Abstract
In order to tackle the problems on low water solubility of teniposide, involvement of toxic surfactant in its injection, and the poor stability during infusion, a Cremophor-free teniposide self-microemulsified drug delivery system (TEN-SMEDDS) was prepared for the first time, characterized, and evaluated in comparison with teniposide injection (VUMON) in vitro and in vivo. The optimized formulation contained N, N-dimethylacetamide, medium-chain triglyceride, lecithin, and dehydrated alcohol besides teniposide. The TEN-SMEDDS could form fine droplets with mean diameter of 282 ± 21 nm and zeta potential of −7.5 ± 1.7 mV after dilution with 5% glucose, which were stable within 4 h. The release of teniposide from TEN-SMEDDS and VUMON was similar. However, the pharmacokinetic behavior of TEN-SMEDDS in rats was different from that of VUMON, evidenced by the lower area under the concentration–time curve and larger volume of distribution in emulsion group. Finally, TEN-SMEDDS was found to distribute more teniposide in most tissues, especially in reticuloendothelial system, after intravenous administration to rats. Importantly, brain drug level in TEN-SMEDDS group was higher than or similar to that in control group, although the emulsion system had a lower plasma drug concentration. In conclusion, the novel SMEDDS prepared here, without toxic surfactant and as an oil solution before use, may be potential for clinical use due to its low toxicity and high store stability. It may be favorable for the treatment of some tumors like cerebroma, since it may achieve the relatively higher drug level in brain but lower blood concentration.
KEY WORDS: characterization, pharmacokinetics, self-microemulsified drug delivery system, teniposide, tissue distribution
INTRODUCTION
Teniposide 4′-demethylepipodophylotoxin-4-(4,6-O-thenylidine-β-d-glucopyranoside), which is a semisynthetic derivative of podophyllotoxin resin, is effective for treatment of various malignancies, such as neuroblastoma (1,2), small cell lung cancer, non-Hodgkin's and Hodgkin's lymphoma, and acute leukemia, especially cerebroma (3). However, its application in clinic was limited due to the poor aqueous solubility. In addition, teniposide was unstable in an aqueous solution. Currently, its intravenous injection available in market (VUMON, Bristol-Myers Squibb S.r.l.) contains high concentration of polyethoxylated castor oil (Cremophor EL) as a solubilization agent (4). VUMON can cause several severe side effects such as hypersensitivity, hypertension, hypoeosinophilia, and hematological toxicity (5–10). These are most probably caused by the surfactant Cremophor contained in the vehicle, rather than the drug itself (11). Also, Cremophor may affect red blood cells because the surfactant can penetrate biological membranes to cause an increase in permeability and cell damage. A change in the shape of human white blood cells (WBCs) has also been reported in the studies on the Cremophor (12–14). Hence, monitoring the WBC and platelet count needs to be executed regularly during VUMON administration in clinic (15). In order to avoid these disadvantages, several kinds of nanocarriers, such as liposomes, submicron lipid particles, and lipid emulsions, have been investigated. These explorations partially overcame the problem on the poor solubility of teniposide; however, the stability of these delivery systems was still a big challenge (4,16).
Self-microemulsified drug delivery system (SMEDDS) has recently attracted much attention (17–21). It holds promise for pharmaceutical industry as a safer and an efficacious alternative for drugs with poor solubility (22). With oil and surfactant, SMEDDS was a concentrated solution without water, so it is physically or chemically more stable than emulsion. This system can dissolve many water-insoluble drugs in a proper formulation, while it is less in volume, leading to the easy storage and transportation. Before use, it can form fine oil-in-water microemulsions with gentle agitation following dilution by aqueous phases (23,24), which is favorable for clinical use as intravenous injection. It was reported that such system was superior to micelle or co-solvent system in terms of drug solubilization and stability (25–27). Nevertheless, there is no SMEDDS available for intravenous injection right now, possibly due to the difficulty in getting rid of the toxic surfactants, such as Cremophor.
In the present study, we try to develop a teniposide self-microemulsified drug delivery system (TEN-SMEDDS) with a good stability and low toxicity by a simple method. All the excipients used in TEN-SMEDDS are commercially available and proven to be safe for i.v. administration by FDA. The physicochemical characteristics of the TEN-SMEDDS were investigated, and the in vitro release, in vivo pharmacokinetics, and tissue distribution were assessed using VUMON as a reference. The results proved the possibility of making the water-insoluble teniposide into a Cremophor-free SMEDDS and demonstrated the characteristics of TEN-SMEDDS in vitro and in vivo, laying a good foundation for its clinical use in the future.
MATERIALS AND METHODS
Materials and Animals
Teniposide was kindly donated by Kelun Pharmaceutical Co., Ltd (Sichuan, China). Lipoid E80 was purchased from Lipoid (German). Medium-chain triglyceride (MCT) was provided by Magna-Kron Co. (USA), and N,N-dimethylacetamide (DMA) was from Acros (Geel, Belgium). Methanol and acetonitrile of HPLC-grade were obtained from Promptar (Elk Grove, USA). All other reagents were of analytical grade.
Preparation of TEN-SMEDDS
TEN-SMEDDS was prepared by a simple process at room temperature. Firstly, 50 mg of teniposide was dissolved in 300 μL DMA. Next, E-80 (2,000–3,000 mg) and MCT (0–500 mg) were added and an appropriate amount of dehydrated alcohol was used to make the formulation up to 5 mL (or 10 mL). Finally, the solution was uniformly mixed and filtered through a 0.22-μm filter.
HPLC Analysis of Teniposide In Vitro
A reversed-phase HPLC system composed of an LC-20AT pump (Shimadzu, Kyoto, Japan), a SPD-20A UV detector (Shimadzu, Kyoto, Japan), and an analysis column (octa decyl silane (ODS) column, 5 μm, 200 × 4.6 mm) was used for the determination of teniposide. The mobile phase consisted of acetonitrile and double-distilled water (45/55, v/v). The elution was carried out at a flow rate of 1.0 mL/min at 35°C and the detect wavelength was 240 nm. The HPLC method was validated by the studies on precision, accuracy, and standard curve (data not shown).
Characterization of TEN-SMEDDS
Transmission Electron Microscopy
The morphology of TEN-SMEDDS after dilution was observed using a transmission electron microscope (TEM) (JEM-1200, JEOL Co., Ltd., Japan). The samples were diluted with purified water at a ratio of 1:200 by gentle shaking. Then, a drop of sample was deposited on a copper grid. The excess fluid was drawn off with filter paper. Subsequently, the grid was stained with 1% (w/v) uranyl acetate and allowed to dry before examination.
Droplet Size and Zeta Potential
A certain volume of TEN-SMEDDS was diluted with 5% glucose injection to a definite volume and shaken gently to mix thoroughly before measurement. The droplet size, polydispersity index (PDI), and zeta potential of so-formed microemulsion were determined by dynamic light scattering (DLS) analysis in a Brookhaven ZetaPlus (Brookhaven, USA) at 25°C. The correlation decay functions were analyzed by the cumulant method to determine the Z-average size, and the regularized CONTIN method was used to obtain the droplet size distributions. The results were the mean values of three experiments for the same sample.
Compatibility with Different Medium
Clinically, the commercial injection of teniposide (VUMON) was diluted with 5% glucose or 0.9% sodium chloride injection before intravenous administration. In order to evaluate the stability of the TEN-SMEDDS when diluted with different medium, it was diluted in 5% glucose or 0.9% sodium chloride injection to the final concentrations of 0.1, 0.2, 0.4, 0.8, and 1.0 mg/mL, respectively, and the physicochemical properties of the so-formed microemulsion, including appearance, droplet size, size distribution, and stability, were then investigated.
In Vitro Release
The release behavior of teniposide from the TEN-SMEDDS or VUMON was determined in phosphate buffer (0.01 M, pH 7.4 ± 0.1) containing 1.0% (w/v) Tween 80. Briefly, 1 mL of TEN-SMEDDS or VUMON diluted with 5% glucose (equivalent to 0.2 mg of teniposide) was put into dialysis bags (MW cut off 12,000–14,000 kDa) which were placed in 100 mL release medium stirred at 100 rpm at 37°C. The dialysis bags were soaked in double-distilled water for 24 h before use. An aliquot of 1 mL release medium was withdrawn at intervals of 0.5, 1, 2, 4, 6, 8, and 12 h and replaced by 1 mL of fresh medium. Each sample was passed through a 0.45-μm syringe filter and then determined by HPLC method described above.
In Vivo Pharmacokinetic Study
Animals and Dosing Protocol
Male Sprague–Dawley rats were provided by the Animal Institute of Peking University Health Science Center (Beijing, China). All care and handling of animals were approved by the Ethics Committee of Peking University. Twelve rats weighting 200 ± 10 g were fasted overnight with free access to water. The animals were then divided into two groups, with six rats in each group. After dilution with 5% glucose, TEN-SMEDDS or VUMON was given intravenously to rats via the tail vein at a dose of 10 mg/Kg. Blood samples were taken into heparinized tubes at pre-designed time points (5, 15, 30, 60, 120, 240, and 360 min) after administration. The plasma was immediately collected by centrifugation at 5,000 rpm for 10 min and stored at −20°C until analysis.
Plasma Processing and Analysis
Plasma processing and HPLC analysis were performed according to literatures with a little modification (28). An aliquot of 3 mL acetoacetate was added into 200 μL of plasma. The mixture was vortexed for 1 min followed by centrifugation at 10,000 rpm for 10 min at 4°C, and then the organic layer was separated and evaporated under a stream of nitrogen gas. The residue was dissolved with 200 μL methanol. The same HPLC system as mentioned above was used except the equipment of an ODS pre-column (12.5 × 4.6 mm, 5 μm). The mobile phase consisted of water and acetonitrile (60:40, v/v), and 20 μL of sample was injected into the HPLC system for the detection of teniposide.
Pharmacokinetic Parameters
Standard non-compartmental pharmacokinetic parameters were calculated using DAS software (ver. 2.1.1, Mathematical Pharmacology Professional Committee of China). The calculated pharmacokinetic parameters included the maximum peak concentration of the drug in plasma (Cmax), the area under the concentration–time curve (AUC0 − ∞), the total elimination rate (CL), and the apparent volume of distribution at a steady state (Vd).
Tissue Distribution
The animals and dosing protocol were the same as in pharmacokinetic study. At predetermined time intervals (5, 30, and 120 min) after i.v. administration, three rats were sacrificed in each group, and the tissues of interest, including heart, liver, spleen, lungs, kidneys, and brain, were harvested. The collected tissues were dried, weighed, and frozen at −20°C. Then, each tissue was homogenized in normal saline before analysis. Sample processing and HPLC analysis were the same as in pharmacokinetic study.
Statistics
Quantitative data were expressed as means ± standard deviation. Statistical significance on the difference of pharmacokinetic parameters between treatment and control group was evaluated by Student's t test. A p value less than 0.05 was considered to be significant, while less than 0.01 was highly significant.
RESULTS AND DISCUSSION
Effect of the Oil, Emulsifier, and Co-emulsifier on the Droplet Size and Stability
The TEN-SMEDDS must be diluted to form microemulsion prior to infusion. The droplet size and the stability of the formed microemulsion are critical to clinical use. As the components of this formulation, MCT, emulsifier E-80, and co-emulsifier dehydrated alcohol might have effect on the droplet size and stability of the microemulsion. In order to investigate the effect of dehydrated alcohol, the final volume of the system containing 50 mg teniposide was made with 5 and 10 mL of dehydrated alcohol, respectively. However, it was difficult to form a microemulsion in 5 mL specification because the concentrated solution was so viscous when diluted with 5% glucose. Therefore, in the following study, the 10-mL formulation containing 50 mg teniposide was used. And in the characterization of TEN-SMEDDS, the final concentration of teniposide in all the samples was 0.4 mg/mL after dilution with 5% glucose.
Table I shows the effect of E-80 and MCT on the appearance, droplet size, and stability of the TEN-SMEDDS. The stability was evaluated according to the time that teniposide forms crystal in microemulsion under microscope. The stability of TEN-SMEDDS, as well as its droplet size and PDI, was found to increase as the amount of E-80 and MCT increased. The formulation with 2,000 mg E-80 and 50 mg MCT was considered to be suitable here since its droplet size was relatively smaller and uniform, and it was stable within 4 h which might be enough for clinical application. Therefore, based on the screening here by the single factor method, the optimal formulation of TEN-SMEDDS was composed of 50 mg teniposide, 300 μL DMA, 2,000 mg E-80, 250 mg MCT, and a suitable amount of dehydrated alcohol to make a final volume of 10 mL.
Table I.
The Effect of the Oil and Emulsifier on the Droplet Size and Stability of TEN-SMEDDS (n = 3)
| Formulation | E-80 (mg) | MCT (mg) | Appearance | Droplet size (nm) | PDI | Stablity (h) |
|---|---|---|---|---|---|---|
| 1 | 2,000 | 0 | Opalescence | 222 ± 15 | 0.273 ± 0.032 | <2 |
| 2 | 2,500 | 0 | Opalescence | 294 ± 20 | 0.413 ± 0.026 | <4 |
| 3 | 3,000 | 0 | Ivory | 441 ± 23 | 0.509 ± 0.021 | >4 |
| 4 | 2,000 | 250 | Ivory | 282 ± 21 | 0.423 ± 0.035 | >4 |
| 5 | 2,500 | 250 | Ivory | 354 ± 29 | 0.624 ± 0.035 | >6 |
| 6 | 3,000 | 250 | Ivory, great viscosity | 432 ± 31 | 0.797 ± 0.031 | >8 |
| 7 | 2,000 | 500 | Ivory | 394 ± 36 | 0.909 ± 0.030 | >6 |
| 8 | 2,500 | 500 | Ivory, great viscosity | 278 ± 41 | 0.911 ± 0.028 | >8 |
| 9 | 3,000 | 500 | Ivory, great viscosity | 390 ± 38 | 0.885 ± 0.030 | >8 |
Characterization of TEN-SMEDDS
Measurement of Droplet Size and Zeta Potential
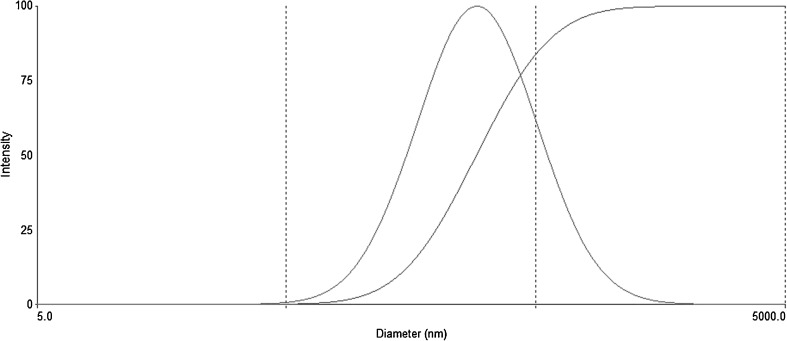
As the result of DLS analysis, the mean diameter, PDI, and zeta potential of teniposide microemulsion were 282 ± 21 nm, 0.423 ± 0.035, and −7.5 ± 1.7 mV, respectively. The droplet size distribution was shown in Fig. 1.
Fig. 1.
Droplet size distribution of the optimized TEN-SMEDDS determined by DLS
Morphological Investigation
TEM image of teniposide microemulsion is shown in Fig. 2, which confirmed that microemulsion droplets were well dispersed without any aggregation or cluster and were almost spherical in shape. In the TEM image, the droplet size was about 150 nm, smaller than that by DLS. This was understandable because these two methods were based on different mechanisms and the samples were also different: DLS using liquid sample where the microemulsion remains unchanged, while TEM involving a drying step that results in dehydration or shrinkage of microemulsion droplet (29,30).
Fig. 2.
TEM image of the TEN-SMEDDS
Compatibility with Different Dilution Medium
In this study, TEN-SMEDDS was diluted with 5% glucose and 0.9% NaCl injection, respectively. After diluted with normal saline to 0.1–1.0 mg/mL (calculated as teniposide), the microemulsion appeared flocculated within 2 h, indicating that teniposide microemulsion was unstable in physiologic saline. The phenomenon may be caused by high ionic strength. The effect of dilution with 5% glucose on the properties of TEN-SMEDDS is presented in Table II. As shown in Table II, as the teniposide concentration increased, the mean diameter and PDI of microemulsion increased, while its stability decreased. When the concentration was more than 0.4 mg/mL, the droplets of microemulsion enlarged dramatically, and its stability was less than 4 h. In conclusion, the TEN-SMEDDS should be emulsified by 5% glucose to produce a 0.1–0.4-mg/mL microemulsion which should be used within 4 h.
Table II.
The Effect of Dilution with 5% Glucose on the Stability of TEN-SMEDDS (n = 3)
| Concentration of teniposide (mg/mL) | Appearance | Droplet size (nm) | PDI | Stability (h) |
|---|---|---|---|---|
| 0.1 | Translucence | 236 ± 22 | 0.416 ± 0.032 | >12 |
| 0.2 | Opalescence | 242 ± 27 | 0.438 ± 0.029 | >12 |
| 0.4 | Ivory | 282 ± 21 | 0.423 ± 0.035 | >4 |
| 0.8 | Ivory, great viscosity | 626 ± 16 | 0.929 ± 0.024 | <4 |
| 1.0 | Ivory, great viscosity | 704 ± 19 | 0.954 ± 0.032 | <4 |
In Vitro Release Study
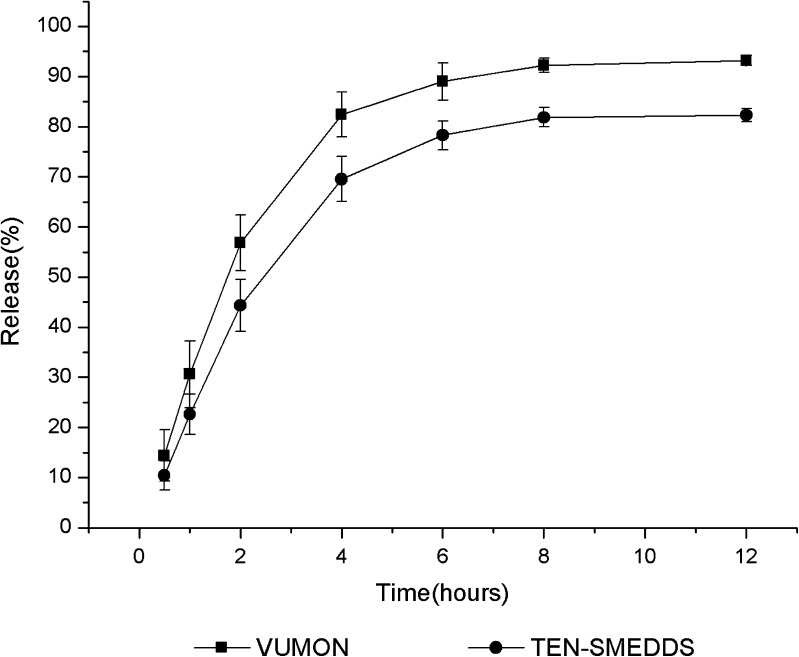
A dialysis technique was used to determine the in vitro release behavior of teniposide from VUMON and SMEDDS. To ensure the sink condition, according to the apparent solubility of teniposide in different media (Table III), 1.0% Tween 80 was added into the release medium. The release profiles of teniposide from VUMON and TEN-SMEDDS are shown in Fig. 3. At 12 h, the cumulative release of teniposide from VUMON and SMEDDS was 92.3% and 83.2%, respectively. Although the rate and extent of teniposide released from SMEDDS were less than those from VUMON, there were no significant differences (p > 0.05). As we know, VUMON is a colloidal solution containing Cremophor, and in TEN-SMEDDS, teniposide is solubilized in the oil phase of the microemulsion. So, in the sink condition, drug diffusion from these two systems was different but somehow similar (15).
Table III.
Apparent Solubility of Teniposide in Double-Distilled Water with Different Percentage of Tween 80 (n = 3)
| % of Tween 80 (v/v) | 0 | 0.1 | 1.0 |
| Solubility (μg/mL) | 0.54 ± 0.32 | 8.35 ± 0.15 | 12.07 ± 0.21 |
Fig. 3.
Release profiles of teniposide from TEN-SMEDDS and VUMON (n = 3)
The obtained release data were then fitted into first-order, Higuchi, Hixcon–Crowell, Nibergull, Ritger–Peppas, and Weibull equations. The regression results indicated that the Ritger–Peppas model best fitted the release data (VUMON, R = 0.9491; SMEDDS, R = 0.9583).
In Vivo Pharmacokinetic Studies
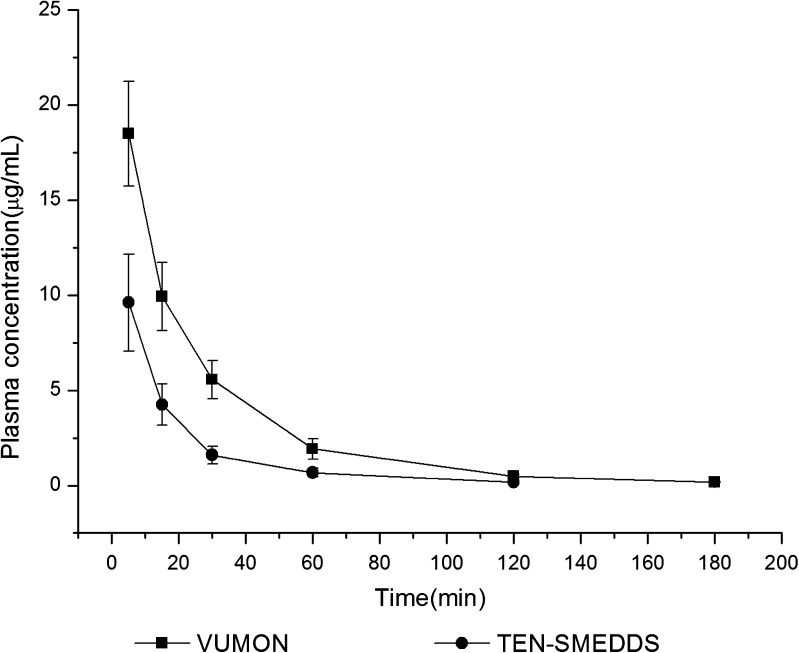
The plasma drug concentration–time profiles and pharmacokinetic parameters of teniposide after a single intravenous administration of VUMON and TEN-SMEDDS are presented in Fig. 4 and Table IV, respectively. As shown in Table IV, there were significant differences between these two formulations in several pharmacokinetic parameters of teniposide, including Cmax, AUC(0 − ∞), CL, and Vd. Typically, the AUC(0 − ∞) and Cmax of TEN-SMEDDS were significantly lower than that of the VUMON (p < 0.01), respectively, while the CL and Vd in TEN-SMEDDS group were significantly larger than that of commercial injection (p < 0.05). This seemed to say that teniposide in SMEDDS could be more widely distributed than that in VUMON and eliminated more quickly.
Fig. 4.
Plasma concentration–time curves after intravenous administration of TEN-SMEDDS or VUMON at a dose of 10 mg/Kg in rats (n = 6)
Table IV.
Pharmacokinetic Parameters of Teniposide after Intravenous Administration of TEN-SMEDDS or VUMON at a Dose of 10 mg/Kg in Rats (n = 6)
| Formulation | C max (mg/L) | AUC0 − ∞ (mg/min/L) | CL (L/min) | V d (L) |
|---|---|---|---|---|
| TEN-SMEDDS | 9.62 ± 2.55** | 235.01 ± 54.84** | 0.0088 ± 0.0019* | 0.27 ± 0.094* |
| VUMON | 18.49 ± 2.75 | 569.29 ± 71.33 | 0.0035 ± 0.00054 | 0.16 ± 0.052 |
*p < 0.05 versus VUMON group; **p < 0.001 versus VUMON group
It was previously reported that solubilization with Cremophor could prolong the drug plasma level after i.v. administration compared to an emulsion system (31–34) because Cremophor was supposed to inhibit P-glycoprotein-mediated biliary secretion and caused lipoprotein dissociation that would alter protein binding (35,36). The significantly higher CL and Vd in TEN-SMEDDS group than that in the commercial injection group indicated that Cremophor might prevent the distribution of teniposide into the tissues (37). On the other hand, the faster clearance of TEN-SMEDDS may partially be explained by the fact that lecithin is an endogenous substance, but Cremophor is not (14). Of course, the wide and fast distribution of teniposide in SMEDDS group to peripheral tissues may also cause additional safety concerns, which needs further studies.
From the literatures cited in our manuscript (5–10), we noticed that the main side effects for VUMON were mainly allergic reaction and hematological toxicity. Without Cremophor EL, our formulation may be favorable for reducing the allergic reaction, and our test proved this also (data not shown). On the other hand, the lower Cmax and AUC values compared to VUMON indicated the lower plasma level of teniposide, likely being advantageous for decreasing the hematological toxicity.
Tissue Distribution
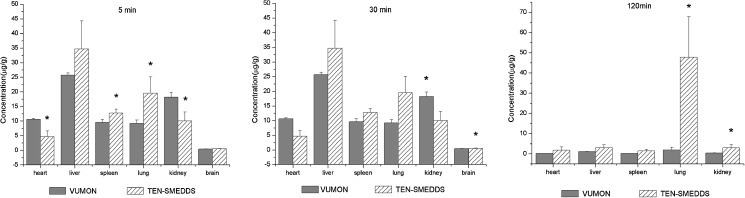
The drug distribution in different tissues after intravenous administration of TEN-SMEDDS and VUMON is shown in Fig. 5. Firstly, the concentrations of teniposide in TEN-SMEDDS group were higher than those in the VUMON group in most tissues tested for 5–120 min and in all tissues at 120 min, confirming the wide distribution of the drug suggested by the larger Vd value in pharmacokinetic study. Secondly, the drug in the TEN-SMEDDS group was found to distribute much more than those in VUMON group in reticuloendothelial system (RES), like liver, spleen, and lung. This reveals that SMEDDS may relatively be easier to accumulate in tissues where phagocytosis cells are rich. Similar observation on rapid uptake of emulsion by the RES after intravenous administration was previously reported (38,39). Several factors, such as droplet size, zeta potential, and opsonization may influence the tissue distribution of intravenously injected TEN-SMEDDS.
Fig. 5.
Concentrations of teniposide in tissues after intravenous administration of TEN-SMEDDS or VUMON at a dose of 10 mg/Kg in rats (n = 3). *p < 0.05 versus VUMON group
Most importantly, it was found here that accumulation of teniposide in the brain was higher in TEN-SMEDDS group than that in its control, although there were no significant differences between these two treatments at 5 and 30 min(p > 0.05), and it could not be detected in the brain at 120 min. It means that the distribution of teniposide in the brain in TEN-SMEDDS group was not less than that in VUMON group though the drug plasma level in the former group was much low. This was significant because teniposide is mostly used for cerebroma in the clinic. So, TEN-SMEDDS may lead to the same drug level in the brain but much low system drug exposure which may result in low system toxicity. For instance, TEN-SMEDDS may be favorable to decrease the main side effect of teniposide, the hematological toxicity (8–10).
CONCLUSIONS
In this study, Cremophor-free TEN-SMEDDS for i.v. injection was designed and successfully constructed. The novel system solved a series of related problems, such as making a water-insoluble drug into an injection, the involvement of toxic surfactant in teniposide injection, and the poor stability of an emulsion system, guaranteeing a low toxicity and a high stability. Its physicochemical characteristics, in vitro release, in vivo pharmacokinetics, and tissue distribution were investigated using VUMON as the reference. It was found that these two systems were similar in drug release but different in pharmacokinetic parameters and tissue biodistribution. After i.v. injection to rats, TEN-SMEDDS achieved a high distribution in most tissues but low drug level in plasma. The brain drug level in SMEDDS group was higher than or similar to its control. This interesting finding may be favorable for teniposide since one of its main indications in clinic is cerebroma and it is a drug with hematological toxicity.
ACKNOWLEDGMENTS
This study was funded by the National Nature Science Foundation (no. 81130059), the National Basic Research Program of China (no. 2009CB930300), and Innovation Team of Ministry of Education (no. BMU20110263). The authors are grateful for the support.
REFERENCES
- 1.McCowage GB, Vowels MR, Shaw PJ, Lockwood L, Mameghan H. Autologous bone marrow transplantation for advanced neuroblastoma using teniposide, doxorubicin, melphalan, cisplatin, and total-body irradiation. J Clin Oncol. 1995;13(11):2789–2795. doi: 10.1200/JCO.1995.13.11.2789. [DOI] [PubMed] [Google Scholar]
- 2.Hayes FA, Abromowitch M, Green AA. Allergic reactions to teniposide in patients with neuroblastoma and lymphoid malignancies. Cancer Treat Rep. 1985;69(4):439–441. [PubMed] [Google Scholar]
- 3.Ruskone-Fourmestraux A, Delmer A, Lavergne A, Molina T, Brousse N, Audouin J, et al. Multiple lymphomatous polyposis of the gastrointestinal tract: prospective clinicopathologic study of 31 cases. Groupe D’etude des Lymphomes Digestifs. Gastroenterology. 1997;112(1):7–16. doi: 10.1016/S0016-5085(97)70212-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Cui Y, Tang X. Chemical stability of teniposide in aqueous and parenteral lipid emulsions. Drug Dev Ind Pharm. 2009;35(4):508–513. doi: 10.1080/03639040802468016. [DOI] [PubMed] [Google Scholar]
- 5.Carstensen H, Nolte H, Hertz H. Teniposide-induced hypersensitivity reactions in children. Lancet. 1989;2(8653):55. doi: 10.1016/S0140-6736(89)90306-1. [DOI] [PubMed] [Google Scholar]
- 6.Nolte H, Carstensen H, Hertz H. VM-26 (teniposide)-induced hypersensitivity and degranulation of basophils in children. Am J Pediatr Hematol Oncol. 1988;10(4):308–312. doi: 10.1097/00043426-198824000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu H, Frankel LS, Culbert SJ. Severe hypertensive reactions to teniposide (VM-26) in infants with congenital leukemia. Am J Pediatr Hematol Oncol. 1987;9(3):239–241. [PubMed] [Google Scholar]
- 8.Schwartsmann G, Sprinz E, Kronfeld M, Vinholes J, Sander E, Zampese M, et al. Phase II study of teniposide in patients with AIDS-related Kaposi's sarcoma. Eur J Cancer. 1991;27(12):1637–1639. doi: 10.1016/0277-5379(91)90434-F. [DOI] [PubMed] [Google Scholar]
- 9.Weller M, Müller B, Koch R, Bamberg M, Krauseneck P. Neuro-Oncology Working Group 01 trial of nimustine plus teniposide versus nimustine plus cytarabine chemotherapy in addition to involved-field radiotherapy in the first-line treatment of malignant glioma. J Clin Oncol. 2003;21(17):3276–3284. doi: 10.1200/JCO.2003.03.509. [DOI] [PubMed] [Google Scholar]
- 10.Mañé JM, Fernández R, Muñoz A, Rubio I, Ferreiro J, López-Argumedo G, et al. Preradiation chemotherapy with VM-26 and CCNU in patients with glioblastoma multiforme. Tumori. 2004;90(6):562–566. doi: 10.1177/030089160409000605. [DOI] [PubMed] [Google Scholar]
- 11.Gelderblom H, Verweii J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–1598. doi: 10.1016/S0959-8049(01)00171-X. [DOI] [PubMed] [Google Scholar]
- 12.Kubisz P, Seghier F, Dobrotora M, Stasko J. Influence of teniposide on platelet functions in vitro. Thromb Res. 1995;77(2):145–148. doi: 10.1016/0049-3848(95)91620-Z. [DOI] [PubMed] [Google Scholar]
- 13.de Vries EG, Mulder NH, Postmus PE, Vriesendorp R, Willemse PH, Sleijfer DT. High-dose teniposide for refractory malignancies: a phase I study. Cancer Treat Rep. 1986;70(5):595–598. [PubMed] [Google Scholar]
- 14.Nornoo AO, Chow DS. Cremophor-free intravenous microemulsions for paclitaxel II. Stability, in vitro release and pharmacokinetics. Int J Pharm. 2008;349(1–2):117–123. doi: 10.1016/j.ijpharm.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 15.Nornoo AO, Osborne DW, Chow DS. Cremophor-free intravenous microemulsions for paclitaxel I: formulation, cytotoxicity and hemolysis. Int J Pharm. 2008;349(1–2):108–116. doi: 10.1016/j.ijpharm.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 16.Liliemark E, Sjöström B, Liliemark J, Peterson C, Kållberg N, Larsson BS. Targeting of teniposide to the mononuclear phagocytic system (MPS) by incorporation in liposomes and submicron lipid particles; an autoradiographic study in mice. Leuk Lymphoma. 1995;18(1–2):113–118. doi: 10.3109/10428199509064930. [DOI] [PubMed] [Google Scholar]
- 17.Gan L, Gan Y, Zhu C, Zhang X, Zhu J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: in vitro and in vivo results. Int J Pharm. 2009;365(1–2):143–149. doi: 10.1016/j.ijpharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Yin YM, Cui FD, Mu CF, Choi MK, Kim JS, Chung SJ, et al. Docetaxel microemulsion for enhanced oral bioavailability: preparation and in vitro and in vivo evaluation. J Control Release. 2009;140(2):86–94. doi: 10.1016/j.jconrel.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Piao HM, Balakrishnan P, Cho HJ, Kim H, Kim YS, et al. Preparation and evaluation of fexofenadine microemulsion for intranasal delivery. Int J Pharm. 2010;395(1–2):309–316. doi: 10.1016/j.ijpharm.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 20.Jogani VV, Shah PJ, Mishra P, Mishra AK, Mishra AR. Intranasal mucoadhesive microemulsion of tacrine to improve brain targeting. Alzheimer Dis Assoc Disord. 2008;22(2):116–124. doi: 10.1097/WAD.0b013e318157205b. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Yue Y, Zhou Y, Fan Y, Fan C, Huang Y, et al. An oil-free microemulsion for intravenous delivery of diallyl trisulfide: formulation and evaluation. Int J Pharm. 2011;407(1–2):158–166. doi: 10.1016/j.ijpharm.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Darole PS, Hegde DD, Nair HA. Formulation and evaluation of microemulsion based delivery system for amphotericin B. AAPS PharmSciTech. 2008;9(1):122–128. doi: 10.1208/s12249-007-9022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Ni X, Sheng J. Comparison of microstructures of microemulsion and swollen micelle in electrokinetic chromatography. J Chromatogr A. 2011;1218(18):2598–2603. doi: 10.1016/j.chroma.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Mrestani Y, Behbood L, Albert H, Neubert RHH. Microemulsion and mixed micelle for oral administration as new drug formulations for highly hydrophilic drugs. Eur J Pharm Biopharm. 2010;74(2):219–222. doi: 10.1016/j.ejpb.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Narang AS, Delmarre D, Gao D. Stable drug encapsulation in micelles and microemulsions. Int J Pharm. 2007;345(1–2):9–25. doi: 10.1016/j.ijpharm.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 28.Nagai N, Shikii T, Mihara K, Ogata H, Sasaki Y. Improved high-performance liquid chromatographic analysis of teniposide in human plasma. J Chromatogr B: Biomed Sci Appl. 1998;709(2):315–319. doi: 10.1016/S0378-4347(98)00059-0. [DOI] [PubMed] [Google Scholar]
- 29.Sharma G, Wilson K, Walle CF, Sattar N, Petrie JR, Kumar R. Microemulsions for oral delivery of insulin: design, development and evaluation in streptozotocin induced diabetic rats. Eur J Pharm Biopharm. 2010;76(2):159–169. doi: 10.1016/j.ejpb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Gong T, Wang CG, Zhong ZR, Zhang ZR. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm. 2007;340(1–2):153–162. doi: 10.1016/j.ijpharm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Ganta S, Paxton JW, Baguley BC, Garg S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in parenteral emulsion. Int J Pharm. 2008;360(1–2):115–121. doi: 10.1016/j.ijpharm.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Choi HK, Suh SP, Lee YB. Pharmacokinetic and pharmacodynamic evaluation of cyclosporin A O/W-emulsion and microsphere formulations in rabbits. Eur J Pharm Sci. 2002;15(5):497–502. doi: 10.1016/S0928-0987(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Zhang Y, Yang ZY, Tang X. Formulation of an intravenous emulsion loaded with a clarithromycin–phospholipid complex and its pharmacokinetics in rats. Int J Pharm. 2009;366(1–2):160–169. doi: 10.1016/j.ijpharm.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Shi S, Chen H, Lin X, Tang X. Pharmacokinetics, tissue distribution and safety of cinnarizine delivered in lipid emulsion. Int J Pharm. 2010;383(1–2):264–270. doi: 10.1016/j.ijpharm.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Henningsson A, Sparreboom A, Sandstrom M, Freijs A, Larsson R, Bergh J, et al. Population pharmacokinetic modelling of unbound and total plasma concentrations of paclitaxel in cancer patients. Eur J Cancer. 2003;39(8):1105–1114. doi: 10.1016/S0959-8049(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 36.Sykes E, Woodburn K, Decker D, Kessel D. Effects of Cremophor EL on distribution of Taxol to serum lipoproteins. Br J Cancer. 1994;70(3):401–404. doi: 10.1038/bjc.1994.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparreboom A, Zuylen L, Brouwer E, Loos WJ, Bruijn P, Gelderblom M, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59(7):1454–1457. [PubMed] [Google Scholar]
- 38.Ma HL, Xu YF, Qi XR, Maitani Y, Nagai T. Superparamagnetic iron oxide nanoparticles stabilized by alginate: pharmacokinetics, tissue distribution, and applications in detecting liver cancers. Int J Pharm. 2008;354(1–2):217–226. doi: 10.1016/j.ijpharm.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 39.Oyewumi MO, Yokel RA, Jay M, Coakley T, Mumper RJ. Comparison of cell uptake, biodistribution and tumor retention of folate-coated and PEG-coated gadolinium nanoparticles in tumor-bearing mice. J Control Release. 2004;95(3):613–626. doi: 10.1016/j.jconrel.2004.01.002. [DOI] [PubMed] [Google Scholar]