Abstract
Background and purpose
Tissue plasminogen activator (tPA) is the only approved therapy for acute ischemic stroke. However, tPA has a brief therapeutic window. Its side effects include intracerebral bleeding and neurotoxicity. Therefore, a combination therapy with tPA and agents that can extend the therapeutic window of tPA and/or counteract its side effects are warranted. Here, we studied whether 3K3A-APC, a neuroprotective analog of activated protein C (APC) with reduced anticoagulant activity, can enhance the therapeutic effects of tPA in models of ischemic stroke in rodents.
Methods
Human recombinant tPA (10 mg/kg), alone or in combination with human recombinant 3K3A-APC (2 mg/kg), was given intravenously 4 hours after proximal or distal transient middle cerebral artery occlusion (MCAo) in mice and embolic stroke in rats. 3K3A-APC was additionally administered for 3–4 consecutive days after stroke. The neuropathological and neurological analyses were performed at 1 to 7 days after stroke.
Results
In all models, tPA alone had no effects on the infarct volume or behavior (i.e., neurological score, foot-fault, forelimb asymmetry, adhesive removal) compared to vehicle. tPA and 3K3A-APC combination therapy reduced the infarct volume 24 hours and 7 days after proximal or distal transient MCAo in mice and 7 days after embolic stroke in rats by 65%, 63% and 52%, respectively, improved significantly (P<0.05) behavior, and eliminated tPA-induced intracerebral microhemorrhages.
Conclusions
3K3A-APC extends the therapeutic window of tPA for ischemic stroke in rodents. Therefore, this combination therapy should also be considered for treating stroke in humans.
Keywords: thrombolytic therapy, ischemic stroke, proteases, neuroprotection
Introduction
The vast majority of strokes are ischemic and thrombotic. The reperfusion therapy with tissue plasminogen activator (tPA) is the only approved therapy for acute ischemic stroke. Still, the rate of recombinant tPA use is unfortunately < 4% of stroke patients1. Problems with tPA include blood-brain barrier (BBB) disruption causing intracerebral bleeding2–4, a brief therapeutic window5 and post-ischemic neuronal toxicity as shown by several studies using transient middle cerebral artery occlusion model in rodents6–8. Therefore, a combination therapy with tPA and agent(s) that can increase the therapeutic window of tPA and/or protect against tPA-induced intracerebral hemorrhage and neurotoxicity are warranted.
Activated protein C (APC) is a protease with systemic anticoagulant activity which is independent of its cell signaling effects resulting in a blockade of various pathological pathways in brain endothelioum, neurons and microglia during an acute or chronic CNS insult9. APC's cell-signaling actions require activation of protease activated receptor 1 (PAR1) by proteolytic cleavage of its extracellular N-terminal tail that generates an intramolecular tethered ligand, which subsequently triggers intracellular signaling9. Endothelial protein C receptor (EPCR) and sphingosine-1-phosphate receptor-1 (S1P1), and PAR3 are also required for APC's actions on brain endothelium and neurons, respectively9. Mutations of APC residues that are not part of the immediate APC enzymatic active site result in diminished APC's anticoagulant activity without altering APC's cell signaling activity, as for example replacement of three lysine residues 191–193 by three alanine residues produces the 3K3A-APC variant causing loss of > 90% of APC's anticoagulant activity10, 11. The significance of such APC engineered mutations is that they provide APC variants for therapeutic purposes in which the risk of serious bleeding caused by APC's systemic anticoagulant activity is diminished, while the cytoprotective activities of APC's direct effects on cells and its pharmacologic benefits are preserved.
APC and its cell-signaling recombinant analogs with reduced anticoagulant activity including 3K3A-APC and 5A-APC exert direct vascular protective effects in the brain endothelium12–16 and can enhance integrity of the endothelial barrier17, 18. Moreover, APC, 3K3A-APC and 5A-APC cross the BBB via EPCR-dependent transcytosis19–21 and have direct neuronal protective actions20, 22, 23. By inhibiting transport of leukocytes across the BBB24 and by suppressing microglia activation20, APC therapy exerts a significant anti-inflammatory activity.
APC is neuroprotective in multiple models of stroke in mice and rats including transient brain ischemia2, 8, 14, 24, 25, permanent distal middle cerebral artery occlusion (MCAo)23, 26, embolic stroke27 and neonatal hypoxic/ischemic brain injury28. After proximal transient and distal permanent MCAo in mice, 3K3A-APC variant with reduced anticoagulant activity had some advantages over the recombinant wild type APC including reduced risk for bleeding that was particularly noticeable when treatments were administered at later time points after MCAo23, 26. In the present study we asked whether 3K3A-APC can enhance tPA therapy for focal ischemic stroke in mice and rats.
Materials and Methods
Reagents
Human recombinant tPA (alteplase) was purchased from Genentech (South San Francisco, CA). Human recombinant 3K3A-APC was prepared in Chinese hamster ovary (CHO) cells and manufactured by the Laureate Biopharmaceutical, Princeton, New Jersey contracted by ZZ Biotech. Methods for the generation, purification, and characterization of recombinant 3K3A-APC have been described (Williams, Zlokovic, Griffin, Pryor and Davis, unpublished data, 2012). Based on analysis of 3K3A-APC using nonreduced and reduced SDS polyacrylamide gels, the protein showed the stained bands typical for disulfide-linked heavy and light chains characteristic of APC. SDS gel analysis and size exclusion chromatography showed that the 3K3A-APC used for these studies was > 96 % pure (data not shown).
Animals
The protocol in mice was approved by the Animal Care Committee at the University of Rochester in compliance with the National Institutes of Health (NIH) guidelines. Male C57Bl6 mice (22–26 g; Jackson Laboratory, Bar Harbor, Maine) were anesthetized intraperitoneally with 100 mg ketamine/10 mg hylazine per kg body weight. Rectal temperature was maintained between 36.5 and 37.0°C using a feedback-controlled heating system. The protocol in rats was approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital in compliance with the NIH guidelines. Adult male Wistar rats (Jackson Laboratory, Bar Harbor, ME) 8–12 weeks of age and weighing 300 to 350 g were anesthetized with 3% isoflurane, and the anesthesia was maintained with 1.0–1.5% isoflurane. Rectal temperature was maintained at 37.0±0.5°C using a feedback regulated water heating system.
Proximal transient middle cerebral artery occlusion (MCAo) in mice
The MCA was occluded for 45 min using a silicon-coated nylon monofilament (DOCCOL CO.) as described2, 8, 14. tPA alone (10 mg/kg, 10% as a bolus and 90% as a 30-min infusion), tPA (10 mg/kg, infused as above) and 3K3A-APC (2 mg/kg, 50% bolus/50% 30-minute infusion), 3K3A-APC alone (2 mg/kg, infused as above) or vehicle were administrated intravenously 4 hours after stroke. For combination therapy, tPA was administered via the femoral vein and 3K3A-APC via the tail vein. Cerebral blood flow was monitored by laser Doppler flowmetry (Transonic Systems). Motor neurological exam was determined after 24 hours2, 8, 14: no neurological deficit, 0; failure to extend left forepaw fully, 1; turning to left, 2; circling to left, 3; unable to walk spontaneously, 4; and stroke-related death, 5. Mice were sacrificed 24 hours after the MCAo. It is of note, the dose of tPA of 10 mg/kg used in this and other studies described below is equivalent to a therapeutic dose of tPA of 1 mg/kg in humans29–31, whereas the dose of human recombinant 3K3A-APC of 2 mg/kg was shown to provide maximal neuroprotection in different stroke models in mice26, 32.
Distal transient middle cerebral artery occlusion (MCAo) in mice
Distal transient MCAo was performed using a modified suture technique as reported33, 34. The right common carotid arteries were transiently occluded for 20 minutes and the MCA for 60 minutes. tPA only (10 mg/kg, 10% as a bolus and 90% as a 30-minute infusion), tPA (10 mg/kg, infused as above) and 3K3A-APC (2 mg/kg, 50% bolus/50% 30-minute infusion), 3K3A-APC alone (2 mg/kg, infused as above), and vehicle were administrated intravenously 4 hours after stroke. When tPA and 3K3A-APC were given together, tPA was administered via the femoral vein and 3K3A-APC via the tail vein. 3K3A-APC (2 mg/kg, intraperitoneally) was additionally administered at 1, 3, 5, and 7 days after stroke. Foot-fault test26, 27 and forelimb asymmetry test26, 27 were performed at 0, 1, 3 and 7 days after the MCAo. Mice were sacrificed 7 days after the MCAo.
Focal embolic stroke in rats
The MCA of male Wistar rats was occluded by placement of an embolus at the origin of the MCA, as described35. Four hours after stroke, tPA (10 mg/kg) was infused as a 10% bolus through the tail vein, and the remainder was infused continuously over a 30-minute interval. 3K3A-APC (2 mg/kg) was administered intravenously 4 hours after stroke as a single bolus (100 μl). 3K3A-APC was additionally injected intravenously for 3 consecutive days. A modified neurological severity score (mNSS), a composite of motor, sensory, reflex, and balance tests (no deficit, score 0; maximal deficit, score 18)36, adhesive removal test, for sensorimotor activity37, and foot-fault test, for locomotor assessment37 were performed 1 and 7 days after stroke. Rats were sacrificed 7 days after stroke.
Neuropathological Analysis
The injury volumes were measured on coronal sections using either cresyl-violet staining (mice) and hematoxylin and eosin staining (rats), as described previously38, 39. The infarct volume was calculated by subtracting the volume of intact area in the ipsilateral hemisphere from the whole volume of the contralateral hemisphere, as reported2, 8, 14, 27, 35–38
Hemoglobin Assay
Hemoglobin levels were determined by a spectrophotometric assay using Drabkin's reagent (Sigma)2, 40.
Microscopic hemorrhage
Microscopic hemorrhage, defined as a cluster of red blood cells outside of the lumen of blood vessels, was measured under a × 40 objective with a Global Laboratory Image (Data Translation, Marlboro, MA) analysis; the area of hemorrhage (μm2/section) was calculated as described27, 35.
Statistics
Data are presented as mean ± SEM. One-way analysis of variance (ANOVA) followed by Tukey's post-hot test was used to determine statistically significant differences. P < 0.05 was considered statistically significant.
Results
It is well known that tPA promotes desirable (thrombolytic) as well as undesirable (neurotoxic) outcomes during stroke6–8. The goal of our first set of studies using 45 min proximal MCAo and 60 min distal MCAo was not to simulate the clinical course of stroke treatment with an approved tPA protocol as presently given to stroke patients, but to investigate 1) tPA's role in ischemia independently of its effects as thrombolytic agent, and 2) whether 3K3A-APC can improve outcome of tPA treatment in an experimental setting chosen to test tPA's undesirable (neurotoxic) effects in isolation of its desirable (thrombolytic) effects.
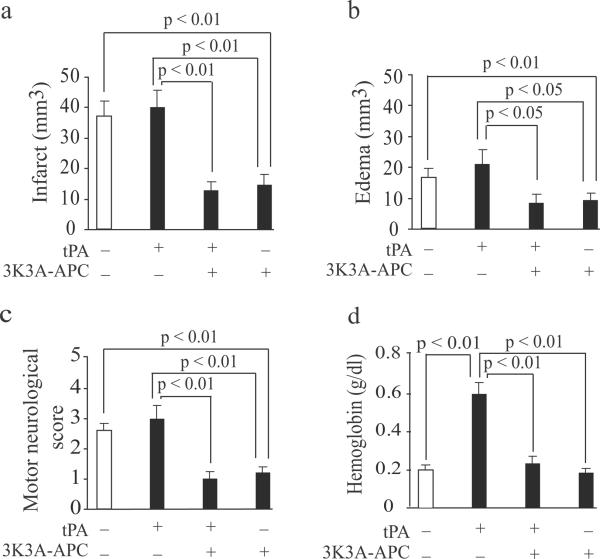
tPA (10 mg/kg) infused 4 h after 45 min of proximal transient MCAo compared to vehicle had no effects on the infract and edema volumes or neurological score 24 hours after stroke (Fig. 1a–c). However, a combined tPA (10 mg/kg) and 3K3A-APC (2 mg/kg) treatment compared to tPA alone reduced the infarct volume by 65–67% and improved neurological score from 3.2 to 1. tPA alone increased intracerebral bleeding as indicated by 3.2-fold increase in hemoglobin levels in the ischemic hemisphere (Fig. 1d), which was normalized by adding 3K3A-APC demonstrating vascular protective effects of 3K3A-APC besides its neuroprotective effects. As reported23, 26, human recombinant 3K3A-APC (2 mg/kg) alone was neuroprotective as shown by 57% reduction in the infract volume and improved neurological score from 2.7 to 1.2 compared to vehicle, respectively.
Figure 1. Human recombinant 3K3A-APC enhances the therapeutic effects of tPA after proximal transient MCAo in mice.
tPA (10 mg/kg) and human 3K3A-APC (2 mg/kg) were administered intravenously 4 hours after 45 min proximal transient MCAo. (a–d) Infarct volume (a), edema (b), motor neurological score (c) and hemoglobin levels in the ischemic hemipshere (d) were determined 24 hours after the MCAo. All values are mean ± SEM, n = 5 mice per group.
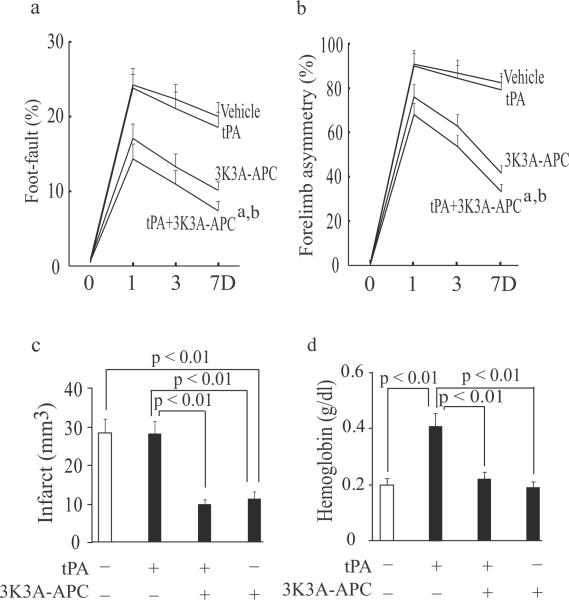
As shown in Fig. 2a–c, in mice subjected to 60 min distal transient MCAo tPA (10 mg/kg) alone compared to vehicle did not improve performance on foot-fault and forelimb asymmetry tests at day 1, 3 and 7 after stroke, neither reduced the infarct size 7 days after stroke. In contrast, tPA and 3K3A-APC combination compared to tPA alone improved significantly performance on foot-fault and forelimb asymmetry tests at day 1, 3 and 7 after stroke, and reduced the infarct volume by 63% as well as edema volume (not shown). tPA alone increased hemoglobin levels in the ischemic hemisphere by 2.1-fold (Fig. 2d), which was normalized by adding 3K3A-APC. 3K3A-APC alone exerted strong neuroprotection compared to vehicle or tPA treatment, consistent with previous findings23, 26, 32. Although the beneficial effects of 3K3A-APC alone after proximal or distal transient MCAo were somewhat less pronounced than of tPA and 3K3A-APC combination, the differences were not significant.
Figure 2. Human recombinant 3K3A-APC enhances the therapeutic effects of tPA after distal transient MCAo in mice.
tPA (10 mg/kg) and human 3K3A-APC (2 mg/kg) were administered intravenously 4 hours after 60 min distal transient MCAo. The additional doses of human 3K3A-APC (2 mg/kg, intraperitoneally) were administered at 1, 3, 5, and 7 days after the MCAo. (a–b) Foot-fault test (a) and forelimb asymmetry test (b) were performed at day 1, 3 and 7 after stroke. (c–d) The infarct volume (c) and hemoglobin levels in the ischemic hemisphere (d) were determined 7 days after the MCAo. All values are mean ± SEM, n = 5 mice per group. aP < 0.01, vehicle versus tPA + 3K3A-APC; bP < 0.01, tPA alone versus tPA + 3K3A-APC.
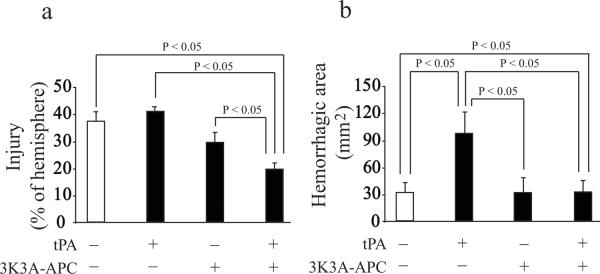
As in other models, tPA (10 mg/kg) standalone therapy compared to vehicle had no effects on modified neurological severity score (mNSS), foot-fault test and adhesive removal test at day 1 or 7 after embolic stroke in rats (Fig. 3a–c). In contrast, tPA and 3K3A-APC combination therapy significantly (p < 0.05) improved performance on all studied behavioral tests both on day 1 and 7 after stroke. 3K3A-APC alone improved modestly performance on behavioral tests compared to vehicle or tPA, but the differences were not significant except for the foot-fault test. tPA alone did not have any effect on the infarct volume determined 7 days after stroke, whereas tPA and 3K3A-APC combination therapy compared to tPA alone reduced the infarct volume by 53% (Fig. 4a). 3K3A-APC reduced the infarct volume by approximately 30% compared to tPA alone. tPA alone increased by 3-fold microscopic hemorrhage compared to vehicle (Fig. 4b) as reported23 which was completely normalized by 3K3A-APC.
Figure 3. Effects of combined tPA and 3K3A-APC treatment on behavior in rats after embolic stroke.
tPA (10 mg/kg) and human 3K3A-APC (2 mg/kg) were administered intravenously 4 hours after embolic stroke. 3K3A-APC (2 mg/kg) was additionally injected intravenously for 3 consecutive days. (a–c) Modified neurological severity score (mNSS) (a), foot-fault test (b) and adhesive removal test (c) were performed at day 1 and 7 after stroke. (e) Microscopic hemorrhage was determined 7 days after stroke. All values are mean ± SEM, n = 10 rats per group.
Figure 4. Effects of combined tPA and 3K3A-APC treatment on the infarct volume and microscopic hemorrhage in rats after embolic stroke.
Rats that were studied for behavior in Figure 3 were sacrificed after 7 days of stroke to determine (a) the infarct volume and (b) microscopic hemorrhage. All values are mean ± SEM, n = 10 rats per group.
Discussion
Thrombolytic therapy for acute ischemic stroke with tPA has clear benefits if administered within a narrow therapeutic window1, 3–5. However, side effects of tPA treatment such as intracerebral hemorrhage limit its use in humans3, 5. Studies in animal models have demonstrated serious side effects of tPA in the ischemic brain including BBB breakdown2, 41, 42, intracerebral bleeding if tPA is administered 3–4 h after either transient MCAo or embolic stroke2, 30, 43 and direct post-ischemic neuronal toxicity after transient MCAo6–8. Earlier reports have shown that tPA reduces neurological damage after cerebral embolism44, 45 and does not exacerbate brain injury after global or focal ischemia46. However, a recent study using a model of angiographically documented recanalization of the rabbit middle cerebral artery occlusion has shown that tPA produces bleeding at all doses in proportion to its thrombolytic potential47. Taken together these studies suggest that beneficial or detrimental effects of tPA therapy for stroke critically depend on the time of its administration after the MCAo embolism48.
Consistent with previous findings, the present study confirmed that tPA treatment administered 4 hours after either proximal or distal transient MCAo in mice or embolic stroke in rats is not neuroprotective as indicated by no changes in performance on multiple behavioral tests at day 1 to 7 after stroke, no change in the infarction volume, and increased risk for intracerebral bleeding compared to vehicle. However, when tPA was administered in combination with 3K3A-APC, the combined therapy exerted a remarkable neuroprotection in all studied models.
Consistent with previous findings demonstrating that APC treatment in addition to being neuroprotective is also vasculoprotective4, 12–16 and can stabilize the endothelial barriers17, 18 including the BBB2, 20, we showed that 3K3A-APC eliminates tPA-induced intracerebral microbleedings. tPA induces post-ischemic brain hemorrhage by activating nuclear-factor κB-dependent matrix metalloproteinase-9 pathway at the BBB which is inhibited by APC both in mice and rats, as reported2. Reduced systemic anticoagulant activity of APC mutants such as 3K3A-APC or 5A-APC by 85–90% and > 95% compared to wild type APC, respectively, has been shown to contribute to reduced risk of bleeding observed with these APC mutants compared to wild type APC in models of ischemic stroke23, brain trauma31 and sepsis49. Interestingly, human recombinant tPA and 3K3A-APC alone had only moderate or no beneficial effects in the rat model of embolic stroke whereas the combination of the two drugs exerted clearly significant synergistic effects on all studied parameters
In conclusion, the present data show that 3K3A-APC extends the therapeutic window of tPA in ischemic stroke models in rodents supporting further development of tPA and 3K3A-APC combination therapy for focal ischemic stroke in humans.
Acknowledgments
Sources of Funding This work was supported by the National Institutes of Health grant HL63290 (B.V.Z.), HL052246 (J.H.G.) and ZZ Biotech LLC. ZZ Biotech LLC also provided human 3K3A-APC mutant.
Footnotes
Disclosures B.V.Z. is the scientific founder of ZZ Biotech, a biotechnology company with a focus to develop APC and its functional mutants for stroke and other neurological disorders. T.P.D. and J.H.G. are members of the Scientific Advisory Board of ZZ Biotech LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National us estimates of recombinant tissue plasminogen activator use: Icd-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 2.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, et al. Activated protein c inhibits tissue plasminogen activator-induced brain hemorrhage. Nature medicine. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 3.Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. The ninds t-pa stroke study group. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter CR, Keim SM, Milne WK, Meurer WJ, Barsan WG. Thrombolytic therapy for acute ischemic stroke beyond three hours. The Journal of emergency medicine. 2011;40:82–92. doi: 10.1016/j.jemermed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saver JL, Levine SR. Alteplase for ischaemic stroke--much sooner is much better. Lancet. 2010;375:1667–1668. doi: 10.1016/S0140-6736(10)60634-4. [DOI] [PubMed] [Google Scholar]
- 6.Nagai N, Vanlinthout I, Collen D. Comparative effects of tissue plasminogen activator, streptokinase, and staphylokinase on cerebral ischemic infarction and pulmonary clot lysis in hamster models. Circulation. 1999;100:2541–2546. doi: 10.1161/01.cir.100.25.2541. [DOI] [PubMed] [Google Scholar]
- 7.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tpa) increases neuronal damage after focal cerebral ischemia in wild-type and tpa-deficient mice. Nature medicine. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 8.Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, et al. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein c. Nature medicine. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 9.Zlokovic BV, Griffin JH. Cytoprotective protein c pathways and implications for stroke and neurological disorders. Trends in neurosciences. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale AJ, Tsavaler A, Griffin JH. Molecular characterization of an extended binding site for coagulation factor va in the positive exosite of activated protein c. The Journal of biological chemistry. 2002;277:28836–28840. doi: 10.1074/jbc.M204363200. [DOI] [PubMed] [Google Scholar]
- 11.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein c variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–1744. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 12.Joyce DE, Grinnell BW. Recombinant human activated protein c attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappab. Critical care medicine. 2002;30:S288–293. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- 13.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein c pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 14.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, et al. Activated protein c blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nature medicine. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 15.Domotor E, Benzakour O, Griffin JH, Yule D, Fukudome K, Zlokovic BV. Activated protein c alters cytosolic calcium flux in human brain endothelium via binding to endothelial protein c receptor and activation of protease activated receptor-1. Blood. 2003;101:4797–4801. doi: 10.1182/blood-2002-12-3680. [DOI] [PubMed] [Google Scholar]
- 16.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein c requires protease-activated receptor-1 and endothelial cell protein c receptor. The Biochemical journal. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein c through par1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 18.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, et al. Activated protein c mediates novel lung endothelial barrier enhancement: Role of sphingosine 1-phosphate receptor transactivation. The Journal of biological chemistry. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 19.Deane R, LaRue B, Sagare AP, Castellino FJ, Zhong Z, Zlokovic BV. Endothelial protein c receptor-assisted transport of activated protein c across the mouse blood-brain barrier. Journal of cerebral blood flow and metabolism. 2009;29:25–33. doi: 10.1038/jcbfm.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, et al. Activated protein c therapy slows als-like disease in mice by transcriptionally inhibiting sod1 in motor neurons and microglia cells. The Journal of clinical investigation. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmon CT, Glass JD. The apcs of neuroprotection. The Journal of clinical investigation. 2009;119:3205–3207. doi: 10.1172/JCI40682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, et al. Activated protein c prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 23.Guo H, Singh I, Wang Y, Deane R, Barrett T, Fernandez JA, et al. Neuroprotective activities of activated protein c mutant with reduced anticoagulant activity. The European journal of neuroscience. 2009;29:1119–1130. doi: 10.1111/j.1460-9568.2009.06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, et al. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein c in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 25.Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein c promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. The Journal of neuroscience. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Thiyagarajan M, Chow N, Singh I, Guo H, Davis TP, et al. Differential neuroprotection and risk for bleeding from activated protein c with varying degrees of anticoagulant activity. Stroke. 2009;40:1864–1869. doi: 10.1161/STROKEAHA.108.536680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zlokovic BV, Zhang C, Liu D, Fernandez J, Griffin JH, Chopp M. Functional recovery after embolic stroke in rodents by activated protein c. Annals of neurology. 2005;58:474–477. doi: 10.1002/ana.20602. [DOI] [PubMed] [Google Scholar]
- 28.Yesilirmak DC, Kumral A, Tugyan K, Cilaker S, Baskin H, Yilmaz O, et al. Effects of activated protein c on neonatal hypoxic ischemic brain injury. Brain research. 2008;1210:56–62. doi: 10.1016/j.brainres.2008.02.088. [DOI] [PubMed] [Google Scholar]
- 29.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Intravenous administration of a gpiib/iiia receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke. 2004;35:2890–2895. doi: 10.1161/01.STR.0000147963.68238.da. [DOI] [PubMed] [Google Scholar]
- 31.Walker CT, Marky AH, Petraglia AL, Ali T, Chow N, Zlokovic BV. Activated protein c analog with reduced anticoagulant activity improves functional recovery and reduces bleeding risk following controlled cortical impact. Brain research. 2010;1347:125–131. doi: 10.1016/j.brainres.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo H, Wang Y, Singh I, Liu D, Fernandez JA, Griffin JH, et al. Species-dependent neuroprotection by activated protein c mutants with reduced anticoagulant activity. Journal of neurochemistry. 2009;109:116–124. doi: 10.1111/j.1471-4159.2009.05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, et al. Vegf antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. The Journal of clinical investigation. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoang S, Liauw J, Choi M, Guzman RG, Steinberg GK. Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia. Journal of cerebral blood flow and metabolism. 2009;29:385–397. doi: 10.1038/jcbfm.2008.128. [DOI] [PubMed] [Google Scholar]
- 35.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain research. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 37.Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: Effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 38.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. Journal of cerebral blood flow and metabolism. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, et al. Vegf overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain : a journal of neurology. 2005;128:52–63. doi: 10.1093/brain/awh325. [DOI] [PubMed] [Google Scholar]
- 40.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr., Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- 41.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the ldl receptor-related protein. The Journal of clinical investigation. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: More than a thrombolytic. Trends in neurosciences. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Ding G, Jiang Q, Zhang L, Zhang ZG, Li L, Knight RA, et al. Analysis of combined treatment of embolic stroke in rat with r-tpa and a gpiib/iiia inhibitor. Journal of cerebral blood flow and metabolism. 2005;25:87–97. doi: 10.1038/sj.jcbfm.9600010. [DOI] [PubMed] [Google Scholar]
- 44.Overgaard K, Sereghy T, Boysen G, Pedersen H, Diemer NH. Reduction of infarct volume and mortality by thrombolysis in a rat embolic stroke model. Stroke. 1992;23:1167–1173. doi: 10.1161/01.str.23.8.1167. discussion 1174. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Carmeliet P, Fay WP. Plasminogen activator inhibitor-1 is a major determinant of arterial thrombolysis resistance. Circulation. 1999;99:3050–3055. doi: 10.1161/01.cir.99.23.3050. [DOI] [PubMed] [Google Scholar]
- 46.Klein GM, Li H, Sun P, Buchan AM. Tissue plasminogen activator does not increase neuronal damage in rat models of global and focal ischemia. Neurology. 1999;52:1381–1384. doi: 10.1212/wnl.52.7.1381. [DOI] [PubMed] [Google Scholar]
- 47.Marder VJ, Jahan R, Gruber T, Goyal A, Arora V. Thrombolysis with plasmin: Implications for stroke treatment. Stroke. 2010;41:S45–49. doi: 10.1161/STROKEAHA.110.595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Q, Zhang RL, Zhang ZG, Ewing JR, Jiang P, Divine GW, et al. Magnetic resonance imaging indexes of therapeutic efficacy of recombinant tissue plasminogen activator treatment of rat at 1 and 4 hours after embolic stroke. Journal of cerebral blood flow and metabolism. 2000;20:21–27. doi: 10.1097/00004647-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein c. The Journal of experimental medicine. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]