Abstract
Optic pathway gliomas represent a specific subtype of astrocytoma with unique clinicopathologic and biological properties but studies of tumors in the optic nerve proper have been hampered by limited tissue availability. We analyzed optic nerve gliomas of 59 patients (median age 9 years, range = 3 months to 66 years; 33 female; 26 male) using formalin-fixed paraffin embedded material in tissue microarrays. Seven patients had the clinical diagnosis of neurofibromatosis type 1 (NF1). Fluorescence in situ hybridization studies were performed for BRAF, PTEN, CDKN2A (p16), and NF1. Immunohistochemistry was performed for glial fibrillary acidic protein, phospho-ERK and mutant IDH1R132H protein. BRAF duplication was present in 11 (of 15) (73%) evaluable tumors including 1 NF1 patient (1 of 4 tested, 25%). The single tumor lacking BRAF duplication or NF1-association had histologic features of a ganglioglioma. Conversely, heterozygous PTEN deletions were present in 2 (of 25) (8%) evaluable cases, one of which was BRAF-duplicated and the other NF1-associated. CDKN2A and NF1 deletions were absent in all tumors tested. Phospho-ERK immunoreactivity was present in 55 (of 57) (96%) tumors, and was mostly strong and diffuse (80%). Only 1 case (of 53) expressed IDH1R132H. Thus, optic nerve gliomas demonstrated molecular alterations typical of pilocytic astrocytomas, including the universal presence of either BRAF duplication or NF1-association and common MAPK pathway activation, but very rare mutant IDH1 expression.
Keywords: BRAF, Fluorescence in situ hybridization (FISH), Glioma, MAPK, Neurofibromatosis, Optic nerve, Pilocytic astrocytoma
INTRODUCTION
Gliomas involving the optic nerve and other optic pathway structures (i.e. optic pathway gliomas) represent a specific subtype of astrocytoma with unique clinicopathologic and biological properties. Optic nerve gliomas may occur in the setting of neurofibromatosis type 1 (NF1) or sporadically, and affect approximately 15% of children with NF1 (1). At the pathological level, NF1-associated and sporadic optic nerve gliomas are predominantly World Health Organization grade I pilocytic astrocytomas (PAs) (2–4).
While optic pathway gliomas are often considered as a group, some clinical data suggest that the site within the optic pathways at which they arise can affect their biology. Tumors in the hypothalamic region can behave aggressively, and these are often of the pilomyxoid variant (5). In contrast, it has long been recognized that tumors involving the optic nerve proper, particularly in the setting of NF1, are typically indolent, and in some instances regress spontaneously (6). Because of the benign behavior and typical radiologic appearance of optic nerve gliomas, in recent decades they have rarely been biopsied, hampering investigations into their molecular basis.
Recent insights into the molecular mechanisms responsible for PA have centered on the importance of MAPK pathway signalling. In NF1-associated tumors, the mechanism is bi-allelic NF1 gene inactivation (7). In sporadic PA, the most frequent molecular alteration is a tandem duplication at chromosomal region 7q34 involving the BRAF kinase domain, which leads to a novel KIAA1549:BRAF fusion (8–11). Other alterations less commonly reported include BRAF (V600E) point mutation (12), K-RAS mutations (13), SRGAP3:RAF1 fusions (13, 14), small BRAF insertions (BRAFinsT ) (14–16) and the recently described FAM131B:BRAF fusion mediated by an interstitial deletion (17). The common biologic effect of all these alterations is MAPK pathway activation, which is an almost universal feature of PA. Conversely, IDH1/2 mutations are absent in almost all instances, in contrast to diffuse gliomas where they are common (18–20).
Of interest, molecular alterations in PA, including optic pathway gliomas, appear to be site-dependent. For example, global gene expression profiles in PA vary according to CNS site of origin (21, 22). In addition, KIAA1549:BRAF fusions are most frequent in cerebellar PA in many studies, with incidences ranging from 72% to 94% (8, 9, 11, 13, 17, 23, 24), whereas BRAF(V600E) is more typical of hemispheric PA (12). Although BRAF alterations are also frequent in optic pathway gliomas, with reported rates ranging from 43% to 69% (9, 17, 24–26), most of the tumors previously profiled were located in the hypothalamic region. The prevalence of BRAF alterations and MAPK pathway activation in gliomas of the optic nerve itself is therefore unclear. In this study, we took advantage of a unique historical archive containing a large number of optic nerve gliomas resected en bloc in order to perform targeted immunohistochemical and molecular analysis of tumors at a site from which tissue is rarely removed in current clinical practice.
MATERIALS AND METHODS
Patients and Tumor Samples
Tumors obtained from 59 patients were retrieved from the archives of the former Armed Forces institute of Pathology (Washington, D.C). In most of these cases the optic nerve was resected en bloc, often with enucleation of the globe. Initial histopathologic evaluation was consistent with PA/low-grade astrocytoma in all cases (Fig. 1). Based on the presence of neoplastic ganglion cells, 2 cases were re-classified after central re-review by several neuropathologists as gangliogliomas. Median age at surgery was 9 years (range = 3 months to 66 years) and there were 33 females and 26 males. Five tumors demonstrated intraocular extension. Storage time for archived tissue ranged from 15 to 78 years (mean = 47 years). A tissue microarray was constructed from formalin-fixed paraffin-embedded material, using 2 to 5 cores 1 mm in diameter per tumor with sampling of different neoplastic regions when possible. Clinical evidence of NF1 was present in 7 of 37 patients (19%) for which detailed clinical records were available. All studies were approved by institutional review boards at the participating institutions.
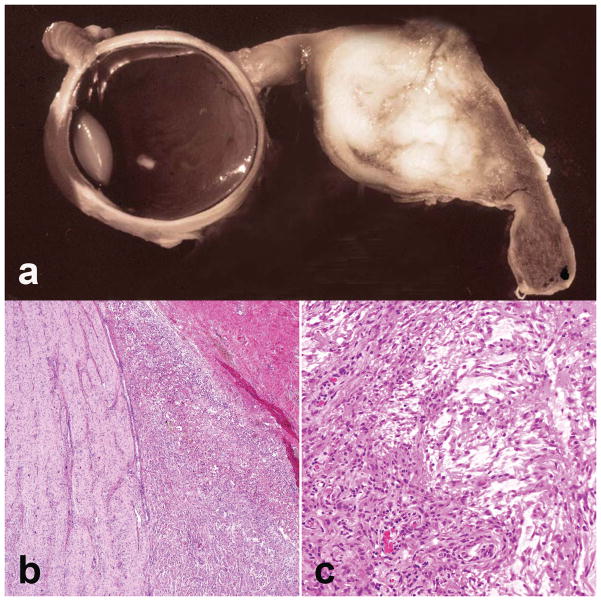
Figure 1.
Optic nerve glioma pathology. (a) Optic nerve gliomas cause fusiform expansions of the optic nerve that in the past often led to large resections. (b) Extension into the subarachnoid space may be a prominent feature (H&E, ×100). (c) The overwhelming majority of optic nerve gliomas demonstrate pilocytic features, including biphasic architecture with alternating microcystic and compact areas (H&E, ×200).
Immunohistochemistry
Immunohistochemical studies were performed using antibodies recognizing phospho-ERK (Rabbit monoclonal antibody (D13.14.4E)XP®, Cell Signaling Technology, Danvers, MA, 1:400), glial fibrillary acidic protein ([GFAP], rabbit monoclonal, Ventana, Tucson, AZ, prediluted), and mutant IDH1R132H protein (clone H09, Dianova, Hamburg, Germany, 1:50). Scoring for pERK and GFAP was performed using a 4-tiered semiquantitative scale (0 to +++) by a single neuropathologist (F.J.R.). The median value of multiple scores was used. IDH1 mutant protein was scored as positive or negative.
Fluorescence In Situ Hybridization
BRAF
Fluorescence in situ hybridization (FISH) studies were performed using home brew probes targeting 3′ BRAF, 5′ BRAF, and a commercial CEP7 probe (Abbott Molecular, Des Plaines, IL), and were scored as previously described (27).
P16, NF1 and PTEN
Commercially available probes that target CDKN2A/p16 (9p21) and PTEN (10q23) were used, with respective control probes CEP9 and CEP10 (Abbott Molecular/Vysis). A custom-made probe targeting NF1 (17q11.2) with associated CEP17 was obtained from Empire Genomics (Buffalo, NY). Appropriate target hybridization was confirmed by hybridization with spread human metaphases. Interpretation of FISH signals was performed using previously established cutoffs (23). In brief, at least 50 non-overlapping nuclei were enumerated per tumor and only cases with clear probe hybridization were scored using a fluorescence microscope. Cutoffs for heterozygous deletion were defined as a target/control ratio of <0.80.
RESULTS
BRAF Duplication is Frequent in Glioma of the Optic Nerve Whereas CDKN2A, NF1 and PTEN Deletions Are Rare to Absent
Only the subset of TMA cases with immunofluorescent signals sufficient for evaluation was scored, and the fact that most cases were over 4 decades old likely limited the technical success rate. Of 59 cases, 26 (44%) lacked successful hybridizations with any probe; 4 (7%) of cases showed successful hybridizations with 1 probe only; and 29 (49%) were successful with 2 or more probes. These findings confirm that hybridization failures/weak hybridizations were not uniform across samples. Duplication of the BRAF kinase domain (3′ portion of the gene) was the most frequent molecular alteration, present in 11 of 15 (73%) of the evaluable tumor samples (Fig. 2a). Conversely, heterozygous PTEN deletions were present in 2 of 25 cases (8%), whereas CDKN2A and NF1 deletions were absent in all cases successfully tested (n = 29 and 23, respectively) (Fig. 2b–d). Both cases with PTEN deletions lacked p16 deletions, 1 had a concurrent BRAF duplication and another was associated with NF1. Polysomies involving chromosomes 7, 9, 10 and 17 were present in 2 (13%), 2 (7%), 1 (4%), and 2 (9%) cases, respectively. Of interest, 17 of 18 cases with successful BRAF FISH studies and/or known NF1 clinical status had either BRAF duplication or NF1 syndrome, and the single case lacking either was histologically classified as a ganglioglioma. Of 4 patients with NF1 syndrome, 1 had a concurrent BRAF duplication (Tables 1, 2).
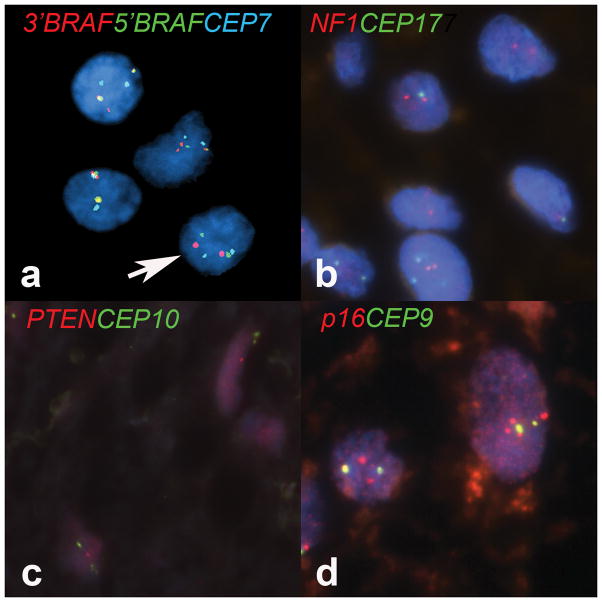
Figure 2.
Molecular cytogenetic findings in optic nerve glioma. (a) The main molecular cytogenetic finding in optic nerve gliomas was duplication of the 3′ region of the BRAF gene, leading to 3 red signals in neoplastic cells (overlapped with green yielding yellow signals in 2 cells). Arrow indicates the normal cell pattern for reference. (b) NF1 deletions were absent in all cases, including those obtained from patients with the clinical diagnosis of NF1. (c) Heterozygous PTEN deletions were present in 2 cases only; 1 of these died with progressive disease. (d) Polysomies involving several chromosomes, including Chr 9 recognized with a probe targeting p16/CEP9, were present in a minority of cases (×1000).
Table 1.
Optic Nerve Glioma Cases with BRAF Duplication and Neurofibromatosis Type 1 Clinical Status Data
| Case | Age | Sex | Clinical Presentation | Site | Pathology | Chiasm involvement | NF status-clinical | BRAF FISH | PTEN FISH | p16 FISH | NF1 FISH | GFAP IHC | IDH1 IHC | pERK IHC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | F | Cough, drowsiness, vomiting and loss of appetite | Optic nerve | Optic glioma | - | NF1 | Polysomy | normal | normal | normal | 3 | Neg | 3 |
| 2 | 5 | F | Painless proptosis for 3 months. | L optic nerve | Optic glioma | - | NF1 | Polysomy | Polysomy | Polysomy | normal | 3 | Neg | 3 |
| 3 | 4 | F | Rapidly progressive proptosis for 9 months reducing vision | Optic nerve | Optic glioma | - | NF1 | normal | normal | - | normal | 2 | Neg | 3 |
| 4 | 2 | M | Gradual blindness right>left | R optic nerve/chiasm | Optic glioma | Yes | NF1 | - | Het del | normal | normal | 3 | Neg | 0 |
| 5 | 6 | F | Exophthalmos | Optic nerve | Optic glioma | Yes | NF1 | - | normal | normal | - | - | Neg | 3 |
| 6 | 2 | F | Bilateral loss of vision, nystagmus and optic atrophy | Bilateral optic nerve | Optic glioma | - | NF1 | - | - | - | - | 3 | Neg | 3 |
| 7 | 7 | M | 3 year history of proptosis, amaurosis, exophthalmos and a mass | R optic nerve | Optic glioma | absent | NF1 | Dup | normal | - | normal | 3 | Neg | 3 |
| 8 | 5 | M | - | Optic nerve | Optic glioma | - | Sporadic | Dup | normal | normal | normal | 3 | Neg | 1 |
| 9 | 7 | M | - | Optic nerve | Optic glioma | - | Sporadic | Dup | normal | normal | normal | - | Neg | 3 |
| 10 | 1 | F | Seizure, increased head circumference | Chiasm | Optic glioma | Yes | Sporadic | Dup | normal | normal | normal | 3 | Neg | 3 |
| 11 | 8 | F | Proptosis of 6 months duration | L optic nerve | Optic glioma | absent | Sporadic | Dup | normal | normal | normal | 3 | Neg | 3 |
| 12 | 30 | F | Blurred vision, visual loss, proptosis | R optic nerve | Optic glioma | Yes | Sporadic | Dup | normal | normal | - | 3 | Neg | 3 |
| 13 | 4 | F | Unilateral intermittent proptosis | L optic nerve | Optic glioma | - | Sporadic | Dup | normal | - | 3 | Neg | 3 | |
| 14 | 6 | F | - | Optic nerve | Optic glioma | - | - | Dup | Het del | normal | - | 3 | Neg | 3 |
| 15 | 16 | M | - | Optic nerve | Optic glioma | - | Dup | normal | normal | normal | 3 | Neg | 3 | |
| 16 | 22 | F | - | Optic nerve | Optic glioma | - | - | Dup | - | - | - | 2 | Neg | 1 |
| 17 | 6 | M | - | Optic nerve | Optic glioma | - | - | Dup | - | - | - | 3 | Neg | 3 |
| 18 | 5 | F | Progressive proptosis, recent pain | R optic nerve | Ganglioglioma | absent | Sporadic | normal | - | normal | - | 3 | Neg | 3 |
| 19 | 13 | F | gradual unilateral visual loss for 3 years, optic atrophy | R optic nerve | Optic glioma | - | - | - | - | - | - | 3 | Pos | 3 |
NF1, neurofibromatosis type 1, FISH, fluorescence in situ hybridization; GFAP, glial fibrillary acidic protein; IHC, immunohistochemistry; Dup, duplication; Het del, heterozygous deletion, Neg, negative; M, male; F, female; R, right; L, left.
Table 2.
Summary of Molecular and Immunohistochemical Markers
| Marker | N (%) |
|---|---|
| BRAF* duplication | 11(73) |
| PTEN* deletion | 2(8) |
| CDKN2A (p16)* deletion | 0 |
| NF1* deletion | 0 |
| pERK¥ | |
| +++ | 46 (81) |
| ++ | 6 (11) |
| + | 3 (5) |
| 0 | 2 (3) |
| IDH1R132H¥ | |
| Negative | 52 (98) |
| Positive | 1 (2) |
Evaluated by fluorescence in situ hybridization.
Evaluated by Immunohistochemistry using a 4-tiered semiquantitative scale (0 to +++) by a single neuropathologist (FJR).
MAPK Activation is Frequent in Optic Nerve Glioma Whereas Mutant IDH1 Protein Expression is Very Rare
Given the prolonged storage age of many of the samples, immunohistochemistry for GFAP was performed to evaluate for adequacy of immunoreactivity. GFAP staining was preserved in 53 of 56 cases (95%), suggesting intact immunoreactivity in the majority of the tumors (Fig. 3a). Cases lacking immunoreactivity for GFAP or the relevant marker were excluded from interpretation.
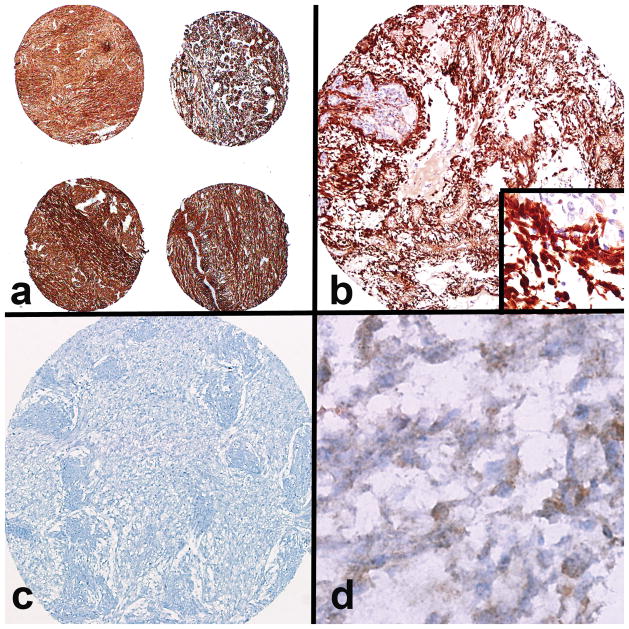
Figure 3.
Immunohistochemical findings in optic nerve glioma. (a) Preserved immunoreactivity was reflected by strong glial fibrillary acidic protein staining in the majority of the cases studied (×40). (b) Strong pERK immunoreactivity, consistent with MAPK pathway activation, was present in the majority of the cases (×200) (inset: ×600 magnification with a negative internal vessel control). (c, d) Almost all cases were negative for mutant IDH1 protein expression (c, ×200), except for a single case with diffuse moderate reactivity (d, ×600).
pERK immunolabeling was present in 55 of 57 cases (97%). When present, pERK immunoreactivity was usually strong and diffuse (81%) (Fig. 3b), supporting MAPK pathway activation in the vast majority of gliomas of the optic nerve and similar to PAs in other anatomical compartments. pERK was also positive in all cases with BRAF duplication (11 of 11); in this group 9 were +++ and 2 were +. Of 7 NF1 cases, 6 demonstrated strong (+++) pERK immunoreactivity, and 1 was negative. Conversely, only 1 case of 53 expressed IDH1R132H mutant protein (Fig. 3c, d), but FISH studies failed in that case. Results are summarized in Table 1.
Optic Nerve Gliomas are Associated with a Relatively Favorable Outcome
Outcome data were available for 15 patients (25%). Ten patients were alive and stable 1 to 12 years after surgery (median follow-up = 6.5 years). Two patients underwent subsequent surgeries for recurrence/progression 3 months and 48 years after the first surgery. Two patients died of perioperative complications, and 1 patient died with progressive disease 5 years after surgery, which was confirmed at autopsy. No firm associations between molecular findings and outcome were identified, although the single patient who died with progressive disease had the clinical diagnosis of NF1 as well as a heterozygous PTEN deletion.
DISCUSSION
Our study supports the feasibility of using older archival material for phenotypic and molecular studies, which may be of particular use in rare tumors for which surgical excess material is frequently not available. Based on GFAP and pERK immunostaining, we demonstrate a high frequency (approximately 95%) of antigen preservation in formalin-fixed paraffin-embedded tumor tissues. Hybridization failures for FISH studies were higher, but objective scoring was possible in almost half of the cases. A particularly high extent of failure for FISH studies was noted in cases stored over 55 years, with only 1 case (of 8) in this group providing interpretable results with 2 FISH probe sets.
BRAF duplications and strong pERK immunostaining were frequent, whereas PTEN, CDKN2A (p16), and NF1 deletions were rare to absent. This confirms MAPK pathway activation through genetic alterations as an essential biological feature of optic nerve gliomas, analogous to PA arising in other anatomical sites such as the cerebellum. The main difference is the greater proportion of NF1 syndrome patients with optic nerve gliomas (19%), whereas cerebellar PAs are almost always sporadic. It is of note that essentially 100% of optic nerve gliomas in this series had either BRAF duplication or NF1-association. The single case lacking BRAF duplication or NF1-association was more consistent with a ganglioglioma than a pilocytic astrocytoma, although it also had strong pERK staining supporting MAPK pathway activation.
PTEN and CDKN2A (p16) deletions are frequent findings in diffusely infiltrating gliomas, particularly glioblastoma (28). Conversely, PTEN deletions were rare in our cohort of optic nerve gliomas and CDKN2A deletions were absent. This is in agreement with prior studies showing these alterations to be relatively rare in conventional PA (29, 30). Of note, these alterations occur at a higher rate in the rare PA with anaplasia (23), which almost never arise in the optic pathways. It is also of interest that the only tumor resulting in patient death with progressive disease in the subset of patients for whom outcome data are available had a heterozygous PTEN deletion.
NF1-associated PAs are characterized at the genetic level by homozygous inactivation of the NF1 gene, which is not a feature of PAs that arise sporadically. In most instances, BRAF alterations and NF1 syndrome are mutually exclusive. Only a single NF1-associated tumor in our cohort had a BRAF alteration, an occurrence that is rare but has been documented (13, 15, 17). We did not identify NF1 deletions by FISH in our cohort, which is consistent with small genetic alterations to be responsible for NF1 biallelic inactivation in these tumors not detectable by FISH. In fact, constitutional NF1 microdeletions occur only in a small subset of NF1 patients (5%–10%) (31).
Why NF1-related gliomagenesis favors the optic nerve and pathways has always been an enigmatic feature of the syndrome. Recent insights have been provided by murine models of NF1 and optic nerve glioma. For example, Warrington et al studied the role of the tumor microenvironment in optic gliomagenesis, where non-neoplastic cells secrete factors that stimulate neoplastic glial cell growth at this specific site (32). Little is known regarding initiation and tumor progression in sporadic PA at this site. However, the high incidence of BRAF duplications in optic nerve PA as compared to those arising in cortex or deep grey matter suggests differences in initiating factors possibly linked to cell of origin.
With respect to biologic behavior, recent studies have described the phenomenon of oncogene-induced senescence to limit growth in PA. Through this mechanism, an initial oncogenic growth stimulus mediated by BRAF activation limits subsequent growth by promoting growth arrest. This is supported by the expression of markers of senescence such as p16 and activation of β-galactosidase in PA cultures and cell cultures after activated BRAF transfection (33, 34). Oncogene-induced senescence may also occur after NF1 loss (35). The specific mechanisms responsible for avoiding senescence in PA are unclear, although p16 protein loss was associated with worse overall survival in a prior study (34).
Few pathologic features have been associated with outcome in tumors involving the optic pathways. Pilomyxoid astrocytoma is a unique pilocytic astrocytoma variant that has a propensity to involve the hypothalamic region of young children, and is associated with a worse clinical outcome (5). However, pilomyxoid astrocytomas centered in the optic nerve proper have not been described. In the setting of NF1, increased mitotic activity and infiltrative features do not seem to be associated with a worse outcome in optic nerve gliomas (2), providing further support for the concept that the optic nerve is a unique anatomic site associated with a distinct biology in gliomas. Outcome data in a subset of patients in our cohort also emphasize this relatively good prognosis of gliomas in the optic nerve.
A major finding of our study is the evidence of MAPK activation in essentially 100% of tumors with available data. This has therapeutic implications given that inhibitors of BRAF and other pathway components are actively being tested in clinical trials, including Sorafenib and AZD6244 (clinicaltrials.gov). In view of the increased morbidity associated with surgery and standard chemo-radiation regimens at this site, pharmacological blockade may be an attractive approach for the optic pathway in specific cases.
In summary, our study supports an important role for BRAF duplication and MAPK pathway activation in gliomas of the optic nerve proper, similar to PA in the cerebellum. Future studies should provide insight into the prognostic and biological features of tumors arising in this unique anatomic compartment and hopefully will lead to much-needed targeted therapies for these patients.
Acknowledgments
This work was funded in part by the Children’s Cancer Foundation and contributors to the Pilocytic/Pilomyxoid Astrocytoma Fund, including Lauren’s First and Goal (C.G.E.); the Childhood Brain Tumor Foundation (F.J.R.), the DFCI Pediatric Low Grade Astrocytoma Foundation (K.L.L., A.H.L.), NCI P01CA142536, and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology of the University of Minnesota (J.D.C.).
The authors thank the Cytogenetic Shared Resource Core at Mayo Clinic, as well as Antoinette Price at Johns Hopkins University for technical assistance.
References
- 1.Listernick R, Charrow J, Greenwald MJ, et al. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114:788–92. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez FJ, Perry A, Gutmann DH, et al. Gliomas in neurofibromatosis type 1: a clinicopathologic study of 100 patients. J Neuropathol Exp Neurol. 2008;67:240–9. doi: 10.1097/NEN.0b013e318165eb75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern J, DiGiacinto GV, Housepian EM. Neurofibromatosis and optic glioma: clinical and morphological correlations. Neurosurg. 1979;4:524–8. doi: 10.1227/00006123-197906000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Cummings TJ, Provenzale JM, Hunter SB, et al. Gliomas of the optic nerve: histological, immunohistochemical (MIB-1 and p53), and MRI analysis. Acta Neuropathol. 2000;99:563–70. doi: 10.1007/s004010051161. [DOI] [PubMed] [Google Scholar]
- 5.Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J NeuropatholExp Neurol. 1999;58:1061–8. doi: 10.1097/00005072-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Parsa CF, Hoyt CS, Lesser RL, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001;119:516–29. doi: 10.1001/archopht.119.4.516. [DOI] [PubMed] [Google Scholar]
- 7.Kluwe L, Hagel C, Tatagiba M, et al. Loss of NF1 alleles distinguish sporadic from NF1-associated pilocytic astrocytomas. J Neuropathol Exp Neurol. 2001;60:917–20. doi: 10.1093/jnen/60.9.917. [DOI] [PubMed] [Google Scholar]
- 8.Bar EE, Lin A, Tihan T, et al. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67:878–87. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 9.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–7. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–49. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19:449–58. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 13.Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–81. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 14.Jones DT, Kocialkowski S, Liu L, et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28:2119–23. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhardt AE, Olbrich H, Roring M, et al. Functional characterization of a BRAF insertion mutant associated with pilocytic astrocytoma. Int J Cancer. 2010 doi: 10.1002/ijc.25893. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Deshmukh H, Gutmann RJ, et al. Alterations of BRAF and HIPK2 loci predominate in sporadic pilocytic astrocytoma. Neurology. 2009;73:1526–31. doi: 10.1212/WNL.0b013e3181c0664a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cin H, Meyer C, Herr R, et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121:763–74. doi: 10.1007/s00401-011-0817-z. [DOI] [PubMed] [Google Scholar]
- 18.Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118:401–5. doi: 10.1007/s00401-009-0550-z. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–53. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma MK, Mansur DB, Reifenberger G, et al. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 2007;67:890–900. doi: 10.1158/0008-5472.CAN-06-0973. [DOI] [PubMed] [Google Scholar]
- 22.Tchoghandjian A, Fernandez C, Colin C, et al. Pilocytic astrocytoma of the optic pathway: a tumour deriving from radial glia cells with a specific gene signature. Brain. 2009;132:1523–35. doi: 10.1093/brain/awp048. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez EF, Scheithauer BW, Giannini C, et al. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011;121:407–20. doi: 10.1007/s00401-010-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob K, Albrecht S, Sollier C, et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer. 2009;101:722–33. doi: 10.1038/sj.bjc.6605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins C, Walker E, Mohamed N, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res. 2011;17:4790–8. doi: 10.1158/1078-0432.CCR-11-0034. [DOI] [PubMed] [Google Scholar]
- 27.Tian Y, Rich BE, Vena N, et al. Detection of KIAA1549-BRAF fusion transcripts in formalin-fixed paraffin-embedded pediatric low-grade gliomas. J Mol Diagn. 2011;13:669–77. doi: 10.1016/j.jmoldx.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horbinski C, Hamilton RL, Nikiforov Y, et al. Association of molecular alterations, including BRAF, with biology and outcome in pilocytic astrocytomas. Acta Neuropathol. 2010;119:641–9. doi: 10.1007/s00401-009-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tada K, Kochi M, Saya H, et al. Preliminary observations on genetic alterations in pilocytic astrocytomas associated with neurofibromatosis 1. Neuro Oncol. 2003;5:228–34. doi: 10.1215/s115285170300005x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasmant E, Sabbagh A, Spurlock G, et al. NF1 microdeletions in neurofibromatosis type 1: from genotype to phenotype. Hum Mutat. 2010;31:E1506–18. doi: 10.1002/humu.21271. [DOI] [PubMed] [Google Scholar]
- 32.Warrington NM, Woerner BM, Daginakatte GC, et al. Spatiotemporal differences in CXCL12 expression and cyclic AMP underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67:8588–95. doi: 10.1158/0008-5472.CAN-06-2220. [DOI] [PubMed] [Google Scholar]
- 33.Jacob K, Quang-Khuong DA, Jones DT, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 2011;17:4650–60. doi: 10.1158/1078-0432.CCR-11-0127. [DOI] [PubMed] [Google Scholar]
- 34.Raabe EH, Lim KS, Kim JM, et al. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res. 2011;17:3590–9. doi: 10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Courtois-Cox S, Genther Williams SM, Reczek EE, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–72. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]