Abstract
Objective
To determine the prevalence of ITP in Oklahoma regardless of age, clinical characteristics, insurance status, and source of health care.
Study Design and Setting
Patients with ITP were identified by the administrative code ICD-9-CM 287.3 in Oklahoma hematologists’ offices for a 2-year period, 2003–2004. Prevalence was estimated separately for children (<16 years old) and adults because of their distinct clinical characteristics. Oklahoma census data for 2000 was used as the denominator.
Results
Eighty-seven (94%) of 93 eligible Oklahoma hematologists participated; 620 patients with ITP were identified. The average annual prevalences were: 8.1 (95% CI 6.7, 9.5) per 100,000 children, 12.1 (95% CI 11.1, 13.0) per 100,000 adults, and 11.2 (95% CI 10.4, 12.0) per 100,000 population. Among children and adults less than age 70 years, the prevalence was greater among women. Among adults aged 70 years and older, the prevalence was greater among men. The highest prevalence of ITP was among men age 80 years and older.
Conclusion
These data document for the first time the prevalence of ITP regardless of age, clinical characteristics, insurance status, and source of health care. The methodology developed for this prevalence analysis may be adaptable for epidemiologic studies of other uncommon disorders which lack specific diagnostic criteria and are treated primarily by medical specialists.
Keywords: primary immune thrombocytopenia, ITP, prevalence, demographics, administrative data, ICD-9-CM 287.3
INTRODUCTION
Primary immune thrombocytopenia (ITP) is an uncommon acquired autoimmune disorder characterized by isolated thrombocytopenia without an apparent alternative etiology.(1–3) Although ITP rarely causes death from bleeding,(4;5) the risks for bleeding,(6) side effects from treatments,(7) and symptoms of fatigue(8) can substantially affect the lives of patients with ITP and their families.(6) Although the effect of ITP on the quality-of-life of individual patients has been documented,(6;9–11) understanding the population burden of ITP requires knowledge of its prevalence. Knowledge of the prevalence of ITP is also important for the development of new treatments, as emphasized by recent pharmaceutical-supported studies of the epidemiology of ITP.(12–14)
The annual incidence of ITP is between 1.9 and 6.4 per 100,000 children and 3.3 per 100,000 adults.(15) Because ITP is a chronic persistent disorder in some children and most adults, the prevalence is expected to exceed the annual incidence. However the prevalence of ITP has only been reported in four studies of four different patient groups.(14;16–18) None of these four studies identified patients with ITP of all ages regardless of their clinical characteristics, insurance status, and their source of health care. Therefore the objective of this study was to determine the prevalence of ITP in the State of Oklahoma regardless of age, clinical characteristics, insurance status, or source of health care.
METHODS
Identification of hematologists
Hematologists in Oklahoma were identified by searching the Oklahoma Board of Medical Licensure and the American Osteopathic Association websites for physicians who listed hematology, oncology, hematology-oncology, and pediatric hematology-oncology as a specialty. Hematologists were included if they had an active Oklahoma license and were currently treating hematology patients in Oklahoma. Current practice was determined by contacting the hematologists’ offices.
Identification of patients with ITP
In our previous study, we developed a definition for a “definite diagnosis of ITP” using administrative data and we identified children and adults who had a definite diagnosis of ITP by a hematologist as an outpatient at the Oklahoma University Medical Center (OUMC) between 1995 and 2004.(19) In the current study, all other hematologists in Oklahoma were surveyed. Data were requested from the hematologists’ office billing managers for all visits of all patients billed with the ICD-9-CM code of 287.3 in the first, second, or third position between 1995 and 2004. If the name of the office or clinic had ‘hospital’ as part of its name, the billing manager confirmed the patients were seen in an outpatient clinic and submitted only outpatient data. Data from the Oklahoma City and Muskogee Veterans Administration (VA) Hospitals were also restricted to billing for outpatient visits to a hematology clinic. Patients in the Oklahoma City VA Hospital hematology clinic were not included in our previous OUMC analysis(19) but were seen by OUMC hematologists; patients in the Muskogee VA Hospital were seen by their medical staff hematologists. No other VA clinics in Oklahoma have hematologists on their staff. The Indian Health Service was contacted to confirm that none of their clinics were practice sites for any of the state’s hematologists. The last four digits of the social security number, date of birth, and gender were requested to identify individual patients, to prevent duplicate counting of patients who had received care by multiple hematology practice groups. Due to confidentiality, some hematology offices would not release the last four digits of the social security number. As a result, exact matching to identify individual patients was performed using gender and date of birth. Patients’ city and state was also requested and patients were excluded if they were not residents of the state of Oklahoma at the time of service.
Definitions
Time period
Since ITP is an uncommon disorder,(15) a period of 10 years, 1995–2004, was initially selected to identify a sufficient number of patients, some of whom may see a hematologist infrequently or only for their initial evaluation. The 10-year period was also used in case complete data for all years were not available for all hematologists’ offices. Therefore it was assumed that there would be some years within this period when data would be available for all or most hematologists in Oklahoma. Period prevalence estimates were calculated for the 10-year period, the five-year period, and the two-year period. The decision for which time period may provide the best estimate of prevalence was based on [1] the precision of the estimates assessed by the width of the 95% confidence intervals and [2] the completeness of the data obtained assessed by the number of hematologists and number of patients included. Ultimately the two-year period (2003–2004) was reported.
Age stratification
Children and adults were analyzed separately because of their distinct clinical features that suggest a different pathogenesis.(1–3) Most children have acute ITP with a sudden onset of severe, symptomatic thrombocytopenia which spontaneously resolves within six to 12 months.(11;20) In contrast, most adults have chronic ITP with an insidious onset of moderate to severe thrombocytopenia which rarely resolves spontaneously.(1–5) Throughout adolescence, chronic ITP becomes more common.(21) Therefore the age defining childhood was set as less than 16 years, the age commonly used in studies of the incidence of ITP.(15) There was no minimum age criterion.
Prevalence estimation
Census year 2000 population data, the midpoint of this analysis period, were used as the denominator for all prevalence calculations. The total population of Oklahoma in 2000 was 3,450,654; for children age less than 16 years, 784,688; for adults 16 years and older, 2,665,966.(22)
Some hematology offices could not retrieve billing information for the entire 10-year period. Due to differences in completeness of the data received, prevalence was estimated for three different time periods: 10-year (initial service dates 1995–2004), five-year (initial service dates 2000–2004), and two-year (initial service dates 2003–2004). Prevalence estimates were calculated for all patients and also separately for children and adults. Age was calculated for each patient at each service date. If a patient was less than 16 years old at the time of the initial service date, he/she was included in the prevalence estimate for children. If during the time period the patient’s age was initially less than 16 years and then greater than or equal to 16 years, two service dates were documented, the initial service date at age less than 16 years and the initial service date after age greater than or equal to 16 years. Therefore some patients were included in analyses of both children and adults if they were seen before and after their sixteenth birthday. However, in the combined prevalence estimates of children and adults, each patient was counted only once. The Poisson asymptotic 95% confidence interval (CI) was calculated and reported.
The 95% confidence intervals were calculated based on a Poisson distribution and the standard error of a Poisson distribution is based on the square root of the numerator. Since more patients were observed over the longer time periods of five and 10 years and since the denominator per year remained the same with each calculation, confidence interval were wider with the longer periods of observation. After the best period prevalence estimate was determined, one-year prevalence estimates were used to calculate an annual average prevalence. Sensitivity analyses to adjust the observed prevalence estimates for the effects of potential limitations of the methods used to identify patients with ITP were performed. Sensitivity analyses adjusted for the following assumptions:(19) [1] that the administrative code ICD-9-CM 287.3 was sufficiently accurate (as determined by calculation of the positive predictive value [PPV] of the code) to identify both children and adults with ITP, [2] that most patients with ITP were diagnosed and/or managed by hematologists, and [3] that most patients with ITP were diagnosed and/or managed as outpatients. Data were stored in a password protected Microsoft Access® database. Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Ethics approvals
This study was approved by the Institutional Review Board (IRB) of the University of Oklahoma Health Sciences Center (OUHSC). Additional approval from the Oklahoma City VA Research and Development Committee was obtained to review data for patients at both the Oklahoma City and Muskogee VA hospitals. Site-specific IRB approval was obtained from two large hematology-oncology offices. For all other hematology offices, OUHSC IRB approval was sufficient.
RESULTS
Identification of hematologists
Ninety-eight hematologists were identified in Oklahoma; five were excluded (one whose office was outside of Oklahoma, three who only treated general internal medicine patients, and one who only treated oncology patients). Therefore 93 hematologists were eligible to participate in this study. Of the 93 eligible hematologists, data were obtained from 88 (95%); five hematologists had no retrievable data from 1995–2004 because of new billing systems established after 2004. Table 1 presents the data from the 15 practice groups for the 88 hematologists who contributed to this analysis.
Table 1.
Oklahoma hematology practice groups and their patients with ITP
| Hematologist groups | Individual hematologists | Patients eligible for analysis | Years of Data | ||
|---|---|---|---|---|---|
| Children | Adults | Total | |||
| 1 (OUMC) | 23 | 220 | 120 | 340 | 1995–2004 |
| 2 (OKC VA*) | 0 | 44 | 44 | 1998–2004 | |
| 3 | 32 | 2 | 348 | 350 | 2000–2004 |
| 4 | 9 | 1 | 120 | 121 | 1995–2004 |
| 5 | 6 | 0 | 6 | 6 | 1997–2004 |
| 6 | 4 | 0 | 40 | 40 | 2003–2004 |
| 7 | 3 | 0 | 71 | 71 | 2003–2004 |
| 8 | 2 | 122 | 16 | 138 | 1999–2004 |
| 9 | 2 | 0 | 8 | 8 | 1996–2004 |
| 10 | 2 | 0 | 11 | 11 | 1996–2004 |
| 11 | 1 | 0 | 3 | 3 | 2003–2004 |
| 12 | 1 | 1 | 0 | 1 | 2001 |
| 13 | 1 | 0 | 10 | 10 | 2000–2004 |
| 14 | 1 | 2 | 35 | 37 | 1995–2004 |
| 15 | 1 | 0 | 12 | 12 | 1999–2004 |
| Total | 88 | 348 | 844 | 1192 | |
| Total individual patients** | 342 | 817 | 1147 | ||
Oklahoma City VA Hospital is staffed by OUMC hematologists
Total individual patients after excluding duplicate patients reported by more than one site. Some patients were included in both the children and adults estimates because they were seen both before and after their sixteenth birthday; these patients were only counted once in the overall prevalence estimates. Therefore the total number is 1,147 rather than 1,159.
In addition to the 93 eligible hematologists, 41 additional hematologists were identified who had practiced in Oklahoma at some time during 1995–2004 but who were no longer active during the time this study was performed, 2007–2008. Nine of these 41 hematologists did not see patients during 1995–2004; 22 were associated with one of the groups participating in this study and it was assumed that their patients would have continued their care within this group and those records were obtained. The remaining 10 hematologists may have had patients who were not included in the prevalence estimates.
Prevalence estimates
The 10-year period prevalence estimate included 342 children and 817 adults. The numerator for the combined 10-year period prevalence for all patients was 1,147 after excluding 12 children who aged into the adult category during these 10 years. The five-year period prevalence estimate included 215 children and 716 adults. The numerator for the combined five-year period prevalence for all patients was 923 after excluding eight children who aged into the adult category during these five years. The two-year period prevalence estimate included 107 children and 515 adults. The numerator for the combined two-year period prevalence for all patients was 620 after excluding two children who aged into the adult category during these two years.
The two-year period prevalence estimates were determined to be better estimates than estimates from 10-year or five-year data, based on better precision assessed by the 95% confidence intervals (Table 2) and because all but one of the 88 participating hematologists could provide data for 2003–2004 (Table 1). For adults, hematology groups 6 and 7 were only able to provide data for these two years and they contributed a large number of patients. The two-year period prevalence estimates were used to calculate annual prevalence estimates. The numerators for annual prevalence estimates for children were 69 for 2003 and 58 for 2004; for adults were 309 for 2003 and 335 for 2004. The average annual prevalence estimates for children, adults and all patients are presented in Table 3.
Table 2.
Comparison of ITP period prevalence estimates by age categories and years of data*
| Patients | 10-year period prevalence 1995–2004 | 5-year period prevalence 2000–2004 | 2-year period prevalence 2003–2004 | Average annual prevalence** |
|---|---|---|---|---|
| Children <16 years | 43.6 (39.0, 48.2) | 27.4 (23.7, 31.1) | 13.6 (11.1, 16.2) | 8.1 (6.7, 9.5) |
| Adults ≥16 years | 30.6 (28.5, 32.7) | 26.9 (24.9, 28.8) | 19.3 (17.6, 21.0) | 12.1 (11.1, 13.0) |
| All Patients | 33.2 (31.3, 35.2) | 26.7 (25.0, 28.5) | 18.0 (16.6, 19.4) | 11.2 (10.4, 12.0) |
Prevalence estimates, with 95% confidence intervals in parentheses, are per 100,000 children for children, per 100,000 adults for adults, and per 100,000 population for all patients.
Average annual prevalence was calculated from the 2003 and 2004 one-year ITP prevalence estimates.
Table 3.
Sensitivity analyses to adjust average annual prevalence estimates for methodology assumptions*
| Methodology assumptions | Prevalence estimates | |
|---|---|---|
| Average annual prevalence estimate per 100,000 children | Average annual prevalence estimate per 100,000 adults | |
| Observed prevalence estimate | 8.1 (95% CI 6.7, 9.5) | 12.1 (95% CI 11.1, 13.0) |
| Independently adjusted for PPV | 6.5 | 8.9 |
| Independently adjusted for exclusion of patients diagnosed and managed only by non-hematologists | 8.3 | 13.1 |
| Independently adjusted for exclusion of patients diagnosed and managed only as inpatients | 8.5 | 13.3 |
| Adjusted for all 3 variables | 7.4 | 11.1 |
The observed average annual 2003 and 2004 prevalence estimates for children and adults are presented with the 95% confidence intervals per 100,000 children and adults, respectively. These estimates were adjusted for the independent effects of positive predictive value (PPV), exclusion of patients managed only by non-hematologists, exclusion of inpatients, and the overall effect of all three variables on the annual average prevalence estimates.
Sensitivity analyses
Table 4 presents the prevalence estimates independently adjusted for PPV of the ICD-9-CM code, exclusion of inpatients, exclusion of patients treated by a non-hematologist, and for all three variables combined, using data previously determined by medical record review at OUMC.(19) When the observed prevalence estimates were adjusted for the previously determined PPV for the ICD-9-CM code of 287.3 of 0.72 for children and 0.69 for adults, the prevalence estimates decreased. When the observed prevalence estimates were adjusted for the 2% of children and 8% of adults who may have been diagnosed and managed for their ITP only by physicians other than hematologists and for the 5% of children and 10% of adults who may have been diagnosed and managed for their ITP only as inpatients, the adjusted prevalence estimates increased. When all three factors were considered together, the adjusted prevalence estimates were lower than the observed prevalence estimates, although they were still contained within the 95% confidence interval of the observed estimates.
Table 4.
Previously reported estimates of the prevalence of ITP*
| Study | Country | Reported Results/105/(year) | Age of subjects | Source of patients | Identification of patients |
|---|---|---|---|---|---|
| 1(17) | USA | 9.6 (2002) | 1–64 years | Medical Care Database of the Maryland Health Care Commission | Two diagnoses with the ICD-9-CM code 287.3 separated by a minimum of 30 days |
| 2 (14) | USA | 7.1 (2002) 9.5 (2004) |
18–64 years 18–64 years |
US Integrated Healthcare Information Database | Two diagnoses with the ICD-9-CM code 287.3 separated by a minimum of six months |
| 3 (18) | United Kingdom | 4.0 (2003) 4.6 (2004) |
18 years and older 18 years and older |
United Kingdom General Practice Research Database (GPRD) | At least one diagnostic Read code (42P2.11, D313.12, D313000, D313012) for ITP in the patient’s electronic medical record in the GPRD. |
In addition to these three reports, one additional report on the prevalence of chronic ITP in children(16) was not included in this Table because it was not comparable to our data.
Age and gender-specific prevalence estimates
The age and gender distributions of patients in 2003 and 2004 are illustrated in Figure 1 and descriptively compared. Although the absolute prevalence estimates were slightly different between these two years, the relative estimates among different age groups and between genders were similar. Prevalence was slightly greater among children less than 10 years old than among children and adolescents 10–19 years old. In both of these groups the prevalence among girls slightly exceeded the prevalence among boys. The prevalence of ITP peaked among women aged 20–29 years relative to women less than age 50 years, while the prevalence of ITP decreased among men aged 20–29 years relative to men less than age 50 years. Among adults aged 20–39 years, the prevalence among women was substantially greater than among men. The prevalence of ITP increased every decade after age 50 years, with the peak occurring among persons 80 years and older. Among persons greater than or equal to 70 years, the prevalence among men was greater than among women.
Figure 1.
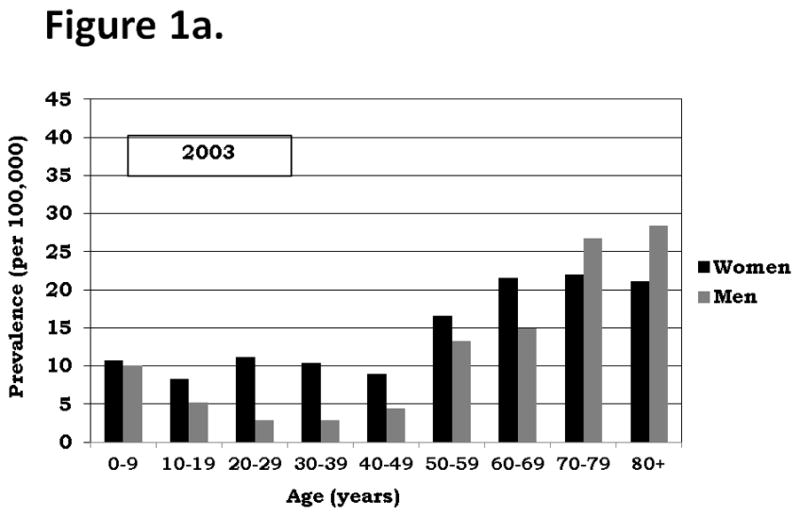
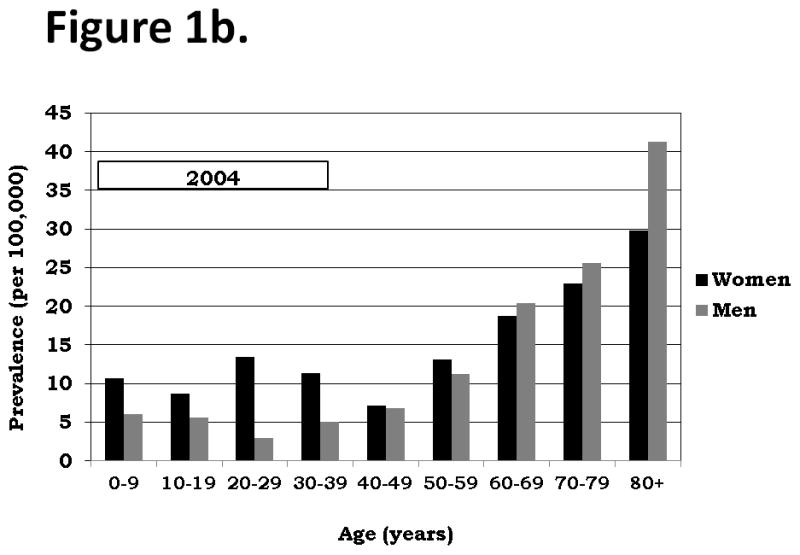
Figure 1a. ITP Gender and Age-Specific Prevalence, Oklahoma
Figure 1b.
DISCUSSION
Estimating prevalence is essential to understand the population burden of a disorder. However estimating the prevalence of ITP is challenging because there are no specific diagnostic criteria that document the presence of ITP; the diagnosis can only be established by excluding other causes of thrombocytopenia.(1–3) In addition, ITP is uncommon(15) and therefore potentially unfamiliar to both physicians and medical record coders.
To determine the prevalence of ITP regardless of age, clinical characteristics, insurance status, or source of health care, we surveyed hematologists in the State of Oklahoma to determine the number of patients identified as outpatients utilizing the administrative code ICD-9-CM 287.3. This method was based on three assumptions: [1] that the administrative code ICD-9-CM 287.3 was sufficiently accurate to identify both children and adults with ITP, [2] that most patients with ITP were diagnosed and/or managed by hematologists, and [3] that most patients with ITP were diagnosed and/or managed as outpatients. The validity of these three assumptions was established by our previous review of OUMC medical records in which we established a definition for a definite diagnosis of ITP.(19) Data from this prior study documented that the PPV of the administrative code ICD-9-CM 287.3 for identifying patients with a definite diagnosis of ITP by a hematologist was 0.72 for children and 0.69 for adults, similar to a previous report on the accuracy of this administrative code for identification of patients with ITP which determined a PPV of 0.71.(23) We also documented that in 95% of children and 92% of adults with a definite diagnosis of ITP, the diagnosis was established by a hematologist; this supported the validity of including only hematologists and not all physicians for our determination of prevalence. Finally, we determined that in 95% of children and 83% of adults with a definite diagnosis of ITP established by a hematologist, the diagnosis was established as an outpatient; this supported the validity of surveying only the offices of hematologists and not records of all hospitals. Therefore these data documented that a survey of hematologists’ offices for patients with ITP identified by the administrative code ICD-9-CM 287.3 was an appropriate method to determine the prevalence of patients with ITP. Because we were successful in obtaining data from 88 (95%) of the state’s 93 hematologists, this was also a feasible method to determine the prevalence of ITP in Oklahoma.
Adjustment of the average annual prevalence estimates for all three of the assumptions in our method resulted in lower average annual prevalence estimates for both children (7.4 per 100,000 children) and adults (11.1 per 100,000 adults) but both adjusted estimates were within the 95% confidence intervals of our observed estimates. Therefore adjustment for these three assumptions did not significantly impact the magnitude of the observed prevalence estimates.
In children, ITP is typically an acute and spontaneously resolving disorder. ITP resolves within six months of initial diagnosis in 70% of children(20) and within 12 months in nearly 80% of children.(11) Consistent with the uncommon persistence of ITP, the annual incidence of chronic ITP in children, defined as persistence for more than 6 months after diagnosis, has been reported to be 0.46 per 100,000 children,(24) while the annual incidence of all (acute and chronic) children with ITP is between 1.9 and 6.4 per 100,000 children.(15) Since the clinical course of ITP in most children is less than one year, it may be expected that the annual prevalence of ITP in children would be similar to the annual incidence. However the average annual prevalence for ITP in children, estimated from our data, 8.1 per 100,000 children, is higher than the reported annual incidence.(15) One possible explanation for the higher prevalence may be continued follow-up of children after the ITP resolves. The prevalence of chronic ITP in children has been reported to be 4.6 per 100,000 children,(16) approximately half of our observed prevalence for all (acute and chronic) children with ITP. Our average annual prevalence estimate for children of 8.1 per 100,000 children (95% CI 6.7, 9.5) is not appreciably different from the previously reported annual prevalence estimate for children of 7.2 per 100,000 children aged 1–14 years.(17)
In adults, ITP is typically a chronic disorder, commonly persistent for many months or years unless effectively treated by splenectomy or immunosuppressive agents.(1–3) Therefore it would be expected that the prevalence would be greater than the incidence. Our average annual prevalence estimate for adults of 12.1 per 100,000 adults (95% CI 11.1, 13.0), is nearly four-fold greater than the incidence of ITP in adults, 3.3 per 100,000 adults per year.(15)
Segal and Powe(17) previously reported the annual prevalence of ITP for privately insured patients ages 1–64 years to be 9.6 (95% CI, 8.6, 11.0) per 100,000 persons (Table 4). Feudjo-Tepie, et al.(14) previously reported the annual prevalence of chronic ITP in privately insured adults ages 18–64 years to be 7.1 for 2002 and 9.5 for 2004 per 100,000 adults. Using data from the United Kingdom General Practice Research Database, Bennett, et al.(18) previously reported the annual prevalence for adults 18 years old and older for 2003 to be 4.0 per 100,000 adults and for 2004 to be 4.6 per 100,000 adults. The annual prevalence of Segal and Powe(17) is lower than our estimate of the prevalence for children and adults and the annual prevalence of Feudjo-Tepie, et al.(14) is lower than our estimate of the prevalence for adults. Because of differences in inclusion criteria these estimates are not directly comparable. However, our estimate of prevalence was higher probably because we included patients over 64 years old and included patients without private insurance. Our annual prevalence estimates are higher than the adult estimates from Bennett, et al. (18) possibly due to the differences between cases identified from administrative data compared to electronic medical records or differences between the USA and UK.
Our prevalence data for children are consistent with previous reports that the annual incidence of ITP in children is greater among children younger than age 10 years than among children 10 years and older.(20;25) The predominance of women among patients age 20–39 years is similar to the increased relative frequency of other autoimmune disorders such as systemic lupus erythematosus in young women.(26) The progressively increasing prevalence among older people and a greater prevalence of men relative to women among people 60 years and older is notable. Segal and Powe(17) and Bennett, et al.(18) also described increased prevalence among older adults. The increased prevalence of ITP among older people is consistent with previous reports that the annual incidence of ITP is greatest among the oldest age cohorts;(5;13;27) in one of these reports the increased incidence among older males was also described.(13)
A limitation of this study was that not all hematology offices across the state were able to provide data for the entire time 10-year time period. However estimates derived for different time periods maximized the use of the available data. The two-year prevalence estimates represented 94% of the state’s hematologists. However, the two-year estimate is a conservative estimate of prevalence because some ITP patients may not visit a hematologist within a two-year period. This study also required diagnosis of ITP by a hematologist, a requirement that could limit the comparability of our results to previous studies. However the requirement for diagnosis by a hematologist reflects the current referral practice in Oklahoma. A survey of primary care providers in the Oklahoma Practice-based Research Network documented that 75% of respondents were ‘likely’ to send a patient with moderate thrombocytopenia (platelet count 30,000/μL) to a hematologist for further evaluation and management. The likelihood of referral increased to 85% when the moderate thrombocytopenia was associated with mild bleeding symptoms (petechiae) and to 92% when the patient presented with severe thrombocytopenia (platelet count 10,000/μL).(28) Some hematology offices may focus more on oncology rather than benign hematology which could raise concern in potential differences in diagnostic coding between groups.
In summary, our prevalence estimates are generalizable beyond privately insured persons and not limited by age. The generalizability of our data is supported by the similarity of our estimates to estimates previously reported from Maryland.(17) This study also provides the best current estimates of the age and gender distribution of ITP throughout the population. These data will be important to assess the population burden of ITP.
Acknowledgments
This project supported in part by the Utay Family Blood Research Fund. Dr. Terrell is partially supported by NIH 1U01HL72283-09S1.
Footnotes
The authors have no conflicts of interest with the data or subject of this manuscript.
Reference List
- 1.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions, and outcome criteria in immune thrombocytopenic purpura (ITP) in adults and children. Report from an international working group. Blood. 2009;113:2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 2.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs PHB, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–86. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 3.Neunert CE, Lim W, Crowther MA, Cohen AR, Solberg LA, Jr, Crowther M. The American Society of Hematology 2011 evidenced-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 4.Portielje JEA, Westendorp RGJ, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97:2549–54. doi: 10.1182/blood.v97.9.2549. [DOI] [PubMed] [Google Scholar]
- 5.Neylon AJ, Saunders PWG, Howard MR, Proctor SJ, Taylor PRA. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population-based cohort of 245 patients. Br J Haematol. 2003;122:966–74. doi: 10.1046/j.1365-2141.2003.04547.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathias SD, Gao SK, Miller KL, Cella D, Snyder C, Turner R, et al. Impact of chronic immune thrombocytopenic purpura on health-realted quality of life” a conceptual model starting with the patient perspective. Health and Quality of Life Outcomes. 2008;6:13. doi: 10.1186/1477-7525-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidry JA, George JN, Vesely SK, Kennison SM, Terrell DR. Corticosteroid side-effects and risk for bleeding in immune thrombocytopenic purpura: patient and hematologist perspectives. Eur J Haematol. 2009;83:175–82. doi: 10.1111/j.1600-0609.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 8.Newton JL, Reese JA, Watson SI, Vesely SK, Bolton-Maggs PHB, George JN, et al. Fatigue in adult patients with primary immune thrombocytopenia. European J Haematol. 2011;86:420–429. doi: 10.1111/j.1600-0609.2011.01587.x. [DOI] [PubMed] [Google Scholar]
- 9.Snyder CF, Mathias SD, Cella D, Isitt JJ, Wu AW, Young J. Health-related quality of life of immune thrombocytopenic purpura patients: results from a web-based survey. Current Medical Research and Opinion. 2008;24:2767–76. doi: 10.1185/03007990802377461. [DOI] [PubMed] [Google Scholar]
- 10.George JN, Mathias SD, Go RS, Guo M, Henry DH, Lyons RM, et al. Improved quality of life for romiplostim-treated patients with chronic immune thrombocytopenic purpura: results from two randomized, placebo-controlled trials. Br J Haematol. 2008;144:409–15. doi: 10.1111/j.1365-2141.2008.07464.x. [DOI] [PubMed] [Google Scholar]
- 11.Imbach P, Kuhne T, Muller D, Berchtold W, Zimmerman S, Elalfy M, et al. Childhood ITP: 12 months follow-up data from the prospective registry I of the intercontinental childhood ITP study group (ICIS) Pediatr Blood Cancer. 2005:1–6. doi: 10.1002/pbc.20453. [DOI] [PubMed] [Google Scholar]
- 12.Schoonen WM, Kucera G, Coalson J, Li L, Rutstein M, Mowat F, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145:235–44. doi: 10.1111/j.1365-2141.2009.07615.x. [DOI] [PubMed] [Google Scholar]
- 13.Abrahamson PE, Hall SA, Feudjo-Tepie M, Mitrani-Gold FS, Logie J. The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur J Haematol. 2009;83:83–89. doi: 10.1111/j.1600-0609.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 14.Feudjo-Tepie MA, Robinson NJ, Bennett D. Prevalence of diagnosed chronic immune thrombocytopenic purpura in the US: analysis of a large US claim database: a rebuttal. J Thromb Haemost. 2008;6:711–12. doi: 10.1111/j.1538-7836.2008.02911.x. [DOI] [PubMed] [Google Scholar]
- 15.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Amer J Hematol. 2010;85:174–80. doi: 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 16.Hedman A, Henter JI, Hedlund I, Elinder G. Prevalence and treatment of chronic idiopathic thrombocytopenic purpura of childhood in Sweden. Acta Paediatr. 1997;86:226–27. doi: 10.1111/j.1651-2227.1997.tb08876.x. [DOI] [PubMed] [Google Scholar]
- 17.Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analysis of administrative data. J Thromb Haemost. 2006;4:2377–83. doi: 10.1111/j.1538-7836.2006.02147.x. [DOI] [PubMed] [Google Scholar]
- 18.Bennett D, Hodgson ME, Shukla A, Logie JW. Prevalence of diagnosed adult immune thrombocytopenia in the United Kingdom. Adv Ther. 2011;28:1096–104. doi: 10.1007/s12325-011-0084-3. [DOI] [PubMed] [Google Scholar]
- 19.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. Determining a definite diagnosis of primary immune thrombocytopenia by medical record review. Amer J Hematol. 2012 doi: 10.1002/ajh.23226. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kühne T, Imbach P, Bolton-Maggs PHB, Berchtold W, Blanchette V, Buchanan GR, et al. Newly diagnosed idiopathic thrombocytopenic purpura in childhood: an observational study. Lancet. 2001;358:2122–25. doi: 10.1016/S0140-6736(01)07219-1. [DOI] [PubMed] [Google Scholar]
- 21.Lowe EJ, Buchanan GR. Idiopathic thrombocytopenic purpura diagnosed during the second decade of life. J Pediat. 2002;141:253–58. doi: 10.1067/mpd.2002.125909. [DOI] [PubMed] [Google Scholar]
- 22.Census factfinder. Census.gov. 2001. 6-25-2008. Ref Type: Electronic Citation. [Google Scholar]
- 23.Segal JB, Powe NR. Accuracy of identification of patients with immune thrombocytopenic purpura through administrative records: a data validation study. Amer J Hematol. 2004;75:12–17. doi: 10.1002/ajh.10445. [DOI] [PubMed] [Google Scholar]
- 24.Reid MM. Chronic idiopathic thrombocytopenic purpura: incidence, treatment, and outcome. Arch Dis Child. 1995;72:125–28. doi: 10.1136/adc.72.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeller B, Helgestad J, Hellebostad M, Kolmannskog S, Nystad T, Stensvold K, et al. Immune thrombocytopenic purpura in childhood in Norway: A prospective, population-based registration. Pediatric Hematol Oncol. 2000;17:551–58. doi: 10.1080/08880010050122816. [DOI] [PubMed] [Google Scholar]
- 26.George JN, Vesely SK, James JA. Overlapping features of thrombotic thrombocytopenic purpura and systemic lupus erythematosus. Southern Med J. 2007;100:512–14. doi: 10.1097/SMJ.0b013e318046583f. [DOI] [PubMed] [Google Scholar]
- 27.Frederiksen H, Schmidt K. The incidence of ITP in adults increases with age. Blood. 1999;94:909–13. [PubMed] [Google Scholar]
- 28.Terrell DR, Beebe LA, George JN, Vesely SK, Mold JW. Referral of patients with thrombocytopenia from primary care clinicians to hematologists. Blood. 2009;113:4126–27. doi: 10.1182/blood-2009-01-200907. [DOI] [PubMed] [Google Scholar]


