Abstract
Products of the COX reaction are frequently elevated in solid tumors and their roles in the malignant phenotype have been extensively investigated. We have shown that COX-2 is essential for the growth of MDA-MB-231 cells in the fat pad of SCID mice and for their extrapulmonary colonization following injection in the tail vein of SCID mice. The molecular changes that follow shRNA-mediated silencing of COX-2 include a significant downregulation of LEF-1, a transcription factor normally activated during development following the Wnt-induced nuclear translocation of β-catenin. We also report that COX-2-silenced cells have reduced nuclear accumulation of LEF-1 protein and that the COX-2 product PGE2 partially restored nuclear LEF-1 expression in COX-2-silenced cells. Further, we demonstrate that, like parental COX-2 containing MDAMB-231 cells, COX-2-silenced cells maintain nuclear localization of β-catenin.
Keywords: Cyclooxygenase-2, Prostaglandin E2, Lymphoid Enhancer Factor-1, β-catenin, Wnt, breast cancer
Introduction
Recent efforts to understand the initiation and progression of breast cancer have focused on the etiology and growth of breast tumors, the interaction of growing epithelial cells with their microenvironment and the steps leading to local and distant colonization of other organs of the human body. Analysis of the molecular pathways promoting breast cancer indicates a wide heterogeneity of mechanisms underlying the initiation, growth and metastasis of tumors [1]. In the last several decades breast tumors have been divided into tumors that even at a small percentage express the estrogen receptor (ER) and those that do not. ER- tumors have generally worse prognosis than ER+ tumors [2]. We have previously demonstrated that the ER/PR/HER2 negative MDA-MB-231 cells displayed delayed tumor onset, reduced frequency of tumor formation following inoculation, and loss of their ability for extrapulmonary colonization when transfected stably with a potent and specific cyclooxygenase (COX)-2 shRNA sequence [3]. We attributed the loss of tumor growth and extrapulmonary colonization to reduced invasive capabilities [3], reduced angiogenesis, altered metabolism [4] consistent with a less aggressive phenotype [3], and differences in the stabilization of hypoxia-inducible factor-1α [5]. These changes were related to the loss of expression of a host of oncogenes, receptors and degradative enzymes associated with tumor growth and metastasis including matrix metalloproteinase-1, CXC receptor-4, roundabout-1, hyaluronan synthase-2, and monocarboxylate transporter 2 [3, 4]. In addition to previously reported transcriptome changes, we observed that the most highly down-regulated transcription factor was lymphoid-enhancer factor (LEF) -1.
LEF-1 is a member of the T-cell specific factor (TCF) family of transcription factors that binds to the promoter of several genes bearing the conserved sequence CTTTGT [6], and promotes oncogenic signaling in breast and other cancers [7-9]. More recently, DNA occupancy analysis identified TCF4, another member of the TCF family of transcription factors, as bound to the A[C/G][A/T]TCAAAG conserved sequence in human colorectal cancer cells [10]. Transcriptional targets of the wingless integration 1 (Wnt) pathway include oncogenes such as Myc and Cyclin D1 [11-14]. The intracellular activating cascade of the Wnt pathway is dependent on the direct interaction of β-catenin with a nuclear complex of core proteins including TCF family members, legless (known as BCL9 in humans) and pygopus [15, 16]. This binding event is preceded by the stabilization and localization of cytoplasmic β-catenin to the nucleus [17]. Following signaling directing the phosphorylation, stabilization and nuclear transport, β-catenin directly displaces the inhibitors Groucho/TLE from the TCF factor nuclear complex [18]. To activate chromatin, TCF factors associate with CBP/p300 proteins, which contain histone acetyltransferase activity [19, 20] that permits the direct binding of DNA by the high mobility group of the β-catenin-TCF nuclear complex. In the absence of stimulation by Wnt or other non-canonical ligands β-catenin is targeted for degradation. β-catenin targeted for degradation is bound by a complex of proteins that include the scaffold protein Axin, the tumor suppressor gene adenomatous polyposis coli product APC, glycogen synthase kinase (GSK) 3, and casein kinase (CK) 1, which control its phosphorylation and stability [21]. It has been proposed that canonical Wnt ligand-receptor interactions result in the phosphorylation and release of β-catenin from the Axin/GSK3/CK1 complex during development.
Activation of the Wnt pathway has been linked to carcinogenesis, notably in familial adenomatous polyposis (FAP) where a mutation in the β-catenin interacting protein APC is thought to contribute to the increase of the levels of cytosolic β-catenin in early adenomas [22]. However, inactivation of APC was not sufficient for nuclear localization of β-catenin, an event seen only in later stages of carcinogenesis of patients with FAP [22]. Subsequently, it was shown that the COX-2 reaction product prostaglandin (PG) E2 could drive the translocation of β-catenin to the nucleus of colon cancer cells [23]. This observation was particularly significant because COX-2 and PGE2 are overexpressed in colorectal cancer and because celecoxib, a selective COX-2 inhibitor, has shown efficacy in patients with FAP and is currently prescribed to patients diagnosed with FAP [24, 25]. More recently PGE2 was shown to promote the phosphorylation of β-catenin on Serine 645 in hematopoietic stem cells [26], an event associated with β-catenin stabilization [27]. Our results demonstrate that while PGE2 is not necessary for the nuclear localization of β-catenin in MDA-MB-231 cells, it can promote the nuclear accumulation of the TCF family member LEF-1, further promoting the non-canonical activation of the Wnt pathway in cancer.
Materials and methods
Silencing of COX-2 in MDA-MB-231 cells
Silencing of COX-2 in MDA-MB-231 cells has been described before [3]. In summary, MDA-MB-231 cells (ATCC, Manassas, VA) were transfected with a vector bearing an shRNA sequence that was identified in-house to specifically down-regulate COX-2 in these cells that were selected with G418 (Invitrogen, Carlsbad, California). Individual clones were isolated using standard protocols. Cells were tested for the presence of COX-2 mRNA by microarray and qPCR experiments, for COX-2 protein by immunoblotting, and for the presence of the COX-2 product PGE2 in the supernatant of cultured media using an immunoassay. Since COX-2 is an inducible gene, we verified that its expression was silenced in the selected clones using the naturally occurring pro-inflammatory cytokine IL-1β and the potent carcinogen 12-O-tetradecanoylphorbol-13-acetate (TPA). TPA is a potent inducer of COX-2 and its carcinogenic effects on the skin have been related to its proinflammatory properties [28, 29].
Microarray experiment
The microarray experiment showing the expression of LEF-1 in uninduced COX-2-silenced cells has been described in [4]. All samples were run in commercial arrays from Affymetrix (Santa Clara, CA), using Human Genome U133Plus 2.0 GeneChip arrays as described in the Affymetrix website.
Quantitative PCR and analysis
Cells were seeded (1.4 × 105) in 6-well plates and allowed to attach to overnight. Cells were induced with TPA (100 nM) for 24 h. RNA was purified using the RNeasy kit (Qiagen, Valencia, CA) as described by the manufacturer and the on-column DNase I digestion done as described by the manufacturer. One hundred nanograms of total RNA were subjected to One-Step Real-time RT-PCR using SYBR Green (Qiagen) in a Bio-Rad iCycler as described by the manufacturer. The primer for LEF-1 was obtained from Qiagen, Cat# QT00021133. Fold differences were calculated using the 2^ΔΔCt method using an HPRT primer for loading control.
PGE2 enzyme immunoassays
Cells were seeded (1.4 × 105) in 6-well plates and allowed to attach overnight. Cells were induced with IL-1β (10 ng/ml) for 24 h. Supernatant was isolated, diluted 1:10 in enzyme immunoassay buffer and assayed for PGE2 expression using a competitive immunoassay detection kit (cat# 514010) as described by the manufacturer (CaymanChem, Ann Arbor, MI).
LEF-1 immunobloting
Parental COX-2 containing MDA-MB-231 and derivative COX-2-silenced Clone 2 cells (1.7×106) were seeded in 100 mm dishes in RPMI 1640 medium containing 8.25% FBS and treated with TPA at a final concentration of 100 nM for 24 h. Cells were washed twice with 1xPBS and the cells scraped from the plates and lysed with M-PER for total cell extracts and NE-PER for nuclear cell extracts (ThermoFisher, Rockford, IL) containing protease inhibitors (SigmaAldrich, St. Louis, MO) for a minimum of 30 minutes. Total cell lysate was separated from membrane components by centrifugation, and the protein content was estimated using the QuickStart Protein Assay (BioRad, Hercules, CA). Nuclear lysate was prepared according the manufacturer's (Thermo-Fisher, Rockford, IL) protocol for the cytoplasmic and nuclear extraction reagents (NE-PER kit). Total cell lysate (50 μg) and nuclear cell extracts (10 μg) were subjected to SDS-PAGE and western blotting on supported nitrocellulose (BioRad) using standard protocols and probed with a goat COX-2 polyclonal antibody (CaymanChem, Ann Arbor, MI), rabbit LEF-1 monoclonal and Histone H3 polyclonal antibodies (Cell Signaling Technologies, Danvers, MA), and a GAPDH mouse monoclonal antibody (SigmaAldrich, St Louis, MO). HRP-labeled goat (Novus Biologicals, Littleton, CO), mouse, and rabbit (GE Healthcare, Little Chalfont, Buckinghamshire, UK) secondary antibodies were added at a concentration of 1:3000. Blotted proteins were visualized using WestPico chemilluminescence reagent (ThermoFisher, Rockford, IL). Densitometric evaluation of the immunoblots shown in Figures 1 and 2 was performed using the ImageJ freeware obtained from the NIH.
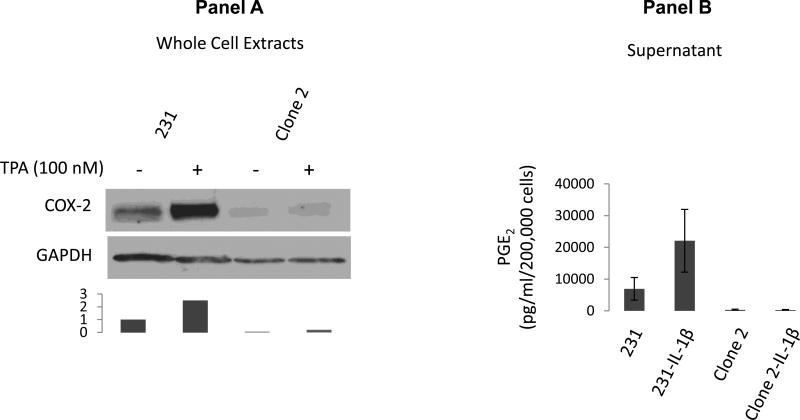
Figure 1. COX-2-silenced cells do not secrete PGE2.
Panel A: Clone 2 cells are silenced for COX-2 expression even in the presence of the phorbol ester TPA; Densitometric evaluation of the expression of COX-2 levels normalized to the loading control is shown. Levels of COX-2 in untreated parental cells are assigned a value of 1. Panel B: PGE2 is not secreted by COX-2-silenced cells even following induction by the pro-inflammatory cytokine IL-1β.
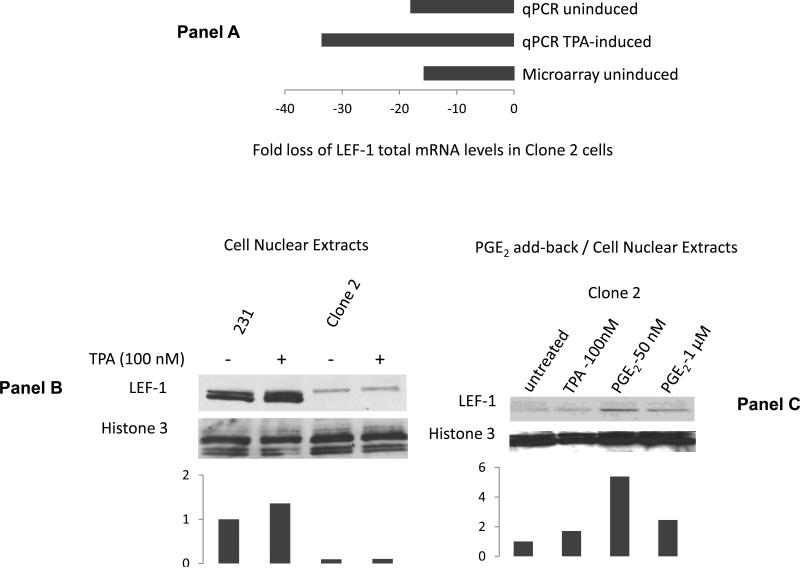
Figure 2. PGE2 promotes the nuclear accumulation of LEF-1.
Panel A. COX-2-silenced cells have reduced levels mRNA levels of LEF-1. Panel B: TPA induces the accumulation of LEF-1 in nuclear cell extracts of COX-2 containing MDA-MB-231 cells, but not in COX-2-silenced derivative Clone 2 cells. Levels of LEF-1 in untreated parental cells are assigned a value of 1. Panel C: Exogenous PGE2 addition induces the accumulation of LEF-1 in nuclear extracts of COX-2-silenced Clone 2 cells. Densitometric evaluation of the expression of LEF-1 levels normalized to the nuclear loading control (Histone 3) is shown for each immunoblot. Levels of LEF-1 in untreated parental cells are assigned a value of 1.
β-catenin immunostaining
Parental COX-2 containing MDA-MB-231 and derivative COX-2-silenced Clone 2 cells were grown in sterile 4-well glass Lab-Tek chamber slides with cover (Nalge Nunc International, Rochester, NY) to 70% confluence. Cells were washed twice with ice-cold phosphate buffered saline (PBS), fixed with 3% paraformaldehyde in PBS for 30 minutes at room temperature, washed again three times with PBS, and incubated with 5% normal donkey serum in antibody dilution buffer (0.5% bovine serum albumin, 0.01% sodium azide, and 0.05% saponin in R/O water) for 30 minutes at room temperature. Cells were incubated with a 1:100 dilution of purified β-catenin mouse monoclonal antibody from BD Biosciences (San Diego, CA) in AB-dilution buffer for 1 hour at room temperature, followed by three washes with AB-dilution buffer. Then, cells were incubated with 1:50 dilution of Cy3-labeled donkey anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature and washed three times with AB-dilution buffer. Cell nuclei were counterstained with a 1:3000 dilution of a 10 mg/ml stock solution of Hoechst H-33342 (Molecular Probes, Eugene, OR) for 10 minutes at room temperature. Cells were washed and a cover glass was attached with Faramount Aqueous Mounting Medium (Dako North America, Inc., Carpinteria, CA). Fluorescence microscopy was performed on a Zeiss LSM 510 Meta laser scanning fluorescence microscope (Zeiss, Göttingen, Germany) using a 63X/1.4 NA Plan-Apochromat oil immersion lens available in the Johns Hopkins University School of Medicine Microscope Facility. Cy3 was excited with a 543-nm laser and fluorescence emission was detected with a photomultiplier using a 560-nm longpass filter. Simultaneously, H-33342 was excited with 405-nm laser, and the fluorescence emission was detected with a second photomultiplier by applying a 545-nm dichroic beam splitter and a 435- to 485-nm bandpass filter. Confocal z sections of 1 μm thickness containing the nucleus were imaged. Cy3 fluorescence was assigned red, H-33342 fluorescence was assigned blue, and both images were superimposed.
Results
LEF-1 expression is reduced in nuclear extracts of COX-2-silenced cells
Silencing of COX-2 was validated at the protein level as shown in Fig. 1, panel A. An enzyme immunoassay for COX-2 product PGE2 showed significant levels of PGE2 present in the media of cultured cells 24 hours following induction with IL-1β (10 ng/ml) while levels of PGE2 in COX-2-silenced Clone 2 cells were very similar to media alone with or without induction of COX-2 (Fig.1, panel B). Similar results have been obtained with the phorbol ester TPA, which we also use to induce COX-2 in our experiments. Our qPCR primers cannot detect a single COX-2 peak in a qPCR melting curve even following induction with IL-1β or TPA (not shown). Since COX-2 protein levels appear extremely low (Fig.1, panel A), and since PGE2 levels are below the limit of detection (Fig. 1, panel B) we consider these cells silenced for the expression of COX-2.
Microarray analysis comparing the transcriptome of parental COX-2 containing MDAMB-231 and derivative COX-2-silenced cells showed LEF-1 as one of the most highly down-regulated transcription factors in COX-2-silenced cells (Fig. 2, panel A). qPCR analysis verified a significant down-regulation of the LEF-1 transcript (Fig. 2, panel A). The expression of LEF-1 protein in COX-2-silenced cells was determined from immunoblotting of nuclear extracts from COX-2 containing parental MDA-MB-231 and COX-2-silenced derivative Clone 2 cells, which showed reduced accumulation of LEF-1 protein in COX-2-silenced cells (Fig. 2, panel B). Further, LEF-1 levels could only be induced by the COX-2-inducing phorbol ester TPA in COX-2 containing, but not in COX-2-silenced cells. Supplementation of COX-2-silenced cells with physiologically relevant PGE2 levels induced the nuclear expression of LEF-1 in COX-2-silenced cells (Fig. 2 panel C) suggesting that a significant amount of LEF-1 expression can be attributed to signaling from the COX-2-catalyzed lipid mediator PGE2 in these cells. We did not examine the supplementation of COX-2-silenced cells with other products of the cyclooxygenase reaction because enzyme immunoassays specific for thromboxane B2 and PGI2 (prostacyclin) did not reveal detectable concentrations of these secondary metabolites in the media of COX-2-containing MDA-MB-231 cells even when induced by IL-1β or TPA. In contrast, PGE2 was present in significant quantities particularly when induced by IL-1β (Fig. 1, panel B) or TPA (not shown).
COX-2-silenced cells maintain the nuclear localization of β-catenin
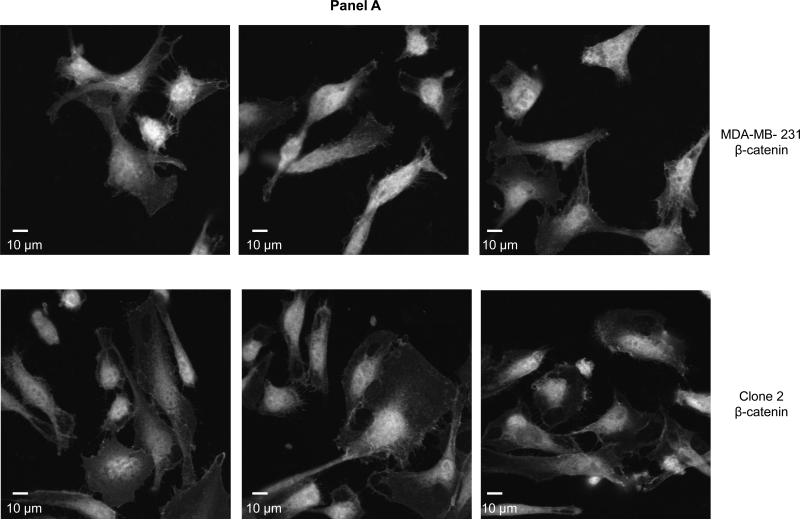
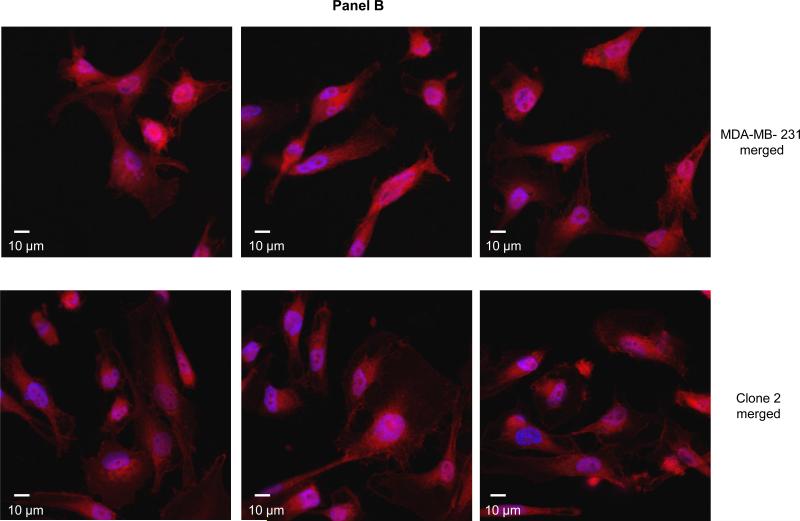
Earlier evidence suggested that COX-2/PGE2 signaled the transcription of the Wnt pathway target genes by promoting the nuclear translocation of β-catenin [23] or its phosphorylation at Serine 645 and subsequent stabilization [26]. To determine whether COX-2 silencing abolished the localization of β-catenin in MDA-MB-231 cells we performed immunofluorescence microscopy in COX-2 containing and COX-2-silenced cells (Fig. 3, panels A and B). In our model, β-catenin is maintained in the nucleus of both parental COX-2 containing and derivative COX-2-silenced breast cancer cells to a similar extent, suggesting that COX-2/PGE2 do not significantly alter the nuclear localization of β-catenin in these cells.
Figure 3. β-catenin is localized in the nucleus of COX-2 containing parental and COX-2-silenced Clone 2 cells.
Panel A: Representative confocal fluorescence microscopy images of β-catenin. Panel B: Superimposed β-catenin (red) and nuclei (blue) stained COX-2 containing (top images) and COX-2-silenced (bottom images). Objective: 63×.
Discussion
The derivation of COX-2-silenced poorly differentiated breast cancer cells has allowed us to demonstrate that COX-2 is necessary for orthotopic tumor growth and extrapulmonary colonization of aggressive, poorly differentiated breast cancer cells [3]. Here, we report that the COX-2 product PGE2 can promote the nuclear accumulation of the Wnt pathway transcription factor LEF-1. This effect is likely mediated through de novo LEF-1 transcription since silencing of COX-2 reduced LEF-1 transcript levels in microarray and qPCR experiments. Two important studies have so far shown that PGE2 regulates the localization of β-catenin by either directing its translocation to the nucleus in colon cancer cells [23] or by promoting its phosphorylation and stabilization in hematopoietic embryonic stem cells [26]. However, the widely used MDA-MB-231 breast cancer cells have β-catenin already present in their nucleus, even in COX-2-silenced cells (Fig. 2) that no longer secrete PGE2 suggesting that PGE2 is not required for the nuclear accumulation of β-catenin in these cells. It has previously been reported that β-catenin may be stabilized by mutations in several colon cancer samples, however the frequency of occurrence of these mutations in breast cancers is low [7]. While the persistence of β-catenin in the nucleus of breast cancer cells has not been widely studied and may be context-specific, we propose that COX-2-derived PGE2 can promote Wnt pathway target gene expression by increasing the nuclear accumulation of the β-catenin nuclear partner LEF-1. The clinical relevance of elevated LEF-1 levels in triple negative or other breast cancers was investigated in a small set of infiltrating ductal carcinomas of the breast where a strong negative correlation was found with the expression of HER2 [30]. In the same study a strong correlation between the expression of LEF-1 and increased invasiveness was described. While absent from adult epithelial cells of the colon, LEF-1 has been shown to be overexpressed in colon cancer [31]. LEF-1 was independently demonstrated to promote increased invasion of endothelial cells [32] consistent with the previously described role in angiogenesis in pathological and physiological processes [33]. Our results suggest that targeting COX-2 in cancer cells expressing stable nuclear β-catenin may alleviate the oncogenic signaling promoted by the Wnt pathway by reducing the levels of the transcription factor LEF-1.
Highlights.
◇ COX-2-silencing reduces the nuclear accumulation of LEF-1 and the effect is partially restored by addition of PGE2.
◇ PGE2 is not required for the nuclear accumulation of β-catenin.
◇ Noncanonical activation of the Wnt pathway in cancer cells may be context-specific.
Acknowledgments
Grant information: This work was supported by NIH research grants R01CA82337 and P50 CA103175.
Abbreviations
- APC
adenomatous polyposis coli
- CK
casein kinase
- COX
cyclooxygenase
- FAP
familial adenomatous polyposis
- FBS
Fetal Bovine Serum
- GSK
glycogen synthase kinase
- IL
interleukin
- LEF
lymphoid enhancer factor
- PG
prostaglandin
- PBS
phosphate buffer saline
- shRNA
short hairpin RNA
- TCF
T-cell factor
- TPA
12-O-tetradecanoylphorbol-13-acetate
- Wnt
wingless integration-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011;378(9805):1812–23. doi: 10.1016/S0140-6736(11)61539-0. [DOI] [PubMed] [Google Scholar]
- 2.Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121(10):3797–803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stasinopoulos I, et al. Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol Cancer Res. 2007;5(5):435–42. doi: 10.1158/1541-7786.MCR-07-0010. [DOI] [PubMed] [Google Scholar]
- 4.Stasinopoulos I, Mori N, Bhujwalla ZM. The malignant phenotype of breast cancer cells is reduced by COX-2 silencing. Neoplasia. 2008;10(11):1163–9. doi: 10.1593/neo.08568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stasinopoulos I, O'Brien DR, Bhujwalla ZM. Inflammation, but not hypoxia, mediated HIF-1alpha activation depends on COX-2. Cancer Biol Ther. 2009;8(1):31–5. doi: 10.4161/cbt.8.1.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X, et al. Systematic discovery of regulatory motifs in human promoters and 3' UTRs by comparison of several mammals. Nature. 2005;434(7031):338–45. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebeshuber CA, Sladecek S, Grunert S. Beta-catenin/LEF-1 signalling in breast cancer--central players activated by a plethora of inputs. Cells Tissues Organs. 2007;185(1-3):51–60. doi: 10.1159/000101303. [DOI] [PubMed] [Google Scholar]
- 8.Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25(57):7492–504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- 9.Ravindranath A, et al. The role of LEF/TCF factors in neoplastic transformation. Curr Mol Med. 2008;8(1):38–50. doi: 10.2174/156652408783565559. [DOI] [PubMed] [Google Scholar]
- 10.Hatzis P, et al. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol. 2008;28(8):2732–44. doi: 10.1128/MCB.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 12.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 13.Sansom OJ, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446(7136):676–9. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 14.Yochum GS, Cleland R, Goodman RH. A genome-wide screen for beta-catenin binding sites identifies a downstream enhancer element that controls c-Myc gene expression. Mol Cell Biol. 2008;28(24):7368–79. doi: 10.1128/MCB.00744-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramps T, et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109(1):47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 16.Thompson B, et al. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4(5):367–73. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- 17.Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol. 2009;1(2):a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12(4):364–71. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 19.Hecht A, et al. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19(8):1839–50. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tutter AV, Fryer CJ, Jones KA. Chromatin-specific regulation of LEF-1-beta-catenin transcription activation and inhibition in vitro. Genes Dev. 2001;15(24):3342–54. doi: 10.1101/gad.946501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 22.Blaker H, et al. Somatic mutations in familial adenomatous polyps. Nuclear translocation of beta-catenin requires more than biallelic APC inactivation. Am J Clin Pathol. 2003;120(3):418–23. doi: 10.1309/4E4W-G3AY-GJNC-D11P. [DOI] [PubMed] [Google Scholar]
- 23.Castellone MD, et al. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310(5753):1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 24.Rostom A, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146(5):376–89. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 25.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 26.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–47. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hino S, et al. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25(20):9063–72. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller MM. Inflammation in epithelial skin tumours: old stories and new ideas. Eur J Cancer. 2006;42(6):735–44. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Sung YM, He G, Fischer SM. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65(20):9304–11. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen A, et al. Wnt pathway component LEF1 mediates tumor cell invasion and is expressed in human and murine breast cancers lacking ErbB2 (her-2/neu) overexpression. Int J Oncol. 2005;27(4):949–56. [PubMed] [Google Scholar]
- 31.Hovanes K, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28(1):53–7. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 32.Planutiene M, Planutis K, Holcombe RF. Lymphoid enhancer-binding factor 1, a representative of vertebrate-specific Lef1/Tcf1 sub-family, is a Wnt-beta-catenin pathway target gene in human endothelial cells which regulates matrix metalloproteinase-2 expression and promotes endothelial cell invasion. Vasc Cell. 2011;3:28. doi: 10.1186/2045-824X-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107(8):943–52. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]