volumes to those provided by Mitchell JP and Nagel MW in cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med. 2005;18(3):364–366. doi: 10.1089/jam.2005.18.364. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JP, Newman S, Chan H-K. In vitro and in vivo aspects of cascade impactor tests and inhaler performance: a review. AAPS PharmSciTechnol. 2007;8(4):237–248. doi: 10.1208/pt0804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunbar C, Mitchell J. Analysis of cascade impactor mass distributions. J Aerosol Med. 2005;18(4):439–451. doi: 10.1089/jam.2005.18.439. [DOI] [PubMed] [Google Scholar]
- 7.Marple VA, Olson BA, Santhanakrishnan K, Mitchell JP, Murray S, Hudson-Curtis B. Next generation pharmaceutical impactor. Part II: calibration. J Aerosol Med. 2003;16:301–324. doi: 10.1089/089426803769017668. [DOI] [PubMed] [Google Scholar]
- 8.Nichols SC. Calibration and mensuration issues for the standard and modified impactor. Pharmeuropa. 2000;12(4):585. [Google Scholar]
- 9.Mitchell JP, Costa PA, Waters S. An assessment of an Andersen mark-II cascade impactor. J Aerosol Sci. 1987;19:213–221. doi: 10.1016/0021-8502(88)90224-8. [DOI] [Google Scholar]
- 10.Beron K, Grabek CE, Jung JA, Shelton CM. Drug delivery to the lungs, 19. Edinburgh: The Aerosol Society; 2008. Flow rate ramp profile effects on the emitted dose from dry powder inhalers; pp. 61–64. [Google Scholar]
- 11.Marple VA, Roberts DL, Romay FJ, Miller NC, Truman KG, Van Oort M, et al. Next generation pharmaceutical impactor. Part 1: design. J Aerosol Med. 2003;16:283–299. doi: 10.1089/089426803769017659. [DOI] [PubMed] [Google Scholar]
- 12.Bird RB, Stewart WE, Lightfoot EN. Transport phenomena. New York: Wiley; 1960. p. 126. [Google Scholar]
- 13.Roberts DL, Chiruta M. Drug delivery to the lungs, 18. Edinburgh: The Aerosol Society; 2007. Transient impactor behavior during the testing of dry-powder inhalers via compendial methods; pp. 202–205. [Google Scholar]