Abstract
Mycobacterium tuberculosis (MTB) remains a leading infectious threat to human health. Macrophages are the cells targeted for infection by the bacterium as well as key effector cells for clearance of the pathogen. Interleukin (IL)-27 opposes macrophage-mediated control of MTB because supplying IL-12 and blocking the activity of IL-27 limits bacterial growth in primary human macrophages. The purpose of this study was to determine the immunological regulators of this macrophage mechanism to restrict MTB growth. Interferon (IFN)-γ, TNF-α, and IL-18 were all demonstrated to be important to the environment that limits bacterial growth when IL-12 is supplied and IL-27 is neutralized. We find IL-18 works in conjunction with IL-12 to achieve optimal IFN-γ production in this system. We also demonstrate novel interactions between these cytokines to influence the expression or responsiveness to one another. Quantitative assays show that IFN-γ enhances expression of the IL-18 receptor signaling chain, as well as TNF expression and secretion. In turn, TNF-α augments expression of the receptor for IFN-γ the amount at the cell surface, and the extent of IFN-γ-induced signaling. We further define how the cytokine environment supports an enhanced state of classical macrophage activation. Collectively, these results describe novel immunological mechanisms that provide additional insights into the effects of IL-12 and IL-27 on macrophage regulation during MTB infection.
Keywords: Human macrophage, Mycobacterium tuberculosis, interleukin-27, interleukin-12, nitric oxide synthase
1. INTRODUCTION
Mycobacterium tuberculosis (MTB) is an intracellular pathogen and the causative agent of tuberculosis. This organism is among the most successful human pathogens and estimated to have infected one-third of the world’s population. Globally in 2009, there were an estimated 9.4 million new cases of tuberculosis [1]. However, in the face of this large incidence of infection, primary tuberculosis only occurs in approximately 5–10% of those infected [2]. This observation suggests that both the innate and specific immune functions of most infected individuals contain the bacterium. Yet despite this containment, they are typically not sufficient to eliminate the pathogen. A greater understanding of immune mechanisms that function to control the bacterium is a necessary prerequisite to development of therapeutic strategies aimed at more effective clearance of mycobacteria.
T lymphocytes are of unquestionable importance to host protection during tuberculosis [3]. They are essential for proper granuloma formation and maintenance [4–6]. Activated Th1 cells are also a dominant source of protective cytokines such as IFN-γ and TNF. As such, significant attention is applied to understanding and improving Th1 responses during tuberculosis. However, host macrophages represent the target cell for MTB in the lungs and the effector immune cell at the heart of the granuloma. MTB is able to subvert phagosome maturation and survive within professional phagocytes. Thus, marked improvement can also be made in understanding how innate cells may better control intracellular bacterial growth and cooperate with adaptive responses to reduce the bacterial burden during infection.
Although macrophages and dendritic cells are prototypical producers of IL-12, macrophages do not produce adequate quantities of IL-12 in response to MTB [7, 8]. In contrast, both EBI3 and p28 subunits of the structurally related heterodimeric cytokine, IL-27, are strongly induced by MTB in human macrophages [9]. The immunobiology of IL-27 has become more appreciated over the last decade, and this cytokine is somewhat paradoxical in that it has both proinflammatory and anti-inflammatory activity towards lymphocytes. IL-27 cooperates with IL-12 to initiate Th1 responses and also augments IFN-γ production by these cells [10–13]. However, the primary function of IL-27 may be to limit inflammatory responses in other circumstances. In experimental models of tuberculosis, mice deficient in IL-27 signaling exhibited improved control of bacteria, but uncontrolled inflammatory responses reduced animal survival over time [14, 15]. This was accompanied by an increase in CD4+ T cell proliferation in the lungs [14, 15], as well as increased proinflammatory cytokine production, including IFN-γ [15]. T cell hyperactivity is also reported in Toxoplasma gondii-infected mice that do not express WSX-1, the ligand binding component of the IL-27 receptor [16]. Other studies have shown direct involvement for IL-27 in negatively regulating TH-17 cells associated with inflammatory responses [17, 18] and in inducing IL-10-producing suppressive T cells [19].
What has been less well understood is the effect of IL-27 on the innate cells responsible for its production. We have previously demonstrated that supplementing IL-12 along with a soluble receptor to neutralize IL-27 (sIL27R) reduces MTB recovered from infected human macrophages [9]. We have extended that study in an effort to better understand the immunological parameters and mechanism involved in this response. Our results demonstrate that production of IFN-γ, TNF-α, and IL-18 are most important for reducing the mycobacterial burden in human macrophages when IL-12 is supplied and IL-27 signaling is blocked. Furthermore, IFN-γ, TNF-α, and IL-18 cooperate in a network such that they influence the expression and/or responsiveness to one another. Finally, this study further highlights the inhibitory nature of IL-27 toward human macrophages. In the case of chronic infections such as tuberculosis, this activity may compromise control of the bacteria to prevent overactive immunological responses.
2. MATERIALS AND METHODS
2.1. Mycobacterium culture conditions
MTB strain Erdman, provided by Dr. JoAnne Flynn (University of Pittsburgh School of Medicine), was maintained in Middlebrook broth containing albumin, dextrose, catalase (ADC) at 37°C with 5% CO2. Mycobacterium tuberculosis mc27000 was described previously [20] and provided by Dr. William R. Jacobs, Jr (Albert Einstein College of Medicine). This strain was grown as described above with the addition of pantothenate (25 g/mL). Gamma-irradiated MTB strain H37Rv was acquired from the Colorado State University TB Vaccine Testing and Research Materials Contract. All operations involving live MTB were performed under standard biosafety level (BSL) 3 laboratory practices.
2.2. Cell culture
Human buffy coats were purchased from Central Blood Bank (Pittsburgh, PA) or New York Blood Center (New York, NY). Eligible donors were 16 years of age or older, at least 110 pounds, and in good physical health. The buffy coat donors were anonymous and deidentified. Human PBMCs were obtained from buffy coats by Ficoll (Amersham Biosciences) density gradient centrifugation. Monocytes were subsequently isolated from human PBMCs by OptiPrep (Axis-Shield) density gradient centrifugation as described previously [21]. Monocytes were allowed to adhere to plastic culture dishes for 1 h in serum-free DMEM. Non-adherent cells were removed and the media was replaced with DMEM supplemented with 2 mM glutamine, 25 mM HEPES, 20% FCS and 10% human serum. Monocyte-derived macrophages were removed from the culture dish with PBS (pH 7.4) that contained 5 mM EDTA and 4 mg/mL lidocaine. Macrophages were routinely harvested for use between days 5 and 8 in culture. The cells were washed with PBS and plated onto Primaria® (Becton Dickinson-Falcon) culture dishes in DMEM supplemented with 2 mM glutamine, 25 mM HEPES, and 1% human serum. When data reported is from pooled experiments, each individual experiment was performed with macrophages isolated from a separate donor.
2.3. Macrophage infection and enumeration of MTB
Human macrophages cultivated in 96-well dishes (5×104/well) were treated with medium alone, IL-12 (5 ng/mL), sIL27R (10 g/mL, R&D Systems, Neutralization Dose [ND]50=1–4 g/mL), or their combination and infected with MTB (~MOI 1). All other neutralizing reagents that were included where indicated were commercially available mouse monoclonal antibodies used at 1 g/mL: IFN-γ (R&D Systems, ND50=0.06–0.3 g/mL), I-TAC (R&D Systems, ND50=0.2–0.8 g/mL), TNF-α (R&D Systems, ND50=0.015–0.06 g/mL), IL-6 (0.05-0.15 g/mL), and IL-18 (MBL International, ND50=0.1 g/mL). Infected cultures were incubated 72h at 37°C with 5% CO2. Culture supernatants were removed and macrophages were permeabilized with 0.05% saponin to release bacteria. Ten-fold serial dilutions were plated on Middlebrook 7H10 agar and incubated 21 days at 37°C with 5% CO2.
2.4. Quantitative PCR
Human macrophages (1.5×105/well) cultivated in 24-well dishes were treated as indicated. At appropriate time points, media was removed from cultures, the cells were lysed with TriReagent® (Molecular Research Center), and RNA was isolated according to commercial product protocol. First strand cDNA synthesis was performed using SuperScript™ III reverse transcriptase (Invitrogen) according to protocol. Real time cycling of reactions that included cDNA diluted 20-fold from above, gene-specific primer-probe sets (Applied Biosystems), and iQ™ Supermix (Bio-Rad) was performed in triplicate using an iQ5™ cycler (Bio-Rad). GAPDH was used as an internal reference gene.
2.5. Immunoblot Analysis
Human macrophages (5×104/well) cultivated in 96-well dishes in triplicate were stimulated as indicated in the presence or absence of -irradiated MTB. Whole-cell lysates were prepared with RIPA buffer (Boston Bioproducts, 50 mM Tris, pH7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, and 0.1% SDS) at 4°C. Equal amounts of protein were separated on 4–20% gradient SDS-PAGE gels and transferred to nitrocellulose according to standard techniques. Primary antibodies used in this study were mouse monoclonal anti-IL18 receptor (R&D Systems), mouse monoclonal anti-IFN-γ receptor beta (Abcam), rabbit polyclonal anti-Stat1 (Cell Signaling Technologies), rabbit polyclonal anti-phospho Stat1 (Cell Signaling Technologies), and goat anti-actin (Sigma). Primary antibodies were revealed with horse radish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies. ECL substrate (Amersham Biosciences) was applied to visualize proteins.
2.6. ELISA analysis
Human macrophages were cultivated in 24-well dishes as indicated above. Supernatants were collected at indicated time points for analysis of TNF or IFN-γ concentrations. Standard curves were performed in parallel.
2.7. Flow Cytometry
For these experiments macrophages were cultivated in low binding 24-well dishes (Nalge-Nunc) and stimulated with γ-irradiated MTB where indicated. Macrophages were mixed for 15 min at room temperature with Fc receptor blocking reagent (Miltenyi Biotec). For surface expression of receptors, macrophages were immunolabeled with mouse monoclonal anti-IL18 receptor conjugated with PE (R&D Systems), mouse monoclonal anti-IFN receptor (Abcam) followed by goat anti-mouse IgG conjugated with Alexa Fluor 488 (Molecular Probes), or an appropriate isotype control. Cells were collected with a FACScan flow cytometer (Becton Dickenson). Approximately 5,000 events were collected for each sample group. The mean channel (green or red) fluorescence intensity of the macrophage population was measured.
2.8. Confocal Microscopy
Human macrophages (2×105) were seeded on glass coverslips and treated with IL-12 (5 ng/ml) and sIL-27R (10 g/ml) or medium alone for 4 h prior to infection with MTB strain mc27000 (~MOI 10). At 72h post-infection, cells were fixed with 4% paraformaldehyde buffered with PBS for 30 minutes. Fixed macrophages were permeabilized and blocked for 15 minutes with PBS that contained 0.05% saponin and 0.2% BSA. Macrophages were immunolabeled with mouse monoclonal anti-NOS2 (BD Bioscience) or anti-ARG1 (Santa Cruz Biotechnology) for 1 hour. After washing, primary antibodies were revealed with anti-mouse Alexa Fluor 468-conjugated secondary antibodies (Molecular Probes). Macrophages fixed to coverslips were mounted to microscope slides using ProLong® Gold anti-fade reagent containing DAPI (Molecular Probes). The macrophages were visualized using a Zeiss Meta 510 laser scanning confocal microscope. A total of 10 fields per treatment condition were examined. The mean fluorescent intensity (MFI) was calculated using the histogram analysis in image J software. The average MFI ± standard error for 10 fields of view was calculated.
2.9. Statistical analysis
A student’s t test was used to compare sample groups within a representative experiment. A two-way ANOVA was used for all other comparisons that involve multiple experimental replicates. The p values ≤0.05 were considered statistically significant.
3. RESULTS
3.1. Restriction of MTB growth by human macrophages requires IFN-γ, TNF, and IL-18
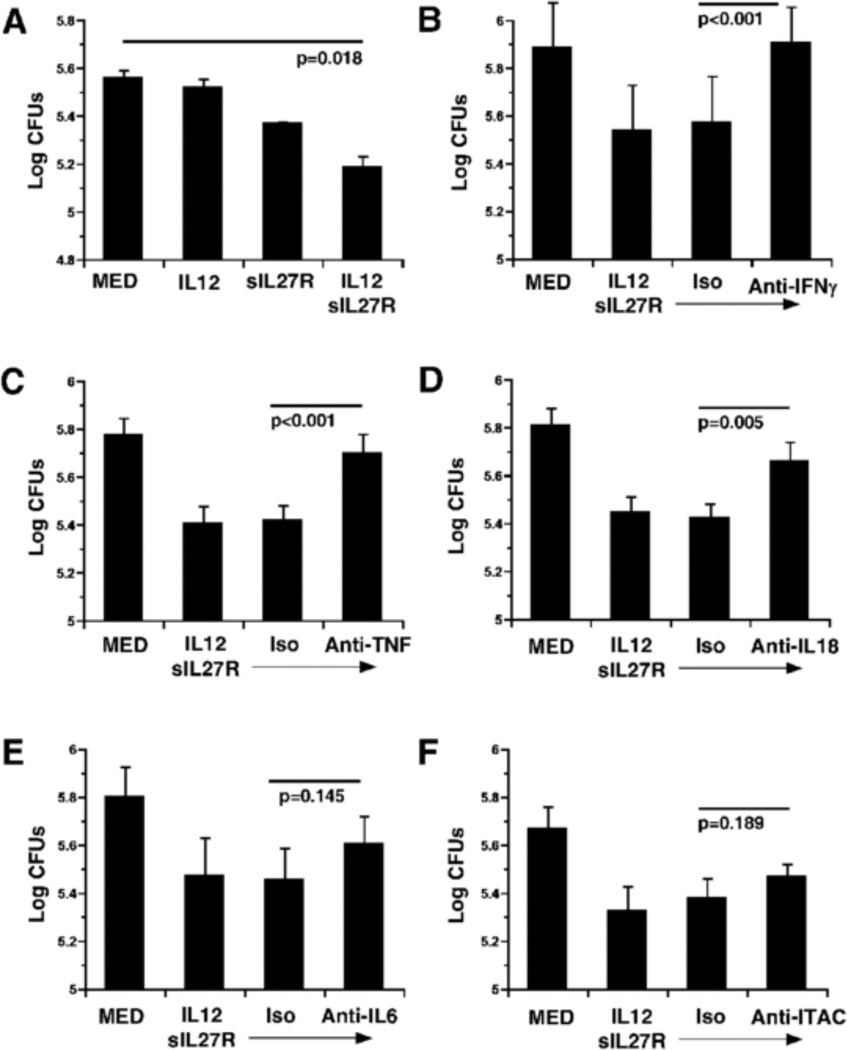
Human macrophages stimulated with IL-12 and a soluble receptor to neutralize IL-27 restrict MTB growth better than untreated macrophages, resulting in a reduced recovery of viable bacteria at 72h (Fig. 1A) [9]. We have demonstrated that this effect on MTB growth is consistent with an elevated inflammatory response that includes factors such as IFN-γ, TNF, IL-6, and I-TAC [9]. Thus, we wanted to understand which inflammatory mediators were required for the restriction of MTB growth. To address this, human macrophages were stimulated with IL-12 and sIL-27R for 4 h and then infected with MTB. Isotype control or neutralizing antibodies to target specific cytokines were also included during the infection and MTB recovery was evaluated at 72 h. Neutralization of IFN-γ completely reversed the anti-mycobacterial activity of human macrophages treated with IL-12 and sIL-27R (p<0.001, Fig. 1B). These results are consistent with previous data showing the importance of IFN-γ [9]. The contribution of other molecules was also determined. TNF production is increased by infected macrophages following stimulation with IL-12 and sIL-27R relative to infected controls [9]. Neutralization of TNF resulted in a significant reversal of the anti-mycobacterial activity (p<0.001) that was comparable to that of IFN-γ (Fig. 1C). Although IL-18 production is not significantly altered by IL-12 or IL-27 in MTB-infected human macrophages, expression of the IL-18 receptor chain is increased by neutralization of IL-27 [22]. Neutralization of IL-18 in this study resulted in an intermediate recovery of MTB that was significantly different than macrophages treated with control antibodies (Fig. 1D, p=0.005). Similar to TNF, secreted IL-6 is present at a higher level in culture supernatants following stimulation with IL-12 and sIL-27R relative to controls [9]. Neutralization of IL-6, however, resulted in a modest change in mycobacterial recovery that was not statistically significant (Fig. 1E, p=0.145). Similarly, I-TAC, is an interferon-γ-induced chemokine heavily expressed in response to IL-12 and sIL27R [9], but did not influence the ability of human macrophages to control MTB growth (Fig. 1F, p=0.189). These results suggest that IFN-γ, TNF, and IL-18 are important to the mechanism that restricts MTB growth when IL-12 and sIL-27R are supplied.
Figure 1.
Identification of factors involved in macrophage restriction of MTB growth. Human macrophages were stimulated with IL-12 (5 ng/mL), sIL-27R (10 g/mL), IL-12+sIL-27R, or medium alone for 4 h. Prior to infection by MTB neutralizing or isotype control antibodies (1 g/ml) were added as indicated. Data is represented as the mean CFUs recovered from infected macrophages ± SE at 72 h for (A) a representative experiment, (B) four, (C) six, (D) five, (E) four, or (F) five independent experiments. A student’s t-test (A) or two-way ANOVA or (B–F) was used to establish statistical significance in the 95% confidence interval between individual sample groups as indicated by the p value.
3.2. Interactions mediated by IFN-γ
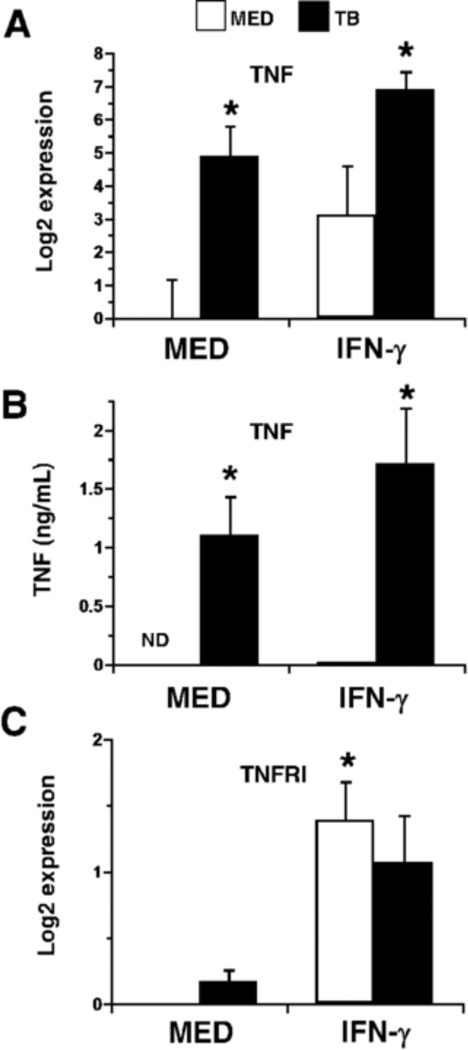
It was interesting that IFN-γ, TNF, and IL-18 each contributed to restriction of MTB growth, yet the independent activities of each cytokine accounted for more than one third of the total MTB growth restriction. This suggested that all three cytokines may cooperate in a manner in which they influence one another. To evaluate possible interactions between these cytokines, IFN-γ TNF, or IL-18 were added individually in the presence or absence of MTB at the time of infection. The addition of IFN-γ increased transcription of TNF in the absence of MTB but also augmented TNF expression during infection at 4 h (Fig. 2A) and 24 h (not shown). To confirm the transcriptional response at the protein level, supernatants were collected at 4 (not shown) and 24 h for analysis of secreted TNF. The presence of IFN-γ also increased the levels of secreted protein (Fig. 2B). In addition, IFN-γ increased expression of the TNFR1 (p55) gene in human macrophages (Fig. 2C). In this way, IFN-γ may not only influence production of TNF, but also increase responsiveness to the change in cytokine concentration.
Figure 2.
The effect of IFN-γ on TNF-α. Macrophages were stimulated with IFN-γ (25 ng/mL) ± MTB. Quantitative analysis of TNF (A) or TNFRI (C) transcripts at 4 h is presented as the mean log2 change in gene expression of triplicate samples ± SEM for three experiments. Values were normalized to the mean expression of GAPDH within a triplicate sample group and expressed relative to that of medium alone. (B) Cytokine concentrations (ng/mL) in supernatants collected from macrophage cultures at 24 h were determined by ELISA. Data is presented as mean ± SEM for three experiments. A sample that did not contain detectable quantities of protein is indicated (ND). (A, B, C) Asterisks indicate statistical significance in the 95% confidence interval relative to medium alone by two-way ANOVA; treatment groups with the same number of asterisks are not statistically different from each another.
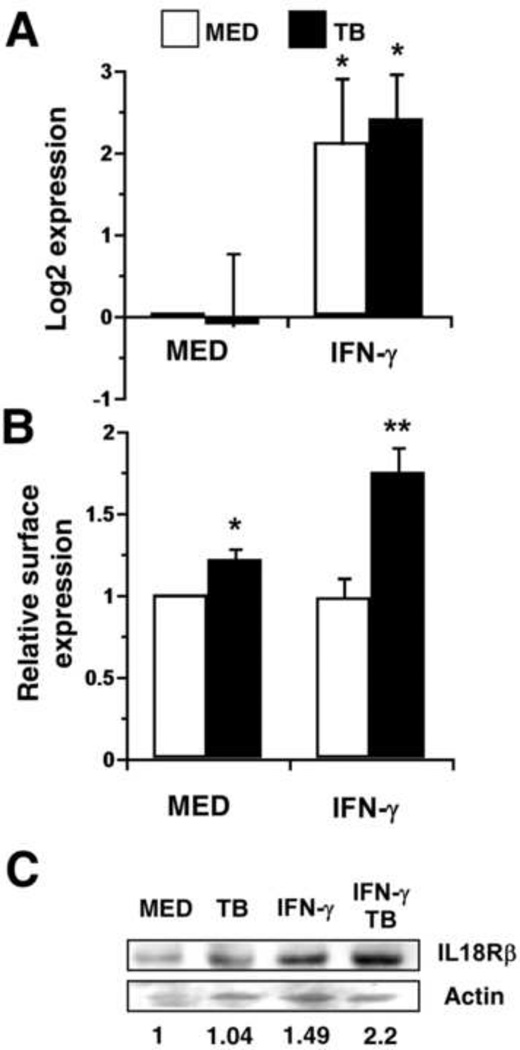
The addition of IFN-γ to human macrophage cultures also increased expression of the IL-18 receptor (IL18R) chain gene in the presence and absence of infection at 4 h (Fig. 3A). To confirm that this trend was also reflected at the cell surface we measured the level of IL-18R by immunolabeling and flow cytometry (Fig. 3B). In this assay, there was a modest increase mediated by MTB alone (Fig. 3B). In the case of MTB alone, this is likely the result of increases in TNF that occur in response to MTB; TNF increased IL-18R gene expression by 2.6-fold (not shown). IFN-γ treatment for 24 h enhanced cell surface expression of IL-18R during infection by MTB (Fig. 3B). However, the change in receptor expression measured by flow cytometry did not reflect the change in gene expression, especially after treatment with IFN-γ alone (Fig. 3A). It was possible that internalization of the receptor complex could account for these discrepancies because we have shown that IL-18 is secreted at a low level in the absence of infection [22]. Therefore, we measured IL-18R levels by immunoblot to determine if the cell surface expression was underestimated relative to the total protein. Human macrophages were treated with IFN-γ in the presence or absence of MTB as before, and total cell lysates were prepared at 24 h. This analysis showed a pattern of IL-18R expression in which IFN-γ increased protein levels that peaked in the presence of MTB (Fig. 3C).
Figure 3.
IFN-γ influences expression of the IL-18 receptor. Macrophages were stimulated with IFN-γ (25 ng/mL) ± MTB. (A) Quantitative analysis of IL-18R gene expression at 4 h is presented as the mean log2 change in gene expression of triplicate samples ± SEM for four experiments. Values were normalized to the mean expression of GAPDH within a sample group and expressed relative to that of medium alone. (B) Macrophages were harvested at 24 h and immunolabeled for surface receptors. The MFI for the cell population corresponding to each treatment condition was normalized to medium alone. The mean data from four separate experiments is shown. (A, B) Asterisks indicate statistical significance in the 95% confidence interval relative to medium alone by two-way ANOVA; treatment groups with a different number of asterisks are also significantly different from each another. (C) Whole-cell lysates were prepared from macrophages treated as indicated for 24 h. Proteins were separated by SDS-PAGE and immunoblotted for IL-18R and γ-actin as described in Materials and Methods. The ratio of IL18R /actin band intensity was expressed relative to medium alone. An image representative of two experiments is shown.
3.3. Interactions mediated by IL-18
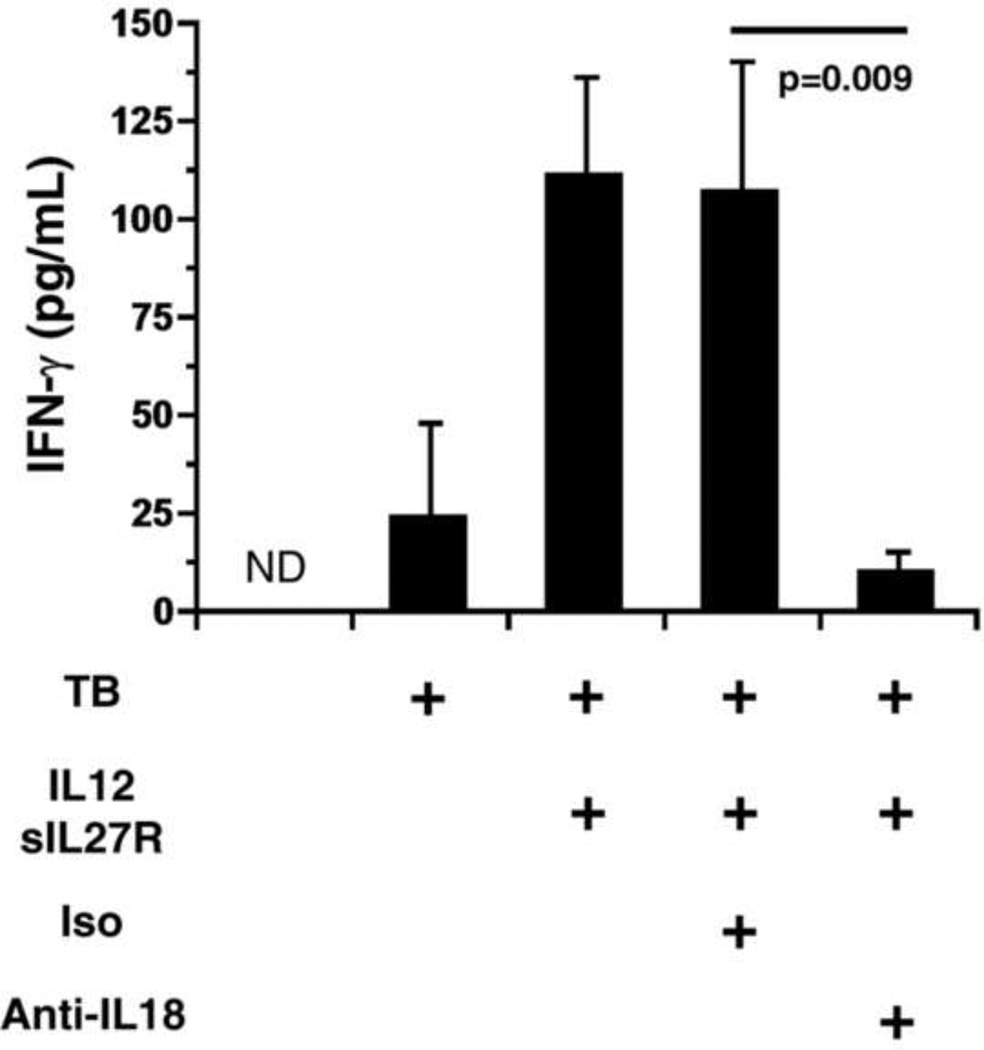
IL-18 is well known for its contribution to IFN-γ production by lymphocytes [23] and has also been implicated in driving IFN-γ production by myeloid cells in both mice and humans [22, 24–25]. Therefore, the effect of IL-18 on levels of secreted IFN-γ was also evaluated in supernatants retained from MTB-infected cultures at 72 h post-infection (Fig. 4). As expected, treatment with IL-12 and sIL-27R induced IFN-γ production in MTB-infected macrophages. The inclusion of antibody to neutralize IL-18 dramatically reduced the concentration of IFN-γ present in culture supernatants (Fig. 4). However, isotype control antibody had no effect on the IFN-γ concentration (Fig. 4). This is consistent with the importance of IL-18 and IFN-γ on restriction of MTB growth (Fig. 1B and D) and previous results investigating IFN-γ secretion at earlier time points [9, 22].
Figure 4.
IL-18 is important for optimal production of IFN-γ. Human macrophages were treated with IL-12+sIL-27R or medium alone and infected with MTB (MOI 1) as indicated. Prior to infection by MTB neutralizing or isotype control antibodies (1 g/ml) were added as indicated. Supernatants were collected at 72 h and IFN-γ concentrations determined by ELISA. Data are presented as the means of triplicate samples from five experiments ± SEM. ND indicates below the limit of detection. A two-way ANOVA was used to establish statistical significance in the 95% confidence interval for the comparison indicated.
3.4. Interactions mediated by TNF-α
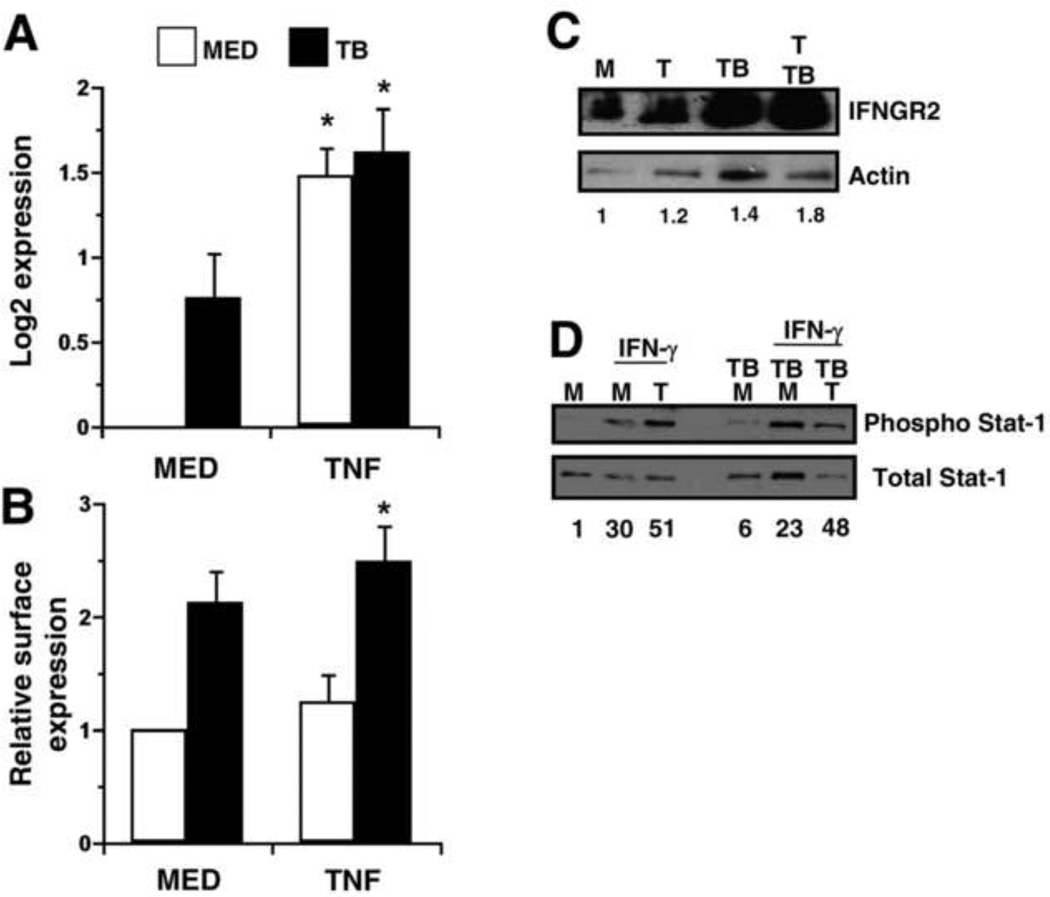
Since blocking TNF signaling had a significant impact on mycobacterial recovery (Fig. 1C), we also explored potential interactions between TNF and the other cytokine systems. The addition of TNF increased gene expression of the IFN-γ receptor 2 chain (IFNGR2 or IFNGR), the signaling molecule in the receptor complex, in the presence or absence of MTB (Fig. 5A). MTB alone also yielded a modest increase in IFNGR2 expression that may be influenced by TNF levels that are induced under this condition (Fig. 5A). We also measured the level of IFNGR2 at the cell surface by immunolabeling and flow cytometry (Fig. 5B). In the presence of MTB, addition of TNF increased IFNGR2 expression above that of MTB alone at 6 h (Fig. 5B). This trend was also confirmed by immunoblot analysis (Fig. 5C). To assess biological function associated with the increase in IFNGR2, we evaluated the phosphorylation state of Stat1 by immunoblot analysis. The macrophages were stimulated with TNF as before, and IFN-γ was supplied for an additional hour. Consistent with the increase in IFNGR2, IFN-γ led to phosphorylation of Stat-1 that was nearly doubled by treatment with TNF (Fig. 5D). This effect was maintained during infection whereby TNF mediated a two-fold enhancement of Stat-1 phosphorylation.
Figure 5.
TNF enhances expression of the IFN-γ-receptor. Macrophages were stimulated with TNF (10 ng/mL) in the presence or absence of MTB. (A) Quantitative analysis of IFNGR2 gene expression at 4 h is presented as the mean log2 change in gene expression of triplicate samples ± SEM for three experiments. Values were normalized to the mean expression of GAPDH within a triplicate sample group and expressed relative to that of medium alone. (B) Macrophages were harvested at 6 h and immunolabeled for surface receptors. The MFI for the cell population corresponding to each treatment condition was normalized to medium alone. The mean data from three separate experiments is shown. (A, B) Asterisks indicate statistical significance in the 95% confidence interval relative to medium alone by two-way ANOVA. (C, D) Macrophages were treated with (T) or without TNF (M) and infected with MTB for 6 h as indicated. (C) Whole-cell lysates were prepared and separated by SDS-PAGE and immunoblotted for IFNGR2 or actin as described in Materials and Methods. The ratio of IFNGR2/actin band intensity was expressed relative to medium alone. An image representative of two experiments is shown. (D) IFN-γ (25ng/mL) was supplied where indicated for an additional hour and whole-cell lysates were prepared. Proteins were separated by SDS-PAGE and immunoblotted for phosphorylated or total Stat-1. The ratio of phospho/total Stat-1 band intensity was expressed relative to medium alone and indicated for each condition. An image representative of three experiments is shown.
3.5. Cytokine effects on M1 and M2 macrophage phenotypes
The cytokines TNF, IL-18, and IFN-γ, shown here to be important for restriction of MTB growth through mutual interactions, are all associated with classical (M1) macrophage activation [26]. Thus, it is possible that the macrophages treated with IL-12 and sIL-27R are polarized toward classical (M1) activation. Resistin-1 (FIZZ1), a secreted cysteine-rich protein, and the mannose receptor (MRC1) that mediates recognition of carbohydrate structures, are associated with alternative (M2) activation [26, 27]. M2-like macrophages are also characterized by arginase-1 (ARG1) expression. In contrast, nitric oxide synthase (NOS2), in conjunction with secretion of proinflammatory cytokines such as TNF, IFN-γ IL-12, IL-γ IL-γ and chemokines CXCL9-11, CCL15, and CCL20, are associated with an M1 macrophage phenotype [26, 27]. The ARG1/NOS2 distinction in humans, however, is not as clear [26].
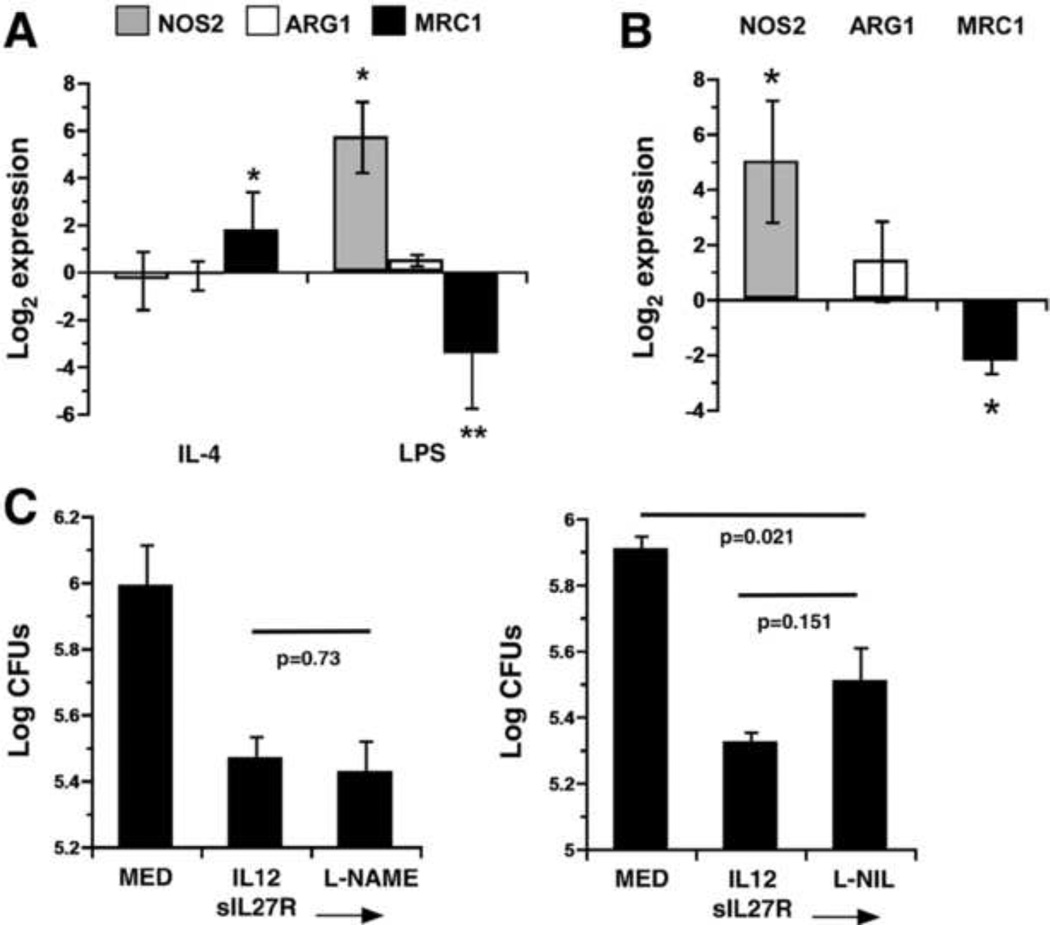
To explore macrophage activation, we investigated cytokine production and gene expression profiles for the markers described above. Production of M1-associated cytokines, IFN-γ and TNF, at 72 h following infection was augmented by treatment with IL-12 and sIL-27R (Table 1). To aid in the interpretation of gene expression data, macrophages were treated with IL-4 (100 ng/mL) or LPS (1 g/mL) for 72 h to serve as positive controls. These treatments are standard conditions for induction of M2 and M1 macrophage phenotypes, respectively. IL-4 induced mrc1 expression (Fig. 6A). Fizz1 was also induced in this condition with a wider variation in the magnitude of expression (data not shown). LPS induced a significant increase in nos2 expression while downregulating mrc1 (Fig 6A);fizz1 was not expressed in any experiment in response to LPS (data not shown). Although arg1 was expressed in response to IL-4 and LPS, there was no significant change in gene expression from medium alone (Fig 6A). In response to IL-12 and sIL-27R during infection nos2 expression was increased approximately 32-fold (Fig. 6B). Mrc1 expression was reduced by the same treatment whereas there was a slight increase in arg1 expression that was not statistically significant (Fig 6B). Fizz1 was not consistently expressed during infection in the presence or absence of IL-12 and sIL-27R (data not shown). These findings demonstrate a pattern of gene expression that is strikingly similar to that seen in response to LPS and suggest a macrophage phenotype that more closely aligns with M1 polarization (Fig 6B).
Table I.
Changes in M1 cytokine production
Cytokine concentration (pg/mL) in culture supernatants at 72h as determined by ELISA.
Data represents the mean ± SEM for 10 combined experiments.
Data represents the mean ± SEM for 4 combined experiments.
Figure 6.
IL-12 and IL-27 regulate the macrophage activation state. (A) Human macrophages were stimulated with IL-4 or LPS as indicated for 72 h. Quantitative analysis of arg1, nos2, and mrc1 transcripts is presented as the mean log2 gene expression ± SEM for three experiments. Values were normalized to the mean expression of GAPDH within a triplicate sample group and expressed relative to that of medium alone. An asterisk indicates the change in gene expression is statistically significant in the 95% confidence interval as determined by two-way ANOVA. (B, C) Human macrophages were stimulated with IL-12+sIL-27R or medium alone and then infected with MTB. (B) Quantitative analysis of transcripts is presented as the mean log2 gene expression in response to IL-12+sIL-27R as compared with medium alone during infection ± SEM for three experiments. Values were normalized to the mean expression of GAPDH within a triplicate sample group and expressed relative to that of medium alone during infection. An asterisk indicates the change in gene expression is statistically significant in the 95% confidence interval as determined by ANOVA. (C) The NOS2 inhibitors L-NAME (2 mM) or L-NIL (2 mM) were added prior to infection. Data is represented as the mean CFUs recovered from infected macrophages ± SE at 72 h for an individual experiment. A student’s t test was used to establish statistical significance in the 95% confidence interval between individual sample groups as indicated.
3.6. Nitric oxide does not have a significant influence on restriction of MTB
Expression data for nos2 suggested that nitric oxide (NO) production may be involved in the mechanism that restricts MTB growth. Therefore, we addressed this possibility by treating macrophages with IL-12 and sIL-27R, as before, in the presence or absence of NOS2 chemical inhibitors. Separate experiments incorporated the arginine analogs L-NAME (L-NG-nitroarginine methyl ester) or L-NIL [N6-(1-iminoethyl)-L-lysine] at concentrations above the IC50 (500 nM and 3.3 M, respectively). The presence of L-NAME did not alter the restriction of MTB growth mediated by IL-12 and sIL-27R (p=0.73, Fig 6C). Similarly, L-NIL resulted in only a modest and statistically insignificant reversal of antimycobacterial activity (p=0.15, Fig 6C). These experiments suggest that nitric oxide does not have a significant role in restriction of MTB growth by human macrophages treated with IL-12 and sIL-27R.
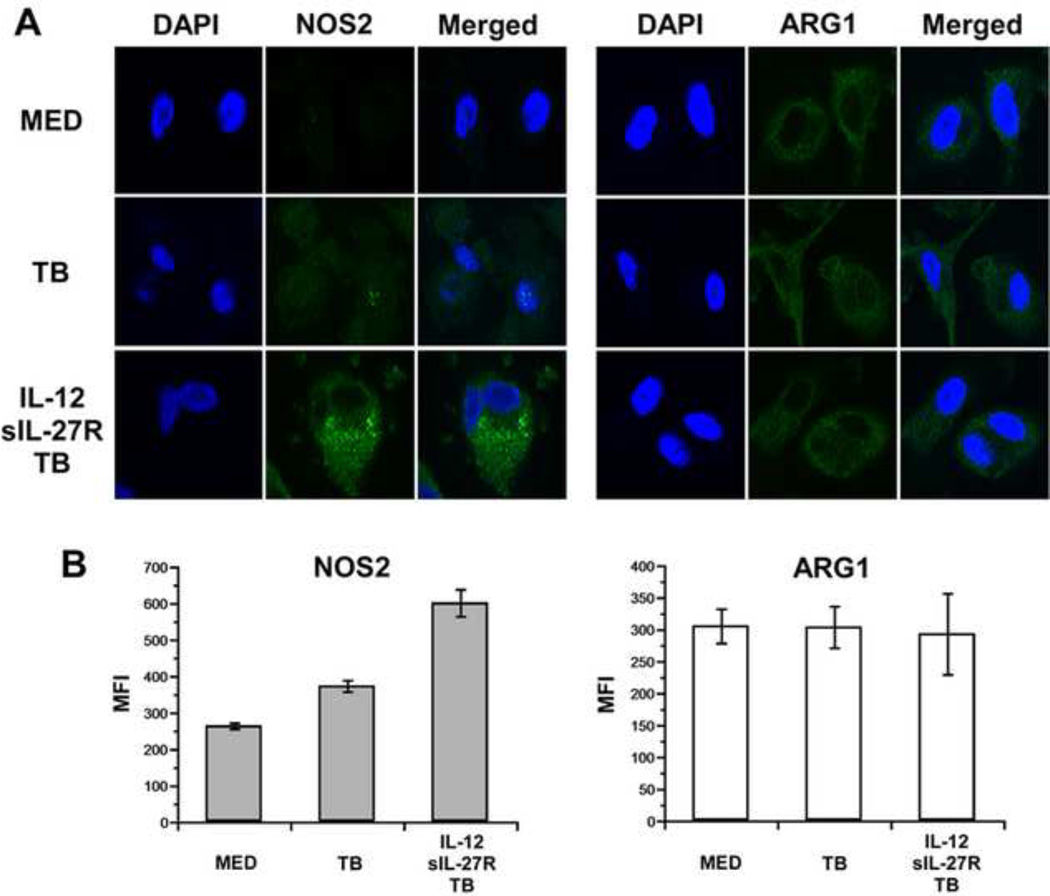
Inhibition of NOS2 did not impact MTB growth within human macrophages. Therefore, we considered the possibility that nos2 gene expression did not lead to significant levels of protein. Macrophages were treated with or without IL-12 and sIL-27R during infection by MTB and immunolabeled for NOS2. For these experiments it was necessary to use a BSL-2 strain of MTB that has been described previously [20], which behaves similarly in the assays described above (data not shown). Consistent with the transcriptional analysis, NOS2 protein increased robustly in response to IL-12 and sIL-27R during infection (Fig. 7A). An analysis of the mean fluorescent intensity (MFI) from 10 distinct fields of view further demonstrates this effect (Fig. 7B). Since ARG1 and NOS2 compete for the substrate arginine, we also investigated ARG1 localization. Although the levels did not change in response to IL-12 and sIL-27R, ARG1 protein was detected under all conditions tested (Fig. 7A). The MFI analysis further highlights this pattern of expression (Fig. 7B). NOS2 and ARG1 levels are therefore consistent with their transcriptional profiles, and create a situation where the two enzymes may compete for substrate with the net result of minimizing nitric oxide production.
Figure 7.
NOS2 and ARG1 expression during infection. Human macrophages were stimulated with IL-12+sIL-27R or medium alone and then infected with MTB mc27000 for 72 h. (A) Macrophages were fixed, permeabilized, immunolabeled as indicated, and visualized by confocal microscopy. NOS2 or ARG1 (green) localization is shown independently and relative to the DAPI stained nucleus in the merged image. Images shown are representative findings from two separate experiments. (B) The MFI was calculated using image J software and the mean of 10 fields of view per treatment condition ± SE is shown.
4. DISCUSSION
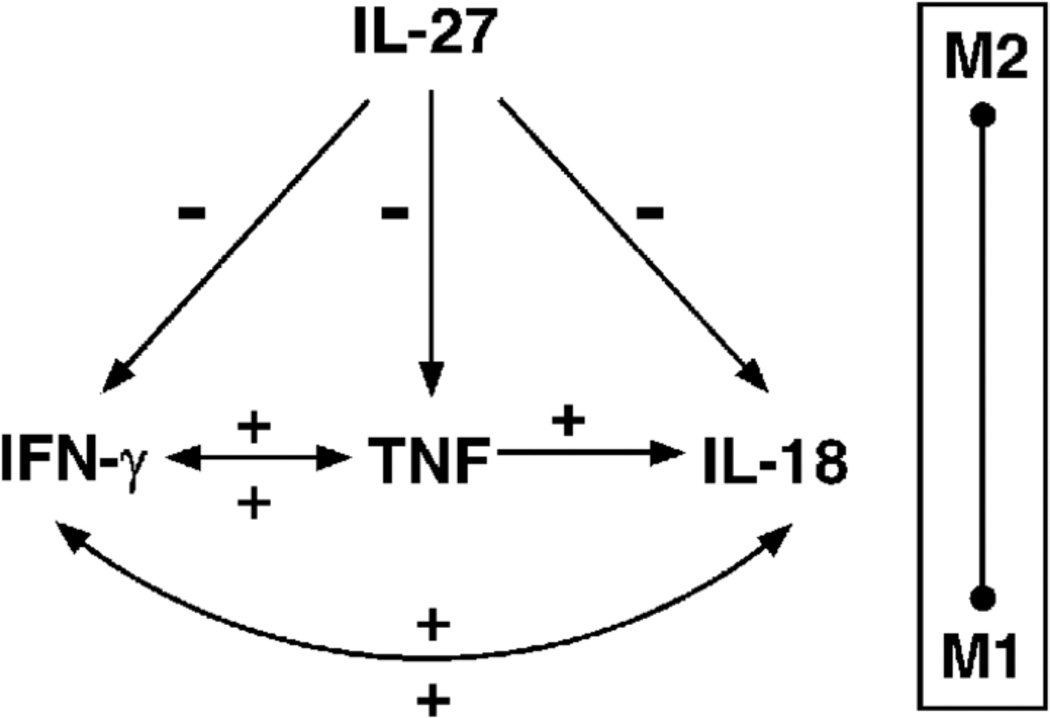
Macrophages produce lower levels of IL-12 in response to MTB than other stimuli [7, 8]. Supplying IL-12 in conjunction with neutralization of IL-27, however, reduces the bacterial burden in infected macrophages (Fig. 1). It has become increasingly clear that IL-27 exerts dominant anti-inflammatory activity toward macrophages during MTB infection. Increased concentrations of proinflammatory cytokines are present in macrophage culture supernatants when IL-12 is supplied and IL-27 signaling is blocked [Table 1, ref. 9]. Those results are recently supported by the findings that IL-27 negatively regulates MAPK signaling, IL-1 expression and processing, IL-8 production, and localization of TNF receptors at the cell surface in human macrophages [28]. We have shown here that when IL-12 is supplied and IL-27 is neutralized, IFN-γ and TNF are critical to the anti-mycobacterial activity with additional contribution made by IL-18 (Fig. 1). These results support a model in which these cytokines interact to influence expression or responsiveness to one another (summarized in Fig. 8). This model highlights that even subtle changes in the cytokine milieu present during infection can lead to meaningful biological changes in signaling molecules and control of pathogens.
Figure 8.
Cytokine interactions that influence MTB growth in human macrophages. IL-27 negatively regulates production of IFN-γ, TNF, and IL-18 signaling through expression of the IL-18R. IFN-γ, TNF, and IL-18 interact to positively influence one another as described here. A sliding scale of macrophage activation that is influenced by these cytokine interactions is depicted. Positive (+) and negative (−) regulation are indicated accordingly.
The results reported here demonstrate cytokine interactions that are important to restrict MTB growth (Fig. 8). Increased production of IFN-γ enhanced TNF and IL-18 signaling. In the presence of IFN-γ, TNF, a potent activator of macrophages, is augmented both at the level of gene expression and secretion (Fig. 2). IL-18R gene expression was also increased by IFN-γ leading to more receptors available at the cell surface, mainly during infection (Fig. 3). IL-18 is known to influence IFN-γ production in a number of cell types in both murine and human systems [22–25]. However, the reciprocal relationship of IFN-γ on IL-18 signaling described in this report was previously undefined. That IFN-γ alone had a greater effect on IL-18R gene expression suggests that other signals received by the macrophage during infection may be necessary to trigger a robust change in IL-18R receptor at the cell surface.
IL-27 negatively regulates the IL-18R chain [22]. This study provides an additional mechanistic explanation for that result. Since IFN-γ production is increased by neutralization of IL-27 [9, 22], it seems that IL-27 regulates IL-18R at least in part by reducing IFN-γ production. In addition, our results show that in the absence of IL-27, IFN-γ can continue to positively regulate its own production through IL-18 responsiveness. IL18 is crucial for optimal IFN-γ production by macrophages (Fig. 4).
The importance of TNF to limiting MTB growth is demonstrated by the effect on the IFN-γ receptor signaling chain (IFNGR2). We have shown for the first time that TNF increases IFNGR2 protein available at the cell surface leading to increased phosphorylation of Stat-1 (Fig. 5). These data demonstrate that while IFN-γ may influence expression of TNF, the latter also enhances responsiveness to IFN-γ. The difference in phosphorylation of Stat-1 is not the result of changes in the IFN-γ receptor 1 chain (IFNGR1) as its expression was unaltered by TNF in the presence or absence of infection (data not shown). An effect of TNF on phosphorylation of JAK kinases has also been reported and may contribute to this result [29].
In total, analysis of the macrophage activation state suggests a polarization in the M1 direction. There is an increase in proinflammatory cytokines as shown with IFN-γ and TNF levels in response to IL-12 and sIL-27R during infection (Table 1). This was accompanied by a decline in mrc1 expression and an increase in nos2 expression that parallel the M1 profile induced by LPS (Fig. 6A, B). However, more typically, macrophages are thought to share characteristics of M1 and M2 populations resulting in a spectrum of activation [27]. This idea is consistent with the modest increase in arg1 expression observed in this study. In bone marrow-derived murine macrophages, treatment with IL-27 was not sufficient to induce an M2 macrophage phenotype [30]. However, WSX-1 gene expression and IL-27 mediated phosphorylation of Stat-3 were increased in IL-4 stimulated M2 macrophages [30]. Classical macrophage activation with LPS or IFN-γ reduced WSX-1 gene expression [30]. Our study addresses the role of IL-27 in human macrophage activation. It is not clear at this time if IL-27 is sufficient to generate an M2 phenotype. However, blocking IL-27 signaling changes the cytokine environment and promotes M1 macrophage activation (Fig. 8). Future experiments will further address this issue. Expression of nos2 in the analysis presented here suggested that nitric oxide may be involved in the restriction of MTB growth. However, inhibition of NOS2 activity did not have a profound impact on bacterial recovery (Fig 6B). Since arg1 transcripts were also detected, we further explored the protein expression and localization of NOS2 and ARG1. Consistent with the transcriptional analysis, NOS2 protein levels were increased by IL-12 and sIL-27R during infection (Fig. 7). However, ARG1 protein was also found throughout the cytosol (Fig. 7). It is possible that ARG1 expression leads to a competition with NOS2 for available substrate. In this scenario, nitric oxide may not be produced at concentrations that are bacteriostatic, or is not reaching the MTB-containing phagosome at concentrations necessary to be effective. These possibilities are under current investigation.
Mycobacterium tuberculosis infections claimed approximately 1.7 million lives worldwide in 2009 [1]. The tuberculosis epidemic is further complicated by HIV coinfection whereby approximately 12% of the annual incident cases are in persons that are HIV-positive [1] and multi-drug or extensive drug resistant (MDR and XDR-TB, respectively). To address the latter, immunotherapeutic strategies that can work with or without traditional chemotherapeutic approaches are a high priority in the global plan to combat MTB [1]. Modulating the cytokine environment during infection by antagonizing IL-27, and perhaps supplying IL-12, may stimulate a more optimal response and represent a future therapeutic approach. In summary, the results described here offer significant insights into the manner in which macrophage control of MTB can be improved as well as novel cytokine interactions that contribute. They also further define the anti-inflammatory activity of IL-27 toward human macrophages.
We studied the mechanisms by which IL-12 and IL-27 regulate human macrophages.
IFN-γ, TNF-α, and IL-18 cooperate to control growth of Mycobacterium tuberculosis.
IFN-γ, TNF-α, and IL-18 interact in a cytokine network.
IL-12 and IL-27 influence the macrophage activation state by altering this network.
ACKNOWLEDGEMENTS
This work was supported by NIH grant HL093300.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.World Health Organization (WHO) Global tuberculosis control. [Accessed 1 March 2011];2010 http://www.who.int/tb/publications/global_report/2010/en/index.html.
- 2.Selwyn PA, Alcabes P, Hartel D, Buono D, Schoenbaum EE, Klein RS, Davenny K, Friedland GH. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. New Engl. J. Med. 1992;327:1697–1703. doi: 10.1056/NEJM199212103272401. [DOI] [PubMed] [Google Scholar]
- 3.Mogues T, Goodrich L, Ryan L, LaCourse R, North R. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bean AGD, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 5.Saunders BM, Cooper AM. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol. Cell Biol. 2000;78:334–341. doi: 10.1046/j.1440-1711.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 6.Scanga CA, Mohan VP, Yu H, Joseph K, Tanaka J, Chan J, Flynn JL. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon-γ and nitric oxide synthase 2. J. Exp. Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Landers ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickman SP, Chan J, Salgame P. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naïve T cell polarization. J. Immunol. 2002;168:4636–4642. doi: 10.4049/jimmunol.168.9.4636. [DOI] [PubMed] [Google Scholar]
- 9.Robinson CM, Nau GJ. Interleukin-12 and Interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J. Inf. Dis. 2008;198:359–366. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, deSauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, Saris CJ, Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 12.Pflanz S, Timans JC, Cheunget J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 13.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosome cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 14.Pearl J, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AD. IL-27 signaling compromises control of bacterial growth in Mycobacteria-infected mice. J. Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 15.Hölscher C, Hölscher A, Rückerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 2005;174:3524–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 16.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–650. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 17.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 18.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage F, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 19.Awasthi A, Carrier Y, Peron JPS, Bettelli E, Kamanaka M, Flavell RA, Kuckroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory cells. Nat. Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 20.Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guerardel Y, Alahari A, Kremer L, Jacobs WR, Jr, Hatfull GF. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson PE, Jr, Carroll JA, O’Dee DM, Nau GJ. Modulation of virulence factors in Francisella tularensis determines human macrophage responses. Microb. Pathog. 2007;42:204–214. doi: 10.1016/j.micpath.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson CM, Nau GJ. Cytokines involved in interferon-γ production by human macrophages. J. Innate Immunity. 2010;2:56–65. doi: 10.1159/000247156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohno K, Kataoka J, Ohtsuki Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J. Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 24.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon-γ upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantuzzi L, Puddu P, Varano B, Del Cornò M, Belardelli F, Gessani S. IFN-γ and IL-18 exert opposite regulatory effects on the IL-12 receptor expression and IL-12-induced IFN-γ production in mouse macrophages: novel pathways in the regulation of the inflammatory response of macrophages. J. Leukocyte Biol. 2000;68:707–714. [PubMed] [Google Scholar]
- 26.Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J. Autoimmun. 2009;33:222–230. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalliolias GD, Gordon RA, Ivashkiv LB. Suppression of TNF-α and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. J. Immunol. 2010;185:7047–7056. doi: 10.4049/jimmunol.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo D, Dunbar JD, Yang CH, Pfeffer LM, Donner DB. Induction of JAK/STAT signaling by activation of the type 1 TNF receptor. J. Immunol. 1998;160:2742–2750. [PubMed] [Google Scholar]
- 30.Rückerl D, Heβmann M, Yoshimoto T, Ehlers S, Holscher C. Alternatively activated macrophages express the IL-27 receptor alpha chain WSX-1. Immunobiology. 2006;211:427–436. doi: 10.1016/j.imbio.2006.05.008. [DOI] [PubMed] [Google Scholar]