Abstract
Background and Purpose
The harsh host brain microenvironment caused by production of reactive oxygen species after ischemic reperfusion injury offers a significant challenge to survival of transplanted neural stem cells (NSCs) after ischemic stroke.
Copper/zinc-superoxide dismutase (SOD1) is a specific antioxidant enzyme that counteracts superoxide anions. Here, we have investigated whether genetic manipulation to overexpress SOD1 enhances survival of grafted stem cells and accelerates amelioration of ischemic stroke.
Methods
NSCs genetically modified to overexpress or downexpress SOD1 were administered intracerebrally 2 days after transient middle cerebral artery occlusion. Histological and behavioral tests were examined from Days 0 to 28 after stroke.
Results
Overexpression of SOD1 suppressed production of superoxide anions after ischemic reperfusion injury and reduced NSC death after transplantation. In contrast, downexpression of SOD1 promoted superoxide generation and increased oxidative stress-mediated NSC death. Transplantation of SOD1-overexpressing NSCs enhanced angiogenesis in the ischemic border zone through up-regulation of vascular endothelial growth factor. Moreover, grafted SOD1-overexpressing NSCs reduced infarct size and improved behavioral performance, compared with NSCs that were not genetically modified.
Conclusions
Our findings reveal a strong involvement of SOD1 expression in NSC survival after ischemic reperfusion injury. We propose that conferring antioxidant properties on NSCs by genetic manipulation of SOD1 is a potential approach for enhancing the effectiveness of cell transplantation therapy in ischemic stroke.
Keywords: neural stem cell, ischemic stroke, copper/zinc-superoxide dismutase, neuroprotection
Introduction
Stem cell therapy is a potential new treatment for stroke.1,2 Recent studies have demonstrated successful engraftment and functional recovery in animal models of ischemic stroke, and stem cell therapy is being evaluated for safety and efficacy in humans.3 A majority of grafted cells, however, dies in the ischemic brain. Only a small fraction (1% to 3%) survives 28 days after transplantation.4 After ischemic reperfusion injury, excessive reactive oxygen species (ROS) are generated and irreversibly oxidize macromolecules, such DNA, lipids, and proteins, leading to severe cell injury.5 Therefore, this hostile host brain environment, which is caused by production of ROS after cerebral ischemia and reperfusion, might be involved in the accelerated death of the grafted stem cells, resulting in the diminished effectiveness of stem cell therapy.
The antioxidant enzymes are among the major mechanisms by which cells counteract the deleterious effects of ROS after cerebral ischemia and reperfusion.6 Copper/zinc-superoxide dismutase (SOD1) is a dimeric cytosolic enzyme that detoxifies superoxide anions to H2O2.7 We have previously shown that SOD1 is highly neuroprotective against reperfusion injury after focal and global cerebral ischemia.8–10 However, it has not yet been determined whether SOD1 plays a critical role in protecting neural stem cells (NSCs) from oxidative stress. Therefore, the purpose of this study was to determine the relationship between SOD1 expression and survival of NSCs after ischemic reperfusion injury using NSCs isolated from wild-type (WT), SOD1 transgenic (Tg), and SOD1 knockout (KO) mice. We also sought to investigate whether SOD1-overexpressing NSCs enhance the effectiveness of transplantation therapy in ischemic stroke.
Materials and Methods
Isolation and Culture of Fetal NSCs
All animals were treated in accordance with Stanford University guidelines and the animal protocols were approved by Stanford University’s Administrative Panel on Laboratory Animal Care. NSCs were isolated from the subventricular zones of postnatal Day 1 green fluorescent protein (GFP) Tg mice, SOD1/GFP double Tg mice, and SOD1 KO/GFP Tg mice as described.11
Induction of Oxygen-Glucose Deprivation
We used oxygen-glucose deprivation (OGD) and reoxygenation (RO), an in vitro model that best mimics in vivo cerebral ischemia and reperfusion. NSCs were subjected to OGD and RO by replacing the medium with a buffered salt solution without glucose. The plates were placed in an anaerobic chamber at 37°C. After 8 hours, the medium was replaced with culture medium with glucose and the plates were returned to a 5% CO2/95% air incubator for various RO periods.
In Situ Detection of Superoxide Anion Production
Hydroethidine (HEt) was used for the detection of early production of superoxide anions as previously described.12 Production of superoxide anions was shown by oxidized HEt as diffuse signals and small particles in the cytosol.
Focal Cerebral Ischemia
Adult male C57BL/6 mice (26 to 30 g) were subjected to transient focal cerebral ischemia by 45 minutes of intraluminal middle cerebral artery blockade with a suture as described previously.13
Cell Transplantation
Three 1.0- μL deposits of NSCs (1×105 cells/μL) were transplanted into the peri-infarct cortex 2 days after stroke.
Statistical Analysis
Behavioral data were assessed using repeated measures ANOVA. We used Scheffé’s post-hoc analysis of a rotarod test and analyzed modified neurologic severity scores (mNSS) using the Steel-Dwass test. For other experimental data, comparisons among multiple groups were performed with one-way ANOVA, followed by Scheffé’s post-hoc analysis. Data are expressed as median for the mNSS and mean ±SD for the other experiments. Significance was accepted with P<0.05.
Full Methods
The full methods are detailed at http://stroke.ahajournals.org.
Results
Characterization of NSCs
We isolated the NSCs from fetal GFP Tg mice (WTNSCs), SOD1/GFP double Tg mice (TgNSCs) or SOD1 KO/GFP Tg mice (KONSCs). These NSCs, grown as adherent cultures, were self-renewing and multipotent (Figure S1A–E). The percentage of neurons (10.4±2.6, 10.7±2.2, and 10.2±2.4%) and astrocytes (72.2±9.9, 70.2±11.1, and 69.2±8.3%) differentiated from the WTNSCs, TgNSCs, and KONSCs was similar. Moreover, no difference was observed in the percentage of Ki-67-positive cells after 8 hours of OGD and 48 hours of RO among the three groups (Figure S1F).
SOD1 Expression in NSCs
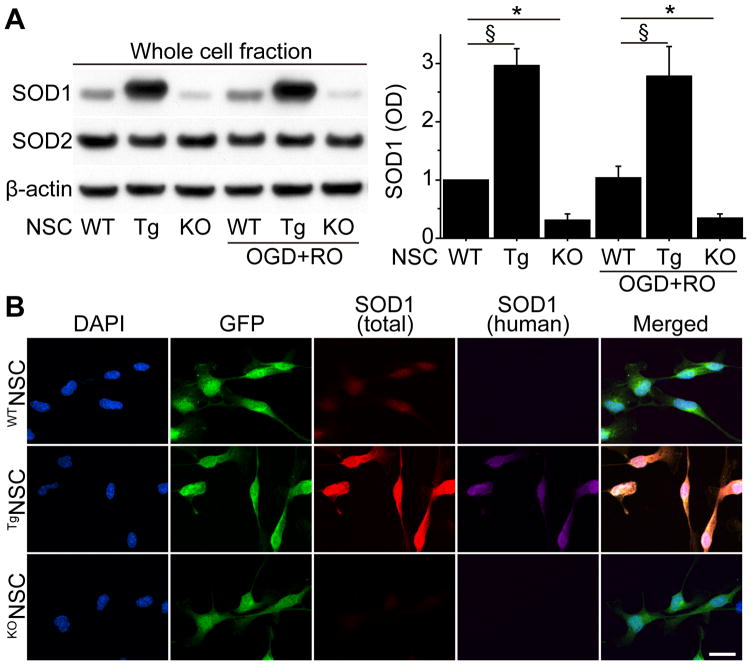
We next investigated the expression of SOD1 in the WTNSCs, TgNSCs, and KONSCs. Western blot analysis revealed that SOD1 expression was significantly higher in the TgNSCs (2.97-, 2.8-fold) and lower in the KONSCs (0.32-, 0.35-fold) than in the WTNSCs under both normal conditions and after OGD and RO, respectively (Figure 1A). In contrast, manganese-superoxide dismutase expression was similar among the three groups. Immunocytochemistry demonstrated that total SOD1 signals (mouse + human) in the cytosol increased in the TgNSCs associated with human SOD1 expression, whereas total SOD1 signals remarkably decreased in the KONSCs (Figure 1B).
Figure 1.
SOD1 expression in NSCs in vitro. A, Western blot analysis of WTNSCs, TgNSCs, and KONSCs. SOD1 expression significantly increased in the TgNSCs and decreased in the KONSCs compared with the WTNSCs under both normal conditions and after 8 hours of OGD and 3 hours of RO. No changes were observed in manganese-superoxide dismutase (SOD2) expression. β-actin was used as an internal control (n=4). OD indicates optical density. B, Fluorescent staining with 4′,6 diamidino-2-phenylindole (DAPI) (blue), GFP (green), total SOD1 (mouse + human) (red), and human SOD1 (magenta) in NSCs under normal conditions. The TgNSCs showed increased cytosolic total SOD1 signals together with human SOD1 signals, whereasthere were less total SOD1 signals observed in the KONSCs compared with the WTNSCs. Scale bar, 20 μm. *P<0.05; §P<0.001.
Involvement of SOD1 Expression in NSC Death In Vitro
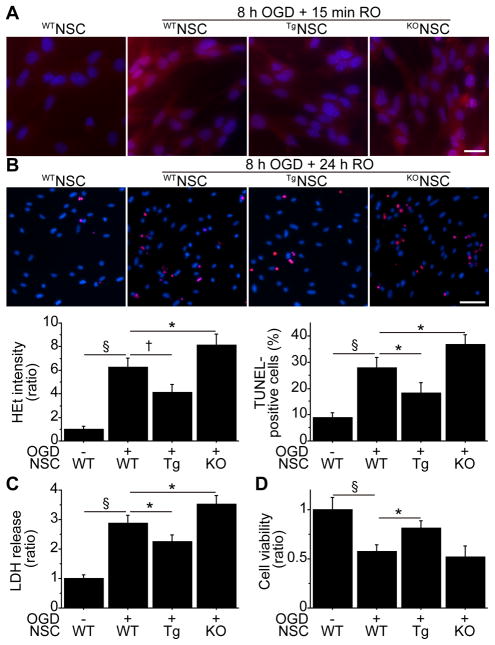
We next asked if the levels of SOD1 expression were associated with NSC death after ischemic reperfusion injury. After 8 hours of OGD and 15 minutes of RO, WTNSCs showed a significant increase in HEt signals in the cytosol, which represents production of superoxide anions (Figure 2A). This signal increase was significantly reduced in the TgNSCs, whereas it was enhanced in the KONSCs. Terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) staining exhibited increased TUNEL-positivity in the WTNSCs after 8 hours of OGD and 24 hours of RO. TUNEL-positivity was decreased in the TgNSCs and was exacerbated in the KONSCs (Figure 2B). Moreover, a lactate dehydrogenase (LDH) assay showed reduced death (by 22%) of the TgNSCs, as well as enhanced death (by 22%) of the KONSCs, after 8 hours of OGD and 24 hours of RO, compared with the WTNSCs (Figure 2C). These findings were also supported by a WST-1 assay (Figure 2D). Furthermore, SOD1 overexpression reduced and SOD1 downexpression increased LDH release from the NSCs subjected to the oxidative stimuli H2O2 and diethylenetriamine/nitric oxide (Figure S2A, S2B).
Figure 2.
Relationship between SOD1 expression and NSC death in vitro. A, Fluorescent staining of NSCs with HEt (red) and 4′,6 diamidino-2-phenylindole (DAPI) (blue). The increase in HEt signals in the WTNSCs after 8 hours of OGD and 15 minutes of RO was reduced in the TgNSCs, whereas they were enhanced in the KONSCs (n=4). Scale bar, 20 μm. B, NSCs analyzed by TUNEL staining (red) and DAPI (blue) after 8 hours of OGD and 24 hours of RO. The cell-counting study revealed a significant decrease in TUNEL-positivity in the TgNSCs, as well as a significant increase in the KONSCs (n=4). Scale bar, 50 μm. LDH (n=4) (C) and WST-1 (n=4) (D) assays showed a significant reduction in NSC death and increased viability in the TgNSCs after 8 hours of OGD and 24 hours of RO. In contrast, LDH release was increased in the KONSCs compared with the WTNSCs. *P<0.05; †P<0.01; §P<0.001.
SOD1 Overexpression Reduced Grafted-Cell Death In Vivo
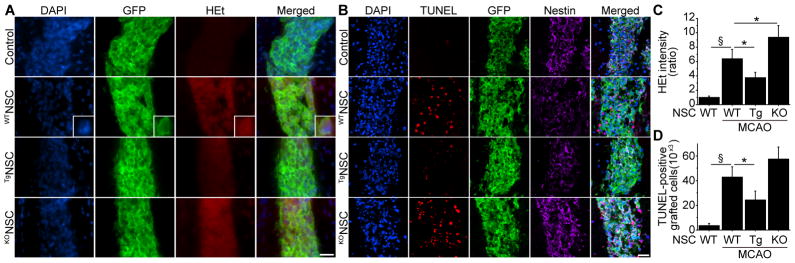
We transplanted WTNSCs, TgNSCs, or KONSCs into the peri-infarct cortex 2 days after stroke (Figure S3). When the WTNSCs were transplanted into the ischemic brains, HEt signals drastically increased 1 hour after transplantation, compared with those in the intact brains (Figure 3A, 3C). SOD1 overexpression significantly diminished this increase, whereas HEt signals were significantly elevated in the KONSC group compared with the WTNSC group. We next counted the number of TUNEL-positive cells that were also GFP-positive 2 days after transplantation. When the WTNSCs were transplanted into the peri-infarct cortex, the number of TUNEL-positive grafted cells increased significantly compared with those in the intact brains. However, this increased TUNEL-positivity was significantly reduced by 43% in the TgNSC group (Figure 3B, 3D).
Figure 3.
Reduced grafted-cell death with SOD1 overexpression in vivo. A and C, Fluorescent staining with 4′,6 diamidino-2-phenylindole (DAPI) (blue), GFP (green), and HEt (red) in brain sections 1 hour after transplantation. HEt signals increased in the WTNSCs under ischemic reperfusion injury, but were significantly reduced in the TgNSCs. Conversely, HEt signals were enhanced in the KONSCs compared with the WTNSCs (n=4). The insets represent high magnification images showing colocalization of HEt with GFP-positive grafted cells. MCAO indicates middle cerebral artery occlusion. B and D, Fluorescent staining with DAPI (blue), TUNEL (red), GFP (green), and nestin (magenta) 2 days after transplantation. SOD1 overexpression significantly reduced the number of TUNEL-positive grafted cells in the ischemic brain (n=4). Scale bars, 20 μm. *P<0.05; §P<0.001.
Increased Survival of Grafted NSCs with SOD1 Overexpression In Vivo
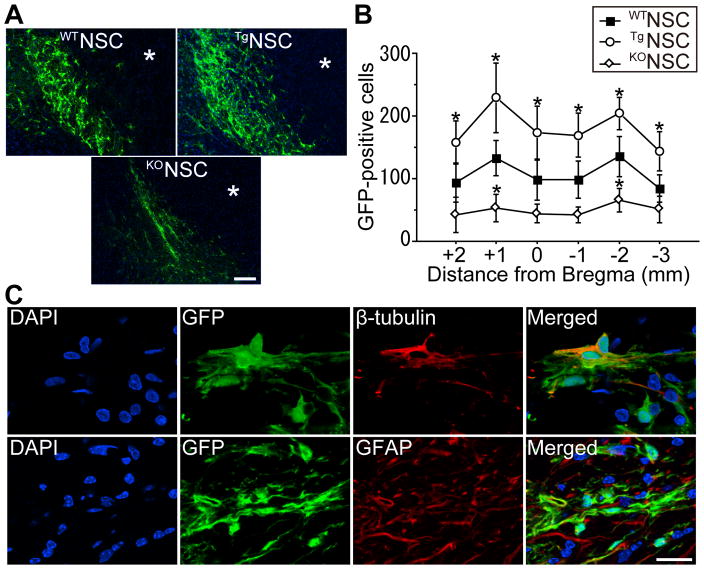
Twenty-eight days after stroke, staining with GFP revealed an extensive migration of grafted cells toward the ischemic lesion border in the WTNSC, TgNSC, and KONSC groups (Figure 4A). GFP-positive cells were not found in the striatum. No mice had signs of tumor formation caused by the transplanted NSCs 28 days, or even 3 months, after stroke. The number of surviving grafted cells in the ischemic brains was significantly large in the TgNSC group compared with the WTNSC group (Figure 4B). In contrast, grafted-cell survival was less in the KONSC group than in the WTNSC group. We next examined the differentiation profiles of the NSCs. Fluorescent double staining of the linage-specific phenotype markers and GFP demonstrated that GFP-positive cells exhibited a neuronal marker, β-tubulin, and an astrocytic marker, glial fibrillary acidic protein (GFAP), 28 days after stroke (Figure 4C). The percentage of neurons (8.9±1.0, 8.8±1.1, and 9.3±1.4%) and astrocytes (40.1±4.3, 37.1±5.9, and 38.3±8.0%) differentiated from the grafted NSCs was similar among the WTNSC, TgNSC, and KONSC groups.
Figure 4.
Increased survival of grafted NSCs with SOD1 overexpression in vivo. A, Fluorescent staining with GFP (green) and 4′,6 diamidino-2-phenylindole (DAPI) (blue) 28 days after stroke. The grafted cells migrated toward the ischemic lesion border. * indicates ischemic lesion. Scale bar, 100 μm. B, Quantification of the number of surviving grafted cells 28 days after stroke. Specimens were picked up every 1 mm and the number of GFP-positive cells was counted. These cells significantly increased in the TgNSC group, whereas less GFP-positive cells were observed in the KONSC group (n=4). C, Fluorescent staining with GFP (green) and β-tubulin or GFAP (red) 28 days after stroke. Grafted NSCs differentiated into neurons (β-tubulin+) and astrocytes (GFAP+). Nuclei were counterstained with DAPI. Scale bar, 20 μm. *P<0.05.
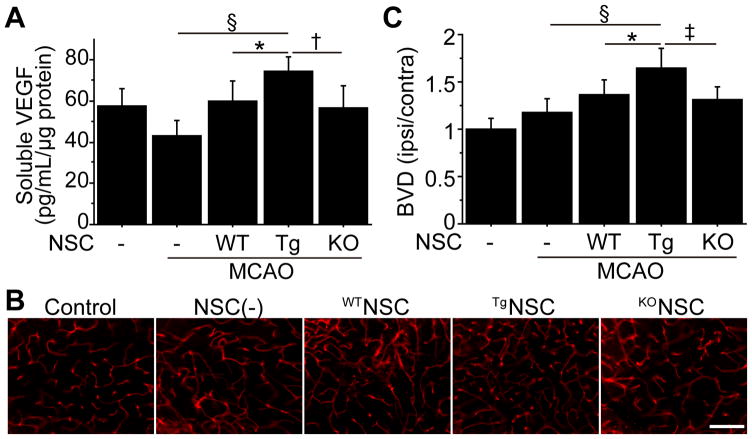
Transplantation of TgNSCs Enhanced Angiogenesis In Vivo
Secretion of vascular endothelial growth factor (VEGF) by transplanted NSCs is involved in functional recovery after ischemic stroke.14 Therefore, VEGF levels in the cortex were measured by a sandwich ELISA 4 days after stroke. These levels significantly increased in the TgNSC group compared with the non-transplanted control and WTNSC groups (Figure 5A). To analyze the effects of the NSCs on angiogenesis, we examined the density of lectin-perfused vessels in the peri-infarct cortex 14 days after stroke, which revealed a significantly higher blood vessel density (BVD) in the TgNSC group than in the non-transplanted control and WTNSC groups (Figure 5B, 5C). However, this enhanced angiogenesis was not observed in the WTNSC or KONSC groups.
Figure 5.
Transplantation of TgNSCs facilitated angiogenesis in vivo. A, In vivo ELISA of thecortex demonstrated a significant increase in VEGF in the TgNSC group 4 days after stroke (n=8). MCAO indicates middle cerebral artery occlusion. Representative images of lectin-perfused vessels (B) and quantification of BVD (C) in the peri-infarct cortex 14 days after stroke. BVD was significantly increased in the TgNSC group (n=8). Scale bar, 100 μm. *P<0.05, †P<0.01, ‡P<0.005, §P<0.001.
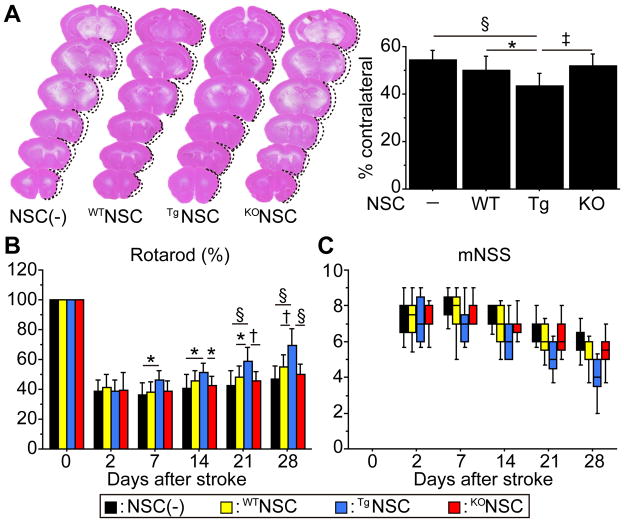
Mice Transplanted with TgNSCs Exhibited Amelioration of Ischemic Stroke
To investigate whether transplantation of NSCs could promote amelioration of ischemic stroke, infarct size was measured by H&E staining 28 days after stroke. Transplantation of TgNSCs significantly reduced the cortical infarct size by 19.8% and 13,1%, compared with the non-transplanted control and WTNSC groups (Figure 6A). No changes were observed in striatal infarct size among the four groups (data not shown). In addition, a significantly larger number of neurons was observed in the peri-infarct cortex in the TgNSC group than the non-transplanted control and WTNSC groups (Figure S4). We next analyzed behavioral performance using the rotarod test and mNSS. The mice that received TgNSCs showed significant functional improvement from Day 7 compared with the non-transplanted control group, and from Day 21 compared with the WTNSC group according to the rotarod test (Figure 6B). Although a statistical significance was not observed, the TgNSC group showed behavioral improvement as indicated by the mNSS (Figure 6C). Significant behavioral improvement was not observed in the WTNSC and KONSC groups at any time point according to either test, compared with the non-transplanted control group.
Figure 6.
Effects of NSCs on infarct size and behavioral performance. A, Measurement of the infarct size by H&E staining 28 days after stroke. Cortical infarct size was significantly decreased in the TgNSC group compared with the non-transplanted control and WTNSC groups (n=12). Behavioral performance using the rotarod test (B) and mNSS (C). Transplantation of the TgNSCs showed the greatest functional improvement in the rotarod test (n=12). Despite the tendency towards enhanced recovery, the TgNSC group did not show a significant behavioral improvement compared with the non-transplanted control and WTNSC groups according to the mNSS (n=12). Black bars denote non-transplanted control group; yellow bars denote WTNSC group; blue bars denote TgNSC group; red bars denote KONSC group. *P<0.05; †P<0.01, ‡P<0.005, §P<0.001.
Discussion
We have demonstrated in this study that SOD1 played a pivotal role in protecting NSCs from ischemic reperfusion injury. The major findings are: 1) SOD1 overexpression increased survival of NSCs after ischemic reperfusion injury by enhancing the antioxidant capacity, 2) downexpression of SOD1 accelerated oxidative stress-mediated NSC death, and 3) transplantation of TgNSCs attenuated infarct size and promoted functional recovery. These findings provide evidence that conferring antioxidant properties on grafted stem cells by genetically manipulating SOD1 expression enhances the effectiveness of stem cell therapy in ischemic stroke.
Although little information exists about the effects of ROS on NSCs, recent reports have shown that NSCs use ROS to regulate proliferation, self-renewal, and neurogenesis under physiological conditions.15 While it is controversial whether NSCs generally have lower or higher endogenous ROS levels than their differentiated progeny,15–17 these cells seem to possess an enhanced antioxidant capacity against oxidative stress compared with more differentiated cells because of the high expression of antioxidant enzymes, including uncoupling protein 2 and gluthathione peroxidase.16 This activity may be a protective mechanism in stem cell populations with active oxidant-mediated signaling to prevent an excess of or toxic levels of ROS from being generated. Regardless of these reports, we have found in this study that a large number of NSCs suffered oxidative stress-mediated death after ischemic reperfusion injury. In line with our data, excessive oxidative stress generated under pathophysiological conditions is reported to be toxic for survival of NSCs.18–21 These findings lead us to surmise that genetic modification of NSCs to overexpress antioxidant enzymes might be beneficial for promoting survival of these grafted cells.
Because the proliferation and differentiation capacity of the NSCs are highly dependent on endogenous ROS levels under physiological conditions, manipulating the expression of antioxidant enzymes may unexpectedly change their profiles.15,22,23 For example, conditional deletion of phosphatase and tensin homolog on chromosome 10 in nestin-expressing NSCs in the developing brain and in GFAP-expressing stem cells in the subventricular zone of the adult brain promoted and sustained NSC self-renewal and neurogenesis, contributing to brain overgrowth.24–26 In contrast, TgNSCs, as well as KONSCs, demonstrated similar proliferation and differentiation capacities in our study compared with WTNSCs. In addition, neither the SOD1 Tg nor the KO mice had any difference from the WT mice in phenotypes, including brain size.27 This discrepancy might be because the basal expression of antioxidant or prooxidant enzymes in TgNSCs and KONSCs is altered in association with SOD1 expression and compensates for the regulation of ROS levels. Further study is needed to clarify this important issue. In the present study, we were able to identify the cell type in less than half the grafted cells in vivo. However, because tumor formation was not observed even 3 months after transplantation indicates that the grafted cells did not transform into tumorigenic cells.
In this study, we focused on the cytoprotective effects of SOD1 in NSCs. First, SOD1 overexpression reduced ROS levels and increased NSC survival after ischemic reperfusion injury. This is consistent with our previous studies showing that SOD1 overexpression exhibited neuroprotection in rodent brains after transient focal cerebral ischemia and transient global cerebral ischemia.8–10,28,29 Second, SOD1 downexpression contributed to enhanced oxidative stress and increased NSC death. This agrees with our previous reports showing that a reduction in SOD1 activity led to larger infarct size and brain swelling after ischemic stroke.30,31 These findings indicate a strong relationship between SOD1 expression and NSC survival under pathophysiological conditions. Thus, we propose that genetic manipulation of SOD1 is a potential molecular target for stem cell therapy in ischemic stroke.
Despite many preclinical studies showing that cell transplantation can ameliorate ischemic stroke, the mechanisms mediating recovery are not well known. The majority of exogenous stem cells under investigation exert so-called “nursing functions” to the injured brain, such as cytoprotection or stimulation of endogenous repair mechanisms.1,32 Considering the reduced cortical infarct size and increased BVD in the ischemic brain, neuroprotection and angiogenesis might be among the main therapeutic actions of transplanted NSCs in the present study. Reduced grafted-cell death with SOD1 overexpression might contribute to the enhanced accumulation of neuroprotective trophic factors, including pro-angiogenic VEGF, that are secreted from NSCs.14 In this study, we performed sub-acute delivery of NSCs, which has more clinical relevance than acute delivery. However, sub-acute delivery might limit the neuroprotective action of the NSCs. We conclude that this is why the behavioral improvement observed in the present study was relatively small and occurred at a later time point.
Conclusions
We have shown the strong involvement of SOD1 expression in NSC survival after ischemic reperfusion injury. Our findings indicate that conferring antioxidant properties on NSCs by genetic manipulation of SOD1 is a potential approach for enhancing the effectiveness of cell transplantation therapy in ischemic stroke.
Supplementary Material
Acknowledgments
We thank Liza Reola and Bernard Calagui for technical assistance, Cheryl Christensen for editorial assistance, and Elizabeth Hoyte for assistance with the figures.
Sources of Funding
This work was supported by NIH grants PO1 NS014543, RO1 NS038653, and RO1 NS025372, and by the James R. Doty Endowment.
Footnotes
Disclosures
None.
References
- 1.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders — time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savitz SI, Chopp M, Deans R, Carmichael ST, Phinney D, Wechsler L. Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke. 2011;42:825–829. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- 3.Lindvall O, Kokaia Z. Stem cell research in stroke. How far from the clinic? Stroke. 2011;42:2369–2375. doi: 10.1161/STROKEAHA.110.599654. [DOI] [PubMed] [Google Scholar]
- 4.Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, et al. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185–2195. doi: 10.1002/stem.161. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 7.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 8.Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, et al. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, et al. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- 10.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller F-J, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim GS, Jung JE, Niizuma K, Chan PH. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci. 2009;29:14779–14789. doi: 10.1523/JNEUROSCI.4161-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madhavan L, Ourednik V, Ourednik J. Increased “vigilance” of antioxidant mechanisms in neural stem cells potentiates their capability to resist oxidative stress. Stem Cells. 2006;24:2110–2119. doi: 10.1634/stemcells.2006-0018. [DOI] [PubMed] [Google Scholar]
- 17.Limoli CL, Rola R, Giedzinski E, Mantha S, Huang T-T, Fike JR. Cell-density-dependent regulation of neural precursor cell function. Proc Natl Acad Sci U S A. 2004;101:16052–16057. doi: 10.1073/pnas.0407065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya MM, Lan ML, Kan VH, Patel NH, Giedzinski E, Tseng BP, et al. Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic Biol Med. 2010;49:1846–1855. doi: 10.1016/j.freeradbiomed.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Sahlgren CM, Pallari H-M, He T, Chou Y-H, Goldman RD, Eriksson JE. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25:4808–4819. doi: 10.1038/sj.emboj.7601366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee C-S, et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–193. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Paik J-h, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renault VM, Rafalski VA, Morgan AA, Salih DAM, Brett JO, Webb AE, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 27.Huang T-T, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann N Y Acad Sci. 1999;893:95–112. doi: 10.1111/j.1749-6632.1999.tb07820.x. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara T, Noshita N, Lewén A, Gasche Y, Ferrand-Drake M, Fujimura M, et al. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22:209–217. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito A, Hayashi T, Okuno S, Ferrand-Drake M, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic mice protects against neuronal cell death after transient focal ischemia by blocking activation of the Bad cell death signaling pathway. J Neurosci. 2003;23:1710–1718. doi: 10.1523/JNEUROSCI.23-05-01710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo T, Reaume AG, Huang T-T, Carlson E, Murakami K, Chen SF, et al. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawase M, Murakami K, Fujimura M, Morita-Fujimura Y, Gasche Y, Kondo T, et al. Exacerbation of delayed cell injury after transient global ischemia in mutant mice with CuZn superoxide dismutase deficiency. Stroke. 1999;30:1962–1968. doi: 10.1161/01.str.30.9.1962. [DOI] [PubMed] [Google Scholar]
- 32.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.