Abstract
FluoroMyelin™ Red is a commercially available water-soluble fluorescent dye that has selectivity for myelin. This dye is marketed for the visualization of myelin in brain cryosections, though it is also used widely to stain myelin in chemically fixed tissue. Here we have investigated the suitability of FluoroMyelin™ Red as a vital stain for live imaging of myelin in myelinating co-cultures of Schwann cells and dorsal root ganglion neurons. We show that addition of FluoroMyelin™ Red to the culture medium results in selective staining of myelin sheaths, with an optimal staining time of 2 hours, and has no apparent adverse effect on the neurons, their axons, or the myelinating cells at the light microscopic level. The fluorescence is bright and photostable, permitting long-term time-lapse imaging. After rinsing the cultures with medium lacking FluoroMyelin™ Red, the dye diffuses out of the myelin with a half life of about 130 minutes resulting in negligible fluorescence remaining after 18–24 hours. In addition, the large Stokes shift exhibited by FluoroMyelin™ Red makes it possible to readily distinguish it from popular and widely used green and red fluorescent probes such as GFP and mCherry. Thus FluoroMyelin™ Red is a useful reagent for live fluorescence imaging studies on myelinated axons.
Keywords: FluoroMyelin™, fluorescence, myelin, myelination, myelinating culture, Schwann cell, neuron, axon, cell culture, live imaging
1. INTRODUCTION
Co-cultures of Schwann cells and dorsal root ganglion neurons are used widely for studies on myelination and axon-glial interactions (Kleitman et al., 1998). Under suitable conditions the Schwann cells form compact myelin sheaths remarkably similar in morphology to myelin sheaths in vivo. The myelin sheaths can be visualized by light microscopy of chemically fixed cultures using immunofluorescence or lipophilic dyes such as Sudan Black, but they are also sufficiently refractile that they can often be detected in living cultures using bright field illumination. However, bright field illumination generates low contrast and is not completely reliable, particularly in dense cultures or for thin sheaths. Thus, for live imaging studies there is a need for vital dyes that can stain myelin in living cultures.
There have been several reports of fluorescent dyes that stain myelin. Schmued et al. (1982) described a number of dyes, including neutral red, that stain myelin in tissue sections, and Pereyra and Roots (1988) reported staining of myelin in tissue sections using tetracycline hydrochloride. Bilderback et al. (1997) used a fluorescent ceramide analog as a metabolic tracer to measure the rate of myelin formation in myelinating cultures. Schmahl et al. (1999) described staining of myelin in tissue sections using a cyanine dye 5,5'-diphenyl-9-ethyl-oxacarbocyanine (DEOC), and Xiang et al. (2005) described the synthesis and characterization of two squarylium-based near infra-red myelin (NIM) dyes with selectivity for myelin. For in vivo imaging, Wang and colleagues described a number of fluorescent dyes including (E,E)-1,4-bis(4'-aminostyryl)-2-dimethoxy-benzene (BDB), 3-(4-aminophenyl)-2H-chromen-2-one (termed Case Myelin Compound), and 3,3’-diethylthiatricarbocyanine iodide (DBT), which penetrate the blood-brain barrier and bind selectively to myelin in the central and peripheral nervous systems (Wang et al., 2010; Wang et al., 2011; Wu et al., 2006).
In 2004, Molecular Probes Inc. (now part of Life Technologies Corporation, Grand Island, NY) introduced the FluoroMyelin™ Red and FluoroMyelin™ Green dyes (Kilgore, 2006; Kilgore and Janes, 2004), which have selectivity for myelin. These fluorescent dyes are water soluble and can be applied directly to sections of frozen or chemically fixed tissue, and have been reported to stain myelin selectively in as little as 20 minutes (e.g. Kanaan et al., 2006; Watkins et al., 2008). In the present study we have explored the potential of FluoroMyelin™ Red as a vital stain for live imaging of myelin in long-term myelinating co-cultures of Schwann cells and dorsal root ganglion neurons. We show that FluoroMyelin™ Red provides bright, photostable and selective fluorescent labeling of myelin in live myelinating cultures with no apparent toxicity and no apparent adverse effects on the myelin sheaths or the axons that they invest.
2. MATERIALS AND METHODS
2.1. Cell culture
Co-cultures of Schwann cells and dorsal root ganglion neurons were established using the method of Svenningsen et al. (2003) with some modifications. Timed pregnant female Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN). The rats were sacrificed in the 17th day of pregnancy (E16.5) using carbon dioxide followed by cervical dislocation. Three embryos were dissected in Petri dishes containing Leibovitz’s L-15 medium (GIBCO Life Technologies, Grand Island, NY), yielding about 120–150 dorsal root ganglia. The ganglia were pooled, transferred to fresh L-15 medium in a 15 ml disposable polystyrene conical centrifuge tube, rinsed twice with PBS, and then treated with 2.5 mg/ml trypsin in phosphate buffered saline (PBS) at 37°C. After 45 minutes, the trypsin solution was removed and the ganglia were treated with 25% fetal bovine serum in PBS for 15 minutes at 37°C. Finally, the ganglia were rinsed twice with L-15 medium containing 0.5 mg/ml bovine serum albumin (BSA Fraction V, EMD4 Biosciences, Darmstadt, Germany) and dissociated by trituration using a fire-polished Pasteur pipet. The density of viable cells was determined using by Trypan Blue exclusion using a hemocytometer and then the cell suspension was diluted in L-15 medium containing 0.5 mg/ml BSA and plated onto glass-bottomed dishes coated with Matrigel™ at a density of 2,000–12,000 cells/cm2. The cells were allowed to settle down onto the coverslips under gravity for 2 hours and then they were fed with myelination medium, consisting of Neurobasal™ medium containing 2% B27 supplements (GIBCO Life Technologies), 20mM L-glutamine, and 100 ng/ml nerve growth factor (BD Biosciences), and maintained in an incubator at 37°C in an atmosphere of 5% CO2. To prepare the glass-bottomed dishes, we drilled 13/32 inch diameter holes in the bottoms of 35 mm plastic Petri dishes (Nalge Nunc International, Rochester, NY) and then attached an acid-washed No. 1.5 borosilicate glass coverslip to the base of each dish using paraffin wax or a platinum-cured medical-grade silicone adhesive (A-103 Medical Grade Elastomer, Factor II Inc., Lakeside, AZ). To prepare the glass for plating, we first treated with 1 mg/ml poly-D-lysine (Sigma-Aldrich, St. Louis, MO) in 0.1M sodium borate buffer pH 8.5 for at least 3 hours, rinsed extensively with PBS, and then treated with Matrigel™ (BD Biosciences, Franklin Lakes, NJ) diluted 1:20 in L15 medium for 4 hours followed by a brief rinse with L15 medium containing 0.5 mg/ml BSA. Five days after plating the cells, the medium was replaced with myelination medium containing a 1:100 dilution of Matrigel™, and three days later half the medium was removed and replaced with myelination medium containing 50 µg/ml ascorbic acid. The cultures were maintained for up to three months, replacing half the medium with myelination medium containing 50 µg/ml ascorbic acid every 2–3 days, with a full medium change once each week.
2.2. Transfection
The pmCherry expression construct was obtained by subcloning mCherry (Shaner et al., 2004) into pEGFP-C1 (Clontech, Mountain View, CA) in place of the EGFP sequence (Alami et al., 2009). The pEGFP-rat neurofilament M expression construct is described by Wang et al. (2000). Myelinating co-cultures were transfected after 4–6 weeks in culture using Lipofectamine 2000 (Life Technologies) cationic lipid reagent according to the manufacturer’s instructions. The DNA:Lipofectamine ratio was 1 µg DNA: 4µl Lipofectamine.
2.3. Staining with FluoroMyelin™ Red
FluoroMyelin™ Red solution in water was purchased from Molecular Probes (Life Technologies, Catalog No. F34652, Lot No. 23512W) and stored in the dark at 4°C. To stain the cultures, we first removed the culture medium and rinsed once with observation medium consisting of Hibernate-E low fluorescence medium (BrainBits, Springfield, IL) supplemented with B27 supplement mixture, 0.3% glucose, 1mM L-glutamine, 66 mM NaCl, and 100 ng/ml 2.5S nerve growth factor. We then added fresh observation medium containing FluoroMyelin™ Red (1:300 dilution) and placed the cultures in an incubator at 37°C with atmospheric CO2. After staining, we withdrew the medium and replaced it with fresh observation medium lacking FluoroMyelin™ Red.
2.4. Microscopy and imaging
Cells were observed in observation medium by epifluorescence, phase contrast or differential interference contrast microscopy on a Nikon TE300 or TE2000 inverted microscope (Nikon Inc., Garden City, NY) using a Nikon 40×/1.0NA Plan Apo oil immersion objective or a Nikon 20×/0.75NA Plan Fluor multi-immersion objective. For epifluorescence, we used ET-EGFP and ET-Texas Red/mCherry filter sets (Filter set numbers 49002 and 49008 respectively, Chroma Technology, Brattleboro, VT), and a custom-made FluoroMyelin™ Red filter set consisting of a D450/40× band-pass exciter, a 505dcxr dichroic mirror, and an E610LPv2 long pass emission filter (Chroma Technology, Brattleboro, VT). The temperature on the microscope stage was maintained using an Air Stream Incubator (Nevtek, Williamsville, VA). A layer of dimethylpolysiloxane fluid (Sigma, 5 centistokes) was floated over the observation medium to prevent evaporation. For live fluorescence imaging, the illumination from the mercury arc lamp was attenuated 8-fold or 12-fold using neutral density filters and images were acquired using Micromax 512BFT or CoolSnap HQ cooled CCD cameras (Roper Scientific, Trenton, NJ) and MetaMorph™ software (Molecular Devices, Downingtown, PA).
2.5. Analysis of fluorescence intensities
The intensity of the FluoroMyelin™ Red fluorescence was measured using MetaMorph™ software. A rectangular region of interest (ROI-1) was drawn manually around a segment of a myelinated axon, encompassing the entire width of the myelin sheath. Additional flanking regions (ROI-2 and ROI-3) were drawn in the background on either side of the sheath, taking care to avoid other fluorescent cells. The contribution of the background fluorescence to ROI-1 was calculated by multiplying the average pixel intensity in ROI-2 and ROI-3 by the area of ROI-1, and then this value was subtracted from the total fluorescent intensity in ROI-1 to yield the background-corrected intensity.
2.6. Analysis of cell viability
The potential toxicity of FluoroMyelin™ Red was evaluated using the LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells (Invitrogen L-3224). The reagent consists of calcein acetomethoxy ester (calcein AM), which is a membrane permeant fluorescent dye that stains live cells green, and ethidium homodimer-1 (EthD-1), which is a membrane impermeant fluorescent dye that stains the nuclei of dead cells red. To perform the assay, the cells were rinsed three times with PBS and then treated with a mixture of 1 µM calcein AM and 1 µM EthD-1 in PBS for 10 minutes at room temperature. After 10 minutes, the cells were transferred to the microscope stage and random fields were imaged using a 40×/1.0NA Plan Apo oil immersion objective. In each image, we counted live cells (green fluorescence in cytoplasm) and dead cells (red fluorescent nuclei). We repeated each treatment three times in three independent experiments (total of 45 image fields in 3 different dishes). The average number of cells counted was about 2700 for each condition.
2.7. Immunostaining
Our immunostaining protocol was based on the method of Dzhashiashvili et al. (2007). Cultures were fixed with a solution containing 1% (w/v) paraformaldehyde and 1% sucrose in phosphate-buffered saline (10 mM phosphate buffer, 2.7 mM potassium chloride, 137 mM sodium chloride, pH 7.4) and then extracted with methanol at −20°C and processed for immunostaining using standard methods. Blocking was performed using 5% normal goat serum (Jackson Immunoresearch, West Grove, PA) and 0.2% Triton X-100 in PBS. Myelin protein P0 was detected using a mouse monoclonal antibody P07 (clone 18) from Juan Jose Archelos (Medical University of Graz). The secondary antibody was goat anti-mouse IgG conjugated with Alexa 488 (Molecular Probes, Eugene, OR). The FluoroMyelin™ Red dye is not fixable and was lost during the fixation and extraction process.
2.8. Fluorometry
The brain of an adult ICR mouse (Harlan Laboratories, Indianapolis, IN) was homogenized in 5 ml of a solution containing 20 mM Tris/HCl, 0.15 M NaCl, 1 mM EGTA, 1 mM EDTA and 10 mg/ml protease inhibitor cocktail (bestatin, E64 and leupeptin; Sigma, St. Louis, MO), pH 7.4. The homogenate was clarified by centrifugation at 3,000g for 40min at 4°C and the pellet was discarded. FluoroMyelin™ Red was diluted 1:300 in water or in clarified brain homogenate and incubated for 2 hours at 37°C. The absorption and emission spectra were measured in 96 well plates with a FlexStation® fluorometer (Molecular Devices, Sunnyvale, CA) using untreated water or brain homogenate as a blank.
3. RESULTS
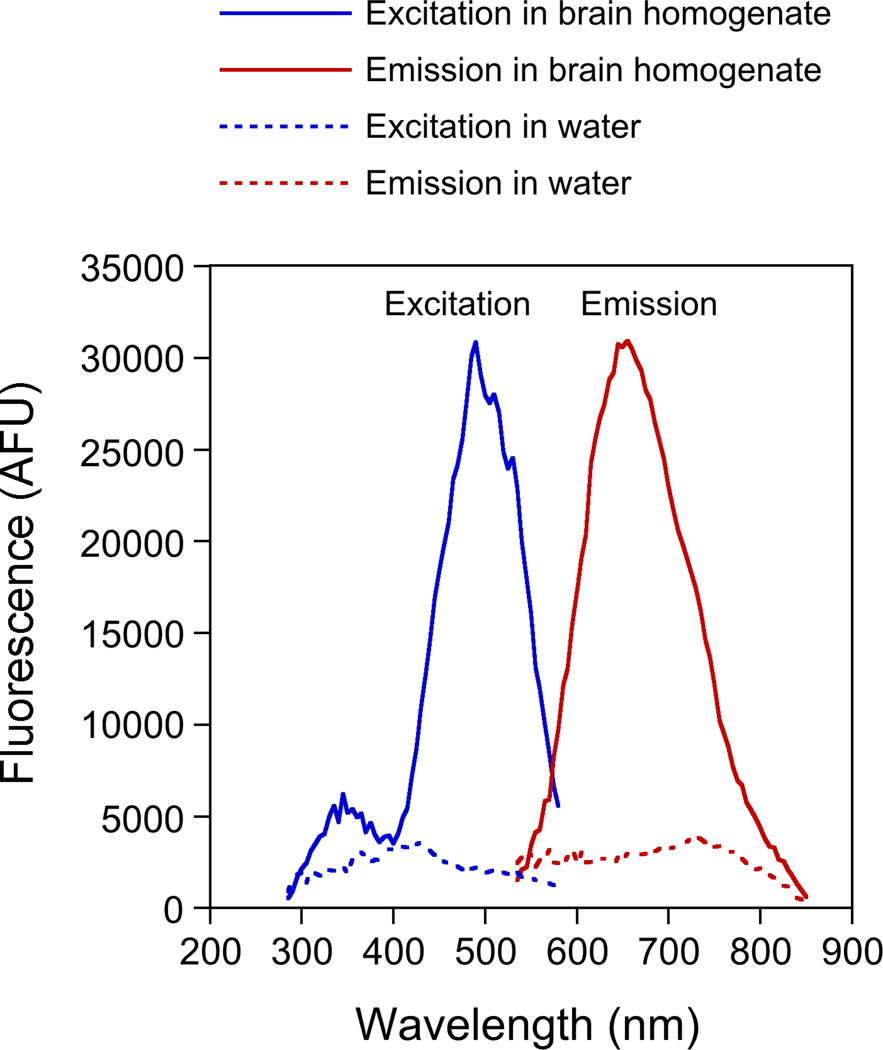
To determine the optimum excitation and emission wavelengths for FluoroMyelin™ Red, we obtained excitation and emission spectra in aqueous solution in the presence or absence of brain homogenate, which is rich in myelin. As expected, the FluoroMyelin™ Red was weakly fluorescent in water but strongly fluorescent in the presence of brain homogenate (Fig. 1). The dye exhibited broad excitation and emission profiles, with a peak excitation at 490 nm (blue light) and a peak emission at 655 nm (red light). Thus this dye has a remarkably large Stokes shift of 165 nm.
Fig. 1. Excitation and emission spectra for FluoroMyelin™ Red.
FluoroMyelin Red™ was diluted 1:300 into either water or brain homogenate. After incubation for 2 hours at 37°C, the absorption and emission spectra were measured by fluorometry using water or brain homogenate as a blank. The FluoroMyelin Red™ fluorescence is enhanced greatly by the myelin in the brain homogenate. The peak excitation is at about 490 nm and the peak emission is at about 655 nm.
To investigate the potential of FluoroMyelin™ Red as a vital stain for myelin, we established long-term myelinating cultures from rat dorsal root ganglia according to the protocol of Svenningsen et al. (2003), with some modifications (see Methods). Compact myelin is sufficiently refractile that myelin sheaths can be detected in these cultures using bright field illumination (Bunge et al., 1989; Kleitman et al., 1998). By this criterion, myelin was first noticeable after 19–21 days and was widespread by 4 weeks. To stain for myelin, we replaced the culture medium with observation medium containing a 1:300 dilution of FluoroMyelin™ Red, which is the manufacturer’s recommended dilution for staining myelin in frozen sections. After 30–45 minutes we observed bright staining of myelin sheaths throughout each culture dish (Fig. 2). Since the FluoroMyelin™ dyes are only weakly fluorescent in aqueous solution, it is possible to observe the fluorescence without removing the dye, but we generally rinse with fresh observation medium before imaging. We have also tested another compound, FluoroMyelin™ Green (also sold by Life Technologies), but in our hands this dye is considerably less bright making it a less favorable choice compared to FluoroMyelin™ Red (data not shown).
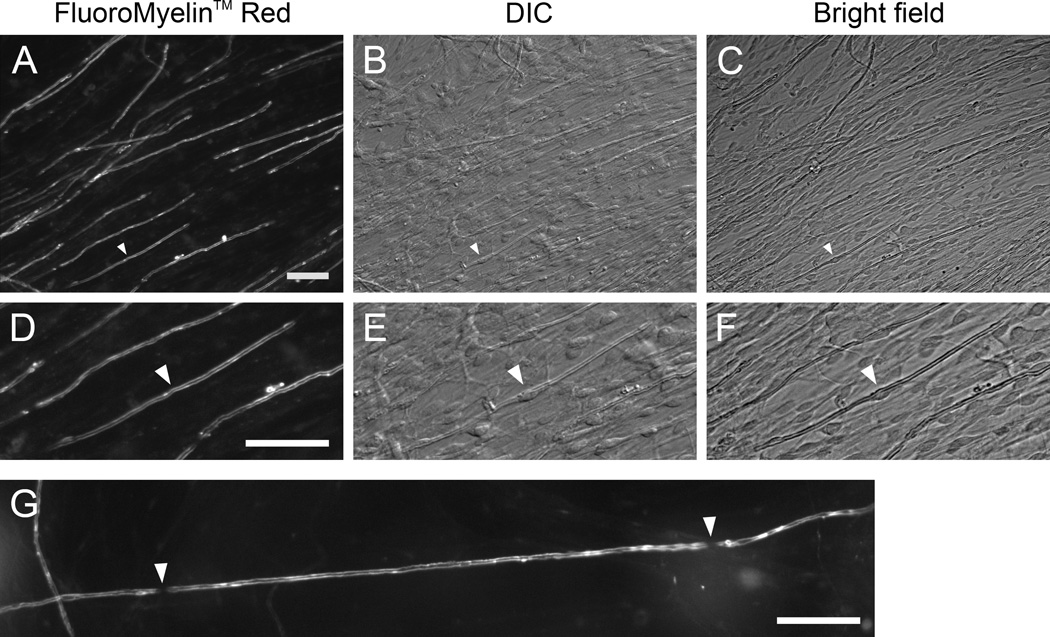
Fig. 2. FluoroMyelin™ Red stains myelin sheaths in living cultures.
Myelinating cultures were incubated for 30 min in observation medium containing FluoroMyelin™ Red (1:300 dilution) and then imaged in fresh observation medium using a 20× objective magnification. A–C. A field of view after 35 days in culture at low magnification, imaged with fluorescence, differential interference contrast and bright field microscopy. D–F. An enlarged sub-region of this field of view. The white arrowheads point to the nucleus of a myelinating Schwann cell, visible in the differential interference contrast (DIC) and bright field images. G. An axon that is continuously myelinated after 46 days in culture. Note the two nodes of Ranvier (white arrowheads). The internode separating these nodes measures approximately 260 µm in length. Scale bars= 40 µm.
Observations of myelinated sheaths at higher magnification revealed no apparent adverse effect of the FluoroMyelin™ Red on the morphology of axons or their myelinating cells at the light microscopic level, as evidenced by the bright and smooth staining of the compact myelin (Fig. 3). In some cases Schmidt-Lanterman clefts were visible (see inset in panel I of Fig. 3). To confirm that the dye incorporated into all myelin sheaths, we treated two culture dishes after 98 days in culture with observation medium containing FluoroMyelin™ Red. We then scanned the dishes under bright field illumination and scored each myelin sheath for the presence or absence of FluoroMyelin™ Red fluorescence. Of a total of 325 myelin sheaths, 100% were stained (data not shown). To corroborate this finding, we treated cultures with FluoroMyelin™ Red in observation medium and imaged myelin sheaths in randomly selected fields. Then we fixed the cultures and imaged the same fields again after processing for immunofluorescence microscopy using a monoclonal antibody specific for the myelin protein P0. In each field there was a direct correspondence between the FluoroMyelin™ Red fluorescence and the myelin immunofluorescence (Fig. 4). To quantify this, we analyzed one dish that was stained after 137 days in culture. Of 359 myelin sheaths that were identified based on their FluoroMyelin™ Red fluorescence, 100% also stained for P0 (data not shown). Thus FluoroMyelin™ Red is a reliable agent for staining myelin in living cultures.
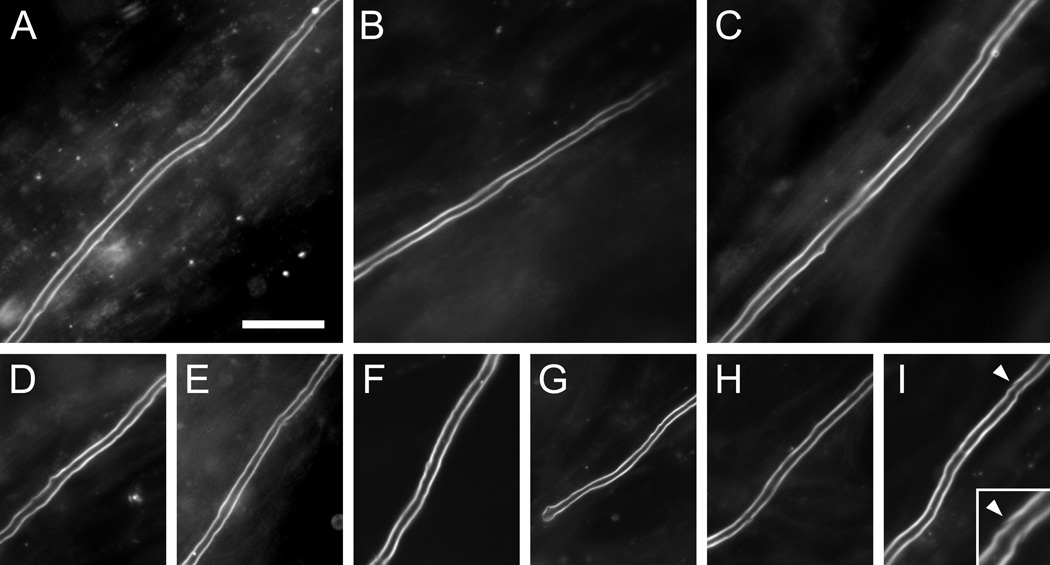
Fig. 3. Examples of myelin sheaths stained with FluoroMyelin™ Red.
Myelinating cultures were incubated for 2 hours in observation medium containing FluoroMyelin™ Red (1:300 dilution) after 34–41 days in culture to visualize the myelin sheaths and then imaged in fresh observation medium using a 40× objective magnification. A–I show examples of 9 different myelin sheaths. Note the smooth appearance of the myelin in these live images. The dye does yield some background staining in these dense cultures, but the staining of the myelin sheaths is much brighter. The inset at the lower right shows an enlarged view of a Schmidt-Lanterman cleft (arrowheads). Scale bar= 25 µm.
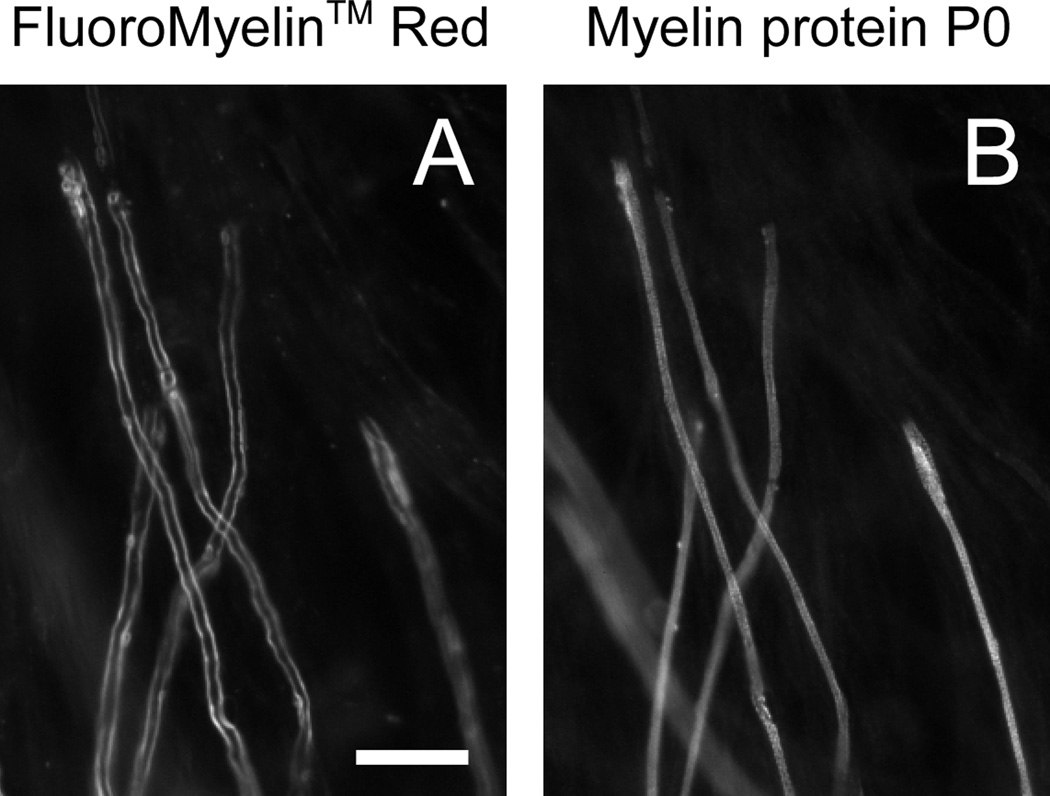
Fig. 4. FluoroMyelin™ Red stains all myelin sheaths.
Myelinating cultures were incubated for 2 hours in observation medium containing FluoroMyelin™ Red (1:300 dilution) after 34 days in culture. After staining we imaged random fields in fresh observation medium using a 40× objective magnification and then we fixed the cultures and imaged the same fields again after processing for immunofluorescence with an antibody to the myelin protein P0 (see Methods). A. FluoroMyelin™ Red fluorescence in living culture. B. Myelin immunofluorescence after fixation. Note that there was some movement and shrinkage of the axons and myelin sheaths during fixation and subsequent processing, but nevertheless it is clear that there is a close correspondence between the FluoroMyelin™ Red fluorescence in the living cultures and the myelin fluorescence in the fixed cultures. Scale bar=10 µm.
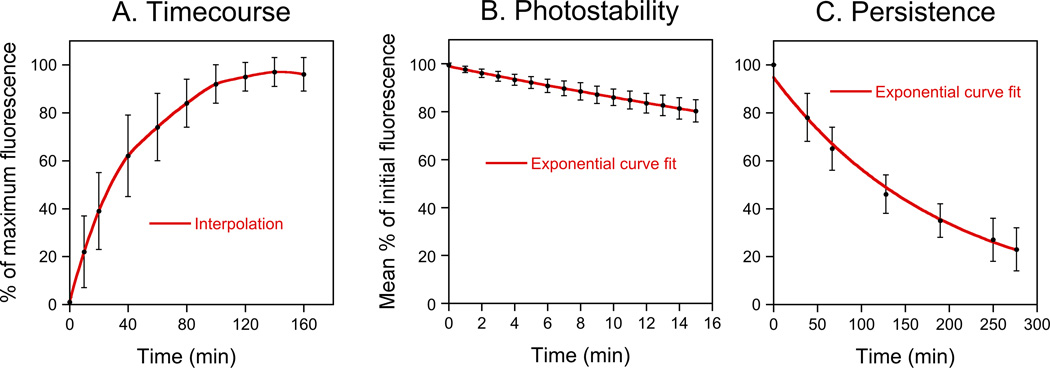
To determine the optimum staining time, we imaged the same myelin sheaths at 10 or 20 minute intervals over a period of 160 minutes (Fig. 5A). Half-maximal fluorescence intensity was attained after 30 minutes and maximal fluorescence intensity was attained after 140 minutes. To characterize the photostability of the dye, we performed repeated imaging of myelin sheaths that had been stained with FluoroMyelin™ Red. As expected, the fluorescence intensity declined with exponential kinetics, consistent with a simple first order photobleaching process (Fig. 5B). However, the kinetics of bleaching were remarkably slow (t1/2=50 minutes), with only a 20% loss of fluorescence intensity after 15 minutes of continuous illumination. Moreover, this is an underestimate of the stability because some of this loss of fluorescence is due to diffusion of the dye out of the myelin (see below). Thus FluoroMyelin™ Red is relatively photostable when incorporated into myelin.
Fig. 5. Characterization of FluoroMyelin™ Red staining.
A. FluoroMyelin™ Red staining time-course. Data from 28 myelin sheaths in 5 dishes that were stained after 31–41 days in culture. B. FluoroMyelin™ Red photostability. The culture was stained for 2 hours and then the excess dye was rinsed off with fresh observation medium and each field of view was imaged continuously for 15 minutes with the light from the mercury arc lamp attenuated 12-fold using neutral density filters. Data from 46 myelin sheaths from 6 different fields of view in one dish that was stained after 67 days in culture. C. Persistence of FluoroMyelin™ Red staining after wash-out. The culture was stained for 2 hours and then the excess dye was rinsed off with fresh observation medium and selected myelin sheaths were imaged at 30–60 minute intervals for 5 hours. Data from 14 different myelin sheaths in one dish that was stained after 45 days in culture.
To determine whether or not the FluoroMyelin™ Red stain is reversible, we stained myelinated axons for two hours and then rinsed off the dye and quantified the fluorescence intensity of identified myelin sheaths at 30–60 minute intervals over a period of 5 hours (Fig. 5C). The intensity declined with exponential kinetics (t1/2=134 minutes), consistent with a simple diffusion-limited process. By extrapolation, this suggests that about 90% of the fluorescence should be lost after 7.4 hours. Thus the FluoroMyelin™ Red incorporation into myelin sheaths is reversible. In several trial experiments we were able to repeatedly stain and destain the same myelin sheaths over a period of a week, though the staining intensity was somewhat variable (data not shown).
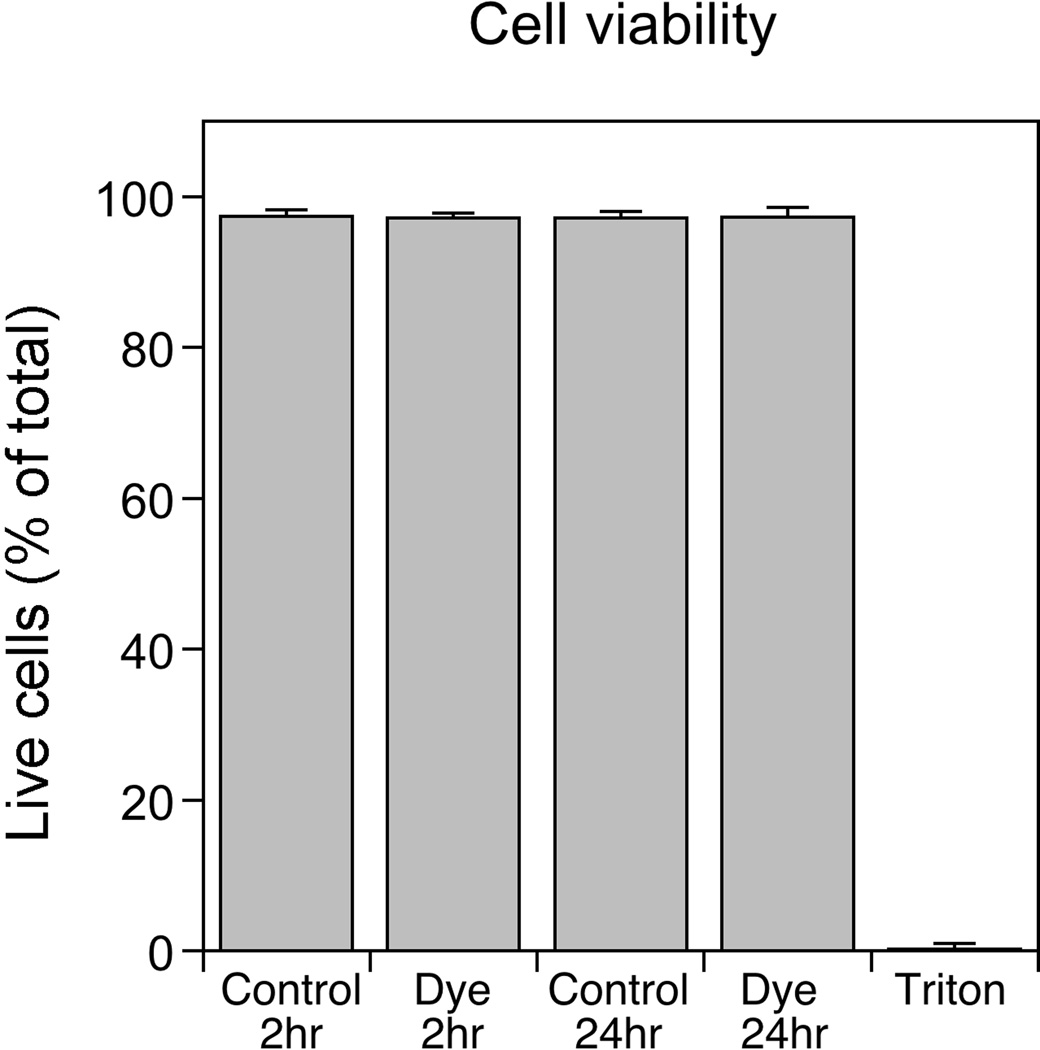
To confirm that the FluoroMyelin™ Red stain is not toxic to our cells, we treated myelinating cultures with FluoroMyelin™ Red for 2 or 24 hours and then analyzed cell viability. About 3% of the cells were dead in control cultures, and this did not change after treatment with FluoroMyelin™ Red, even after 24 hours (Fig. 6). Thus FluoroMyelin™ Red does not appear to be cytotoxic to dorsal root ganglion neurons or Schwann cells under the staining conditions described in this study.
Fig. 6. Analysis of cell viability.
Myelinating cultures after 30–37 days in culture were treated with myelination medium with or without FluoroMyelin™ Red (1:300 dilution) for 2 hours or 24 hours, or they were treated with 0.1% Triton X-100 for 10 minutes to confirm that the assay was capable of detecting dead cells. The percentage of the cells that were alive or dead was determined using Invitrogen’s LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells (see Methods). The data for each treatment are an average of three independent experiments (about 2700 cells in total counted per treatment). On average about 3% of the cells in the untreated cultures were dead, and there was no change after treatment with FluoroMyelin™ Red, even after 24 hours (p>0.3, t-test). Greater than 99% of the cells were scored as dead after treatment with Triton X-100 (positive control).
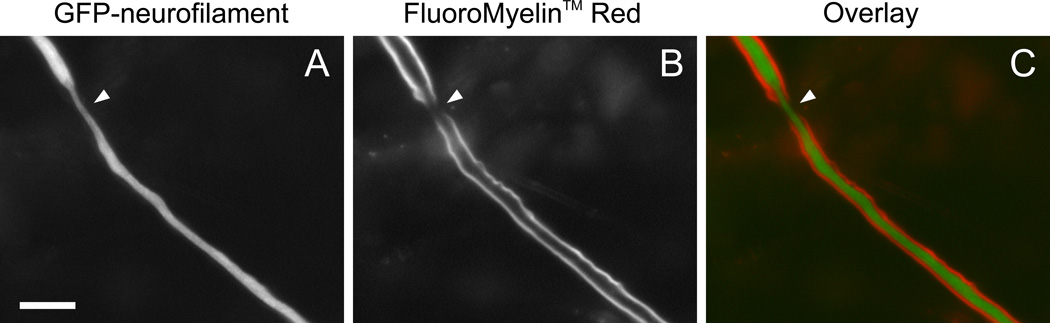
To explore the potential of FluoroMyelin™ Red for live imaging of myelinated axons, we transfected myelinating co-cultures with a green fluorescent neurofilament fusion protein (GFP-tagged rat neurofilament protein M) using Lipofectamine 2000 reagent (Invitrogen). We have shown previously that this fusion protein co-assembles with endogenous neurofilament proteins into neurofilaments (Uchida et al., 2009; Uchida and Brown, 2004; Wang and Brown, 2010, 2001; Wang et al., 2000). Fortuitously, Schwann cells are not transfected readily with the Lipofectamine 2000 reagent, whereas dorsal root ganglion neurons are. Typically we obtain about a dozen transfected neurons per dish, though the efficiency is somewhat variable. Though this number may seem low, the extensive axonal arbor of these neurons in these long-term cultures means that we encounter fluorescent axons in most fields of view. To image the axons live, we treated the cultures with FluoroMyelin™ Red and then observed them by time-lapse imaging on the microscope stage for up to several hours. Fig. 7 shows a node of Ranvier between two myelinated internodes. Note the narrowing of the axon in the node, which is a consistent feature of myelinated axons in the peripheral nervous system in vivo (Berthold, 1968; Sward et al., 1995). Both GFP and FluoroMyelin™ Red excite in the blue, but they can be discriminated using appropriate filter sets (see Methods) because GFP does not emit in the red and FluoroMyelin™ Red does not emit in the green.
Fig. 7. Live imaging of a node of Ranvier.
A myelinating culture was transfected with GFP-tagged neurofilament protein M after 43 days in culture. Three days later, the culture was exposed to FluoroMyelin™ Red (1:300 dilution) in observation medium for 2 hours, and then rinsed and imaged in observation medium at 37°C with a 40× objective magnification. A. Image of GFP-tagged neurofilament protein M fluorescence. B. Image of the FluoroMyelin™ Red fluorescence. C. Pseudocolor overlay. The arrowhead points to a node of Ranvier between two myelin sheaths. Both GFP and FluoroMyelin™ Red excite in the blue, but they can be discriminated using standard filter sets because GFP does not emit in the red and FluoroMyelin™ Red does not emit in the green. Scale bar= 10 µm.
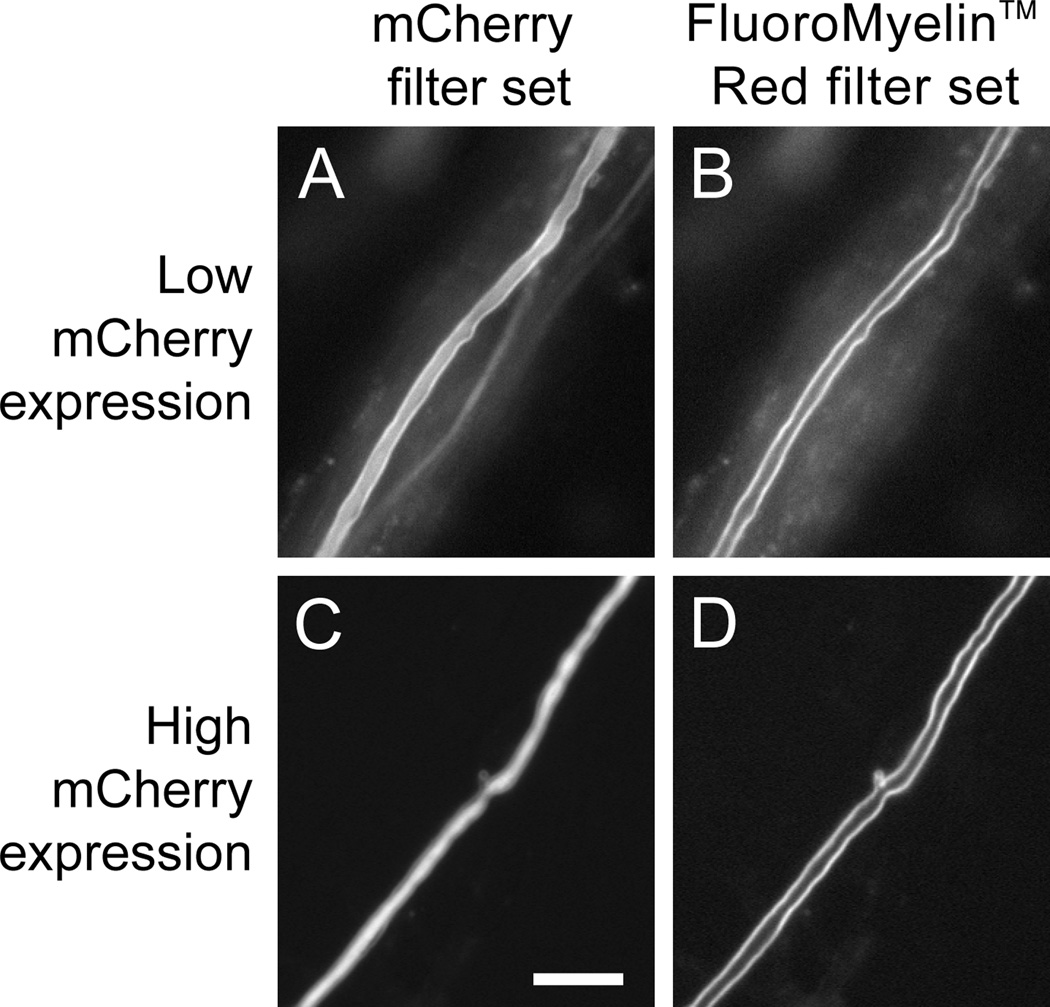
To further examine the utility of FluoroMyelin™ Red for live fluorescence imaging of myelin, we investigated whether the large Stokes shift of this dye would permit the fluorescence of this dye to be distinguished from the fluorescence of a popular red fluorescent protein, mCherry. To do this, we designed a custom band-pass epifluorescence filter set (see Methods) that allows for excitation in the blue and emission in the red (Fig. 8). We found that both mCherry and FluoroMyelin™ Red fluorescence are visible using a standard mCherry filter set because both dyes excite in the green and emit in the red. In contrast, only FluoroMyelin™ Red is visible using the custom FluoroMyelin™ Red filter set because FluoroMyelin™ Red excites in the blue but mCherry does not (Fig. 1). Thus it is possible to perform multi-wavelength live fluorescence imaging of myelinating cultures using GFP, mCherry and FluoroMyelin™ Red, with the proviso that the FluoroMyelin™ Red will be visible on the mCherry channel even though the mCherry will not be visible on the FluoroMyelin™ Red channel.
Fig. 8. Separation of FluoroMyelin™ Red and mCherry fluorescence using a custom FluoroMyelin™ Red filter cube.
A myelinating culture was transfected with mCherry after 40–47 days in culture. Four days later, the culture was exposed to FluoroMyelin™ Red (1:300 dilution) in observation medium for 2 hours, and then rinsed and imaged in observation medium with a 40× objective magnification. A,B. A myelinated axon expressing relatively low levels of mCherry. C,D. A myelinated axon expressing relatively high levels of mCherry. Both mCherry and FluoroMyelin™ Red fluorescence are visible with the mCherry filter set (A,C) because both dyes excite in the green and emit in the red, but only the FluoroMyelin™ Red fluorescence is visible using the custom FluoroMyelin™ Red filter set (B,D) because mCherry does not excite in the blue, whereas FluoroMyelin™ Red does. When the mCherry expression is relatively low, the myelin sheath appears on the mCherry channel as a bright border along either side of a faint axon (A), whereas when the mCherry expression is relatively high the myelin sheath appears on the mCherry channel as a faint halo along either side of a bright axon (C). Scale bar= 10 µm.
4. DISCUSSION
The above data indicate that FluoroMyelin™ Red is a bright, photostable and non-toxic dye that selectively stains compact myelin in long-term myelinating co-cultures of Schwann cells and dorsal root ganglion neurons. The dye is freely soluble in water so staining can be achieved by simply adding it to the culture medium. After rinsing, the dye diffuses out of the myelin with a half-life of about 2 hours. However, the cultures can be imaged without rinsing, if desired, because the dye is only weakly fluorescent in aqueous solution. Moreover, the dye has no adverse effects on the morphology of the axons or the myelin sheaths at the light microscopic level, and no adverse effects on neuronal or Schwann cell viability. Finally, due to its spectral properties and large Stokes shift, the dye can be distinguished from both GFP and mCherry. While it is possible to identify myelin sheaths in living cultures under bright field illumination due to the natural birefringence of the myelin, fluorescence microscopy with FluoroMyelin™ Red affords much better contrast.
An alternative approach to using fluorescent dyes for live imaging of myelin is to express either membrane-tethered fluorescent proteins or fluorescent fusions of myelin proteins in the myelinating cells, either by transfection of myelinating glia in culture (Ioannidou et al., 2012; Pedraza and Colman, 2000; Watkins et al., 2008) or by using transgenic animals (Kirby et al., 2006; Sobottka et al., 2011; Yoshida and Macklin, 2005). The use of transgenic animals has the obvious advantage that it can allow imaging of myelin in vivo. However, for studies in culture FluoroMyelin™ Red also has several advantages. First, it is fast and simple, requiring just a 1–2 hour application. Second, unlike transfection methods, it labels all the myelin sheaths. Third, the myelin can be allowed to develop for days or weeks in the absence of any dye or fluorescent protein prior to staining. Fourth, the dye diffuses readily out of the myelin after rinsing. Together, these features make FluoroMyelin™ Red a useful reagent for live-cell fluorescence imaging of compact myelin in myelinating co-cultures.
Highlights.
FluoroMyelinTM Red is a water-soluble fluorescent dye that has selectivity for myelin in fixed and frozen tissue
Here we report that this dye is also suitable as a vital stain for live imaging of myelin in myelinating co-cultures
The dye incorporates rapidly and reversibly into compact myelin sheaths and is bright and photostable with no apparent adverse effects on the neurons or their myelinating cells
ACKNOWLEDGMENTS
This project was funded by NSF grant IOS-0818653 and NIH grant RO1-NS38526 to A.B., with additional support provided by NIH grant P30-NS045758. We thank Juan José Archelos of the Medical University of Graz for the P0 antibody, and Roger Tsien of the University of California San Diego for the mCherry. We also thank Liliana Pedraza of McGill University for helpful advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing financial interests.
REFERENCES
- Alami NH, Jung P, Brown A. Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. J. Neurosci. 2009;29:6625–6634. doi: 10.1523/JNEUROSCI.3829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold CH. Ultrastructure of the node-paranode region of mature feline ventral lumbar spinalroot fibres. Acta Soc. Med. Ups. 1968;73(Suppl 9):37–70. [PubMed] [Google Scholar]
- Bilderback TR, Chan JR, Harvey JJ, Glaser M. Measurement of the rate of myelination using a fluorescent analogue of ceramide. J. Neurosci. Res. 1997;49:497–507. [PubMed] [Google Scholar]
- Bunge RP, Bunge MB, Bates M. Movements of the Schwann cell nucleus implicate progression of the inner (axon-related) Schwann cell process during myelination. J. Cell Biol. 1989;109:273–284. doi: 10.1083/jcb.109.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J. Cell Biol. 2007;177:857–870. doi: 10.1083/jcb.200612012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidou K, Anderson KI, Strachan D, Edgar JM, Barnett SC. Time-lapse imaging of the dynamics of CNS glial-axonal interactions in vitro and ex vivo. PLoS One. 2012;7:e30775. doi: 10.1371/journal.pone.0030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. American Journal of Physiology and Regulatory Integrative and Comparative Physiology. 2006;290:R1105–R1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- Kilgore J. Lipophilic dyes and their application for detection of myelin. US Patent Application Pub. No: US 2006/0073541 A1. Appl. No. 11/241,323. Filed Sept 30, 2005. Patent pending. 2006
- Kilgore J, Janes MS. A novel myelin labeling technique for fluorescence microscopy of brain sections. Abstract. Program number 124.7. 2004 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2004. Online 2004. [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Kleitman N, Wood PM, Bunge RP. Tissue culture methods for the study of myelination. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge, Mass.: MIT Press; 1998. pp. 545–594. [Google Scholar]
- Pedraza L, Colman DR. Fluorescent myelin proteins provide new tools to study the myelination process. J. Neurosci. Res. 2000;60:697–703. doi: 10.1002/1097-4547(20000615)60:6<697::AID-JNR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Pereyra PM, Roots BI. The use of tetracycline hydrochloride as a rapid fluorescent stain for myelin membranes in vertebrates and invertebrates. Brain Res. 1988;458:377–382. doi: 10.1016/0006-8993(88)90482-9. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Hermel H, Matiasek K, Mohwald H. Selective staining by the fluorochrome 5,5- diphenyl-9-ethyl-oxacarbocyanine. II. Application to paraffin embedded nervous tissue. Biotech. Histochem. 1999;74:229–235. doi: 10.3109/10520299909034658. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Swanson LW, Sawchenko PE. Some fluorescent counterstains for neuroanatomical studies. J. Histochem. Cytochem. 1982;30:123–128. doi: 10.1177/30.2.6174560. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N. CNS live imaging reveals a new mechanism of myelination: The liquid croissant model. Glia. 2011;59:1841–1849. doi: 10.1002/glia.21228. [DOI] [PubMed] [Google Scholar]
- Svenningsen AF, Shan WS, Colman DR, Pedraza L. Rapid method for culturing embryonic neuron-glial cell cocultures. J. Neurosci. Res. 2003;72:565–573. doi: 10.1002/jnr.10610. [DOI] [PubMed] [Google Scholar]
- Sward C, Berthold CH, Nilsson-Remahl I, Rydmark M. Axonal constriction at Ranvier's node increases during development. Neurosci. Lett. 1995;190:159–162. doi: 10.1016/0304-3940(95)11528-5. [DOI] [PubMed] [Google Scholar]
- Uchida A, Alami NH, Brown A. Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol. Biol. Cell. 2009;20:4997–5006. doi: 10.1091/mbc.E09-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A, Brown A. Arrival, reversal and departure of neurofilaments at the tips of growing axons. Mol. Biol. Cell. 2004;15:4215–4225. doi: 10.1091/mbc.E04-05-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Popescu DC, Wu C, Zhu J, Macklin W, Wang Y. In situ fluorescence imaging of myelination. J. Histochem. Cytochem. 2010;58:611–621. doi: 10.1369/jhc.2010.954842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wu C, Popescu DC, Zhu J, Macklin WB, Miller RH, Wang Y. Longitudinal near-infrared imaging of myelination. J. Neurosci. 2011;31:2382–2390. doi: 10.1523/JNEUROSCI.2698-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown A. A hereditary spastic paraplegia mutation in kinesin-1A/KIF5A disrupts neurofilament transport. Molecular Neurodegeneration. 2010;5:52. doi: 10.1186/1750-1326-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown A. Rapid intermittent movement of axonal neurofilaments observed by fluorescence photobleaching. Mol. Biol. Cell. 2001;12:3257–3267. doi: 10.1091/mbc.12.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ho C-L, Sun D, Liem RKH, Brown A. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat. Cell Biol. 2000;2:137–141. doi: 10.1038/35004008. [DOI] [PubMed] [Google Scholar]
- Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Tian D, Feng Y, Polak P, Wei J, Sharp A, Stankoff B, Lubetzki C, Zalc B, Mufson EJ, Gould RM, Feinstein DL, Wang Y. A novel fluorescent probe that is brain permeable and selectively binds to myelin. J. Histochem. Cytochem. 2006;54:997–1004. doi: 10.1369/jhc.5A6901.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Nesterov EE, Skoch J, Lin T, Hyman BT, Swager TM, Bacskai BJ, Reeves SA. Detection of myelination using a novel histological probe. J. Histochem. Cytochem. 2005;53:1511–1516. doi: 10.1369/jhc.5A6704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Macklin WB. Oligodendrocyte development and myelination in GFP-transgenic zebrafish. J. Neurosci. Res. 2005;81:1–8. doi: 10.1002/jnr.20516. [DOI] [PubMed] [Google Scholar]