Abstract
Few therapeutic options exist for meniscus repair after injury. Local delivery of growth factors may stimulate repair and create a favorable environment for engineered replacement materials. In this study, we assessed the effect of basic fibroblast growth factor (bFGF) (a pro-mitotic agent) and transforming growth factor beta 3 (TGF-β3) (a pro-matrix formation agent) on meniscus repair and the integration/maturation of electrospun poly(ε-caprolactone) (PCL) scaffolds for meniscus tissue engineering. Circular meniscus repair constructs were formed and refilled with either native tissue or scaffolds. Repair constructs were cultured in serum-containing media for 4 and 8 weeks with various growth factor formulations, and assessed for mechanical strength, biochemical content, and histological appearance. Results showed that either short-term delivery of bFGF or sustained delivery of TGF-β3 increased integration strength for both juvenile and adult bovine tissue, with similar findings for engineered materials. While TGF-β3 increased proteoglycan content in the explants, bFGF did not increase DNA content after 8 weeks. This work suggests that in vivo delivery of bFGF or TGF-β3 may stimulate meniscus repair, but that the time course of delivery will strongly influence success. Further, this study demonstrates that electrospun scaffolds are a promising material for meniscus tissue engineering, achieving comparable or superior integration compared to native tissue.
Keywords: Meniscus, Tissue Engineering, Growth Factors, Scaffolds
1. Introduction
The meniscus is a C-shaped fibrocartilage in the knee that transmits load from the femur to the tibia [1, 2]. The unique architecture and composition of the meniscus, consisting of aligned collagen bundles and centrally-located proteoglycan, allows it to withstand both tensile and compressive forces in order to transfer loads and maintain joint stability during movement [3]. Due to the high stresses imparted on the tissue [4], both acute and degenerative tears are common, and natural repair capacity is limited, especially in the inner avascular regions [5]. Of the 850,000 meniscus surgeries performed annually in the US [6], resection is the most common technique to alleviate symptoms associated with meniscal tears. However, the procedure can result in joint incongruency and significant stresses on the adjoining cartilage, which can lead to premature degeneration (i.e., osteoarthritis) [7, 8]. Few procedures exist to repair the meniscus, and those that are performed do not restore native tissue structure and function. Thus, there is a need for novel strategies for meniscus repair.
Delivery of biological factors may stimulate tissue repair either alone or in combination with mechanical stabilization. Early work in this area delivered vascular endothelial growth factor (VEGF) from sutures to stimulate blood vessel formation in the damaged region [9]. However, delivery of VEGF from sutures failed to improve healing in vivo in a number of studies, perhaps due to suboptimal time courses of delivery [10, 11]. Rather than modulating the vascular supply, another approach is to alter biosynthesis and matrix assembly at the repair site. During repair, new matrix must be formed by nearby cells to bridge the wound gap, creating a mechanically stable interface. Increasing the amount of matrix deposited by each cell or increasing the overall number of cells (or a combination of the two) may improve repair. One of the most potent stimulators of matrix deposition in meniscal cells is transforming growth factor beta 3 (TGF-β3) [12–16], although other growth factors such as bFGF, PDGF-AB, IGF-1 and EGF all can increase matrix production [17]. Basic fibroblast growth factor (bFGF) strongly stimulates proliferation of meniscus cells in monolayer culture as well as in tissue engineered constructs [17–20]. For this reason, both TGF-β3 and bFGF were identified as potential meniscus repair factors by Kasemkijwattana et al [21], and Imler et al showed that TGF-β3 stimulated protein and proteoglycan deposition to a greater extent than bFGF in meniscus explants [16]. Due to the ability of these growth factors to stimulate matrix deposition and increase cell number, they are promising candidates for promoting repair of avascular meniscus tears as well as the maturation and integration of engineered materials in vivo.
Another important parameter in repair is the time course of delivery of biologic factors. Many growth factors function during a very specific window and at precise doses, and may work in concert with other cues [22]. Clinically, growth factors could be delivered with a bolus injection or via sustained release from a biomaterial over a given period of time; however, continual delivery of a growth factor over very long periods of time is challenging. Fortunately, recent work suggests that short-term exposure to growth factors may actually have superior efficacy compared to continual delivery [23]. These findings suggest that delivery of a biologic factor for enhancing meniscus repair need not occur continuously, but rather need only be applied in the appropriate time frame to exert maximal impact.
Chemical cues alone may not be sufficient to restore meniscus function in situations where repair is not possible, such as when the tissue is severely disrupted or degraded. To address this issue, tissue engineering generates structures that recapitulate native tissue architecture and behavior [24]. Recently, biodegradable scaffolds composed of porous collagen (Menaflex) or porous polyurethane (ActiFit) were introduced into clinical practice to replace regions of resected meniscus [25]. Other pre-clinical materials and scaffolds under investigation include subintestinal submucosa, anatomically shaped alginate hydrogels, and hyaluronic acid based constructs, to name but a few [26–28]. Our lab has focused on the use of electrospun scaffolds, which are fabricated by collecting nano-sized synthetic and biological polymeric fibers on electrically charged surfaces [29]. These scaffolds are amenable to cell attachment, proliferation and infiltration [30–32] and can be functionalized to release biologic agents and growth factors [33–35].
While these nanofibrous scaffolds have shown promise in vitro, additional work is required to evaluate how these scaffolds are colonized by cells from the native tissue, to determine how the scaffolds integrate mechanically with the native tissue, and the key modulators in this integration process. Because large animal studies of meniscus repair are quite costly, smaller in vitro experiments may be beneficial for assessing the potential of new therapies [36]. Early work demonstrated that meniscus tissue remains viable when cultured in proper media conditions [37]. Later, concentric explants were used to test the influence of inflammatory cytokines and matrix metalloproteinases on meniscus integration [38]. More recently, we have used this model to demonstrate that, in accordance to observations made clinically [39], immature meniscus undergoes self-repair to a greater extent than mature meniscus in vitro [30] and that TGF-β3 bolsters this integration process [30, 38]. Further, we explored how electrospun scaffolds in annular meniscus defects are colonized by native tissue cells with time in culture [30].
To further these lines of inquiry, this study evaluated the impact of TGF-β3 and bFGF delivery for short-term (1 week) and sustained (8 weeks) periods, alone and in combination, and assessed the mechanical integration strength of the repair as well as the biochemical content of the repair material. This work was carried out in both juvenile and adult bovine meniscus defects (meniscus to meniscus integration), as well as in meniscus defects `repaired' with electrospun scaffold. We hypothesized that bFGF would improve integration by increasing the cell density in the tissue interface while TGF would act to increase matrix density at the repair interface, and that short-term delivery of growth factors would elicit comparable integration as continual delivery.
2. Materials and Methods
2.1 Evaluation of Meniscus-to-Meniscus Repair with Growth Factor Supplementation
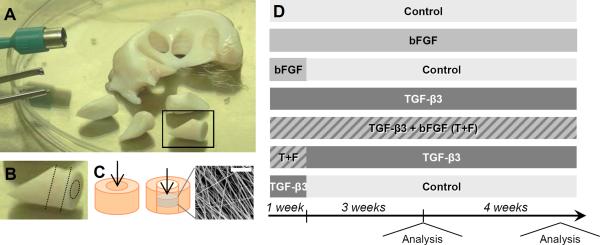
Menisci were dissected from the knee joints of juvenile (0–3 months old) and adult (skeletally mature, > 2 year old) bovine limbs in a sterile manner. Cylinders (8 mm diameter × 3 mm thick) were excised centrally in the axial direction using a dermal punch (Miltex, Plainsboro, NJ), as shown in Figure 1A. To simulate an acute meniscus tear, a full-thickness inner columnar defect (4 mm diameter) was made and the core reinserted with care to maintain fiber alignment, as shown in Figure 1B–C.
Figure 1. Experimental set-up and study design.
(A) 8 mm cylinders were excised from bovine meniscus. (B) Tissue cylinders were flattened and a smaller biopsy punch was used to remove a 4 mm core. (C) This tissue core was either reinserted back into the tissue (left), or replaced with a disc of electrospun PCL (right, inlay scale = 20 μm). (D) Schematic of media formulations and temporal exposure regimens and testing time points over 8 weeks.
Meniscus repair constructs were cultured in control media (Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), 1% penicillin/streptomysin/fungizone (PSF), 50 μg/mL ascorbate-2-phosphate) supplemented with 5 different growth factor regimens: continual 50 ng/mL bFGF, 1 week of bFGF, continual 10 ng/mL TGF-β3, combined continual bFGF and TGF-β3, combined 1 week bFGF and continual TGF-β3, or 1 week of TGF-β3 (Figure 1D). Explants were stored in 6-well plates, covered completely in media that was replaced twice weekly. After 4 and 8 weeks of culture, the mechanical integration strength was evaluated using a custom testing device [30]. Briefly, an Instron 5848 was outfitted with a 3.5 mm diameter indenter in series with a 50 N load cell. This indenter was placed above a plate with a 5 mm diameter through-hole. The meniscus sample was placed onto the plate, and the indenter progressed through the defect site at a rate of 0.0833 mm/sec. Integration strength was calculated as:
where r was the core radius (2 mm). Height (h) of the interface was determined by averaging four caliper measurements of the construct prior to testing (n=5–8/condition). For control, TGF-β3 and bFGF conditions, three replicate studies were performed. For all other conditions, two replicate studies were performed.
2.2 Biochemical and Histological Analysis of Meniscus-to-Meniscus Repair
After mechanical pushout testing, the meniscus-to-meniscus construct was reassembled (to maximize the quantity of tissue analyzed) and prepared for biochemical analysis by lyophilizing and digesting the tissue in a buffer containing 2% papain at 60°C. The resulting digestate was analyzed for DNA content (PicoGreen Assay, Invitrogen, Carlsbad, CA), glycosaminoglycan (GAG) content (DMMB Assay [40]) and collagen content (OHP assay with a conversion factor of 7.14 [41]). Results were normalized to sample dry weight. Histological analysis was conducted on fresh, untested samples for each condition and time point. Constructs were fixed in phosphate-buffered 4% paraformaldahyde (PFA), embedded in paraffin and cut to 8 μm sections and affixed to glass slides. Samples were stained with Alcian blue for proteoglycans (PG), Picrosirius red for collagen, and DAPI (Prolong Gold, Invitrogen, Carlsbad, CA) or a mixture of hematoxylin and eosin (H&E) to identify cell nuclei. Immunohistochemical staining for anti-phospho-histone H3 (Sigma-Aldrich, Saint Louis, MO), a proliferation marker, was performed after antigen retrieval (2% hyaluronidase), followed by exposure to hydrogen peroxide and background blocking (Background Buster, American Master Tech, Lodi, CA) for 10 minutes each, using 8 μm sections of rat spleen as a positive control (data not shown). The primary antibody was incubated for 90 minutes at room temperature, with subsequent secondary antibody incubation and color development using the SuperPicture DAB kit (Invitrogen, Carlsbad, CA). Images were acquired at 20× using a Nikon Eclipse 50i microscope with NIS Elements F3.0 software
2.3 Formation of Electrospun Scaffolds
To generate electrospun scaffolds for meniscus defect repair, 14.3% w/v poly(ε-caprolactone) (PCL, 80 kDa, Sigma-Aldrich, St. Louis MO) in a 1:1 mixture of tetrahydrofuran (THF, Fisher Chemical, Fairlawn NJ) and N,N-dimethylformamide (DMF, Fisher Chemical) was mixed overnight. A 20 mL syringe was filled with PCL electrospinning solution and fitted with a stainless steel 18G blunt-ended needle that served as a charged spinneret. A flow rate of 2.5 mL/h was maintained for 8 hours with a syringe pump (KDS100, KD Scientific, Holliston, MA). A power supply (ES30N-5W, Gamma High Voltage Research, Inc., Ormond Beach, FL) applied a +13 kV potential difference between the spinneret and the grounded mandrel located at a distance of 12 cm from the spinneret. Additionally, two aluminum shields charged to +10 kV were placed perpendicular to and on either side of the mandrel to better direct the fibers towards the grounded mandrel. The mandrel was rotated via a belt mechanism conjoined to an AC motor (Pacesetter 34R, Bodine Electric, Chicago, IL) at a speed of 10 m/sec to form 800 μm thick mats.
2.4 Evaluation of Meniscus-to-Scaffold Repair
After scaffold fabrication, 4 mm scaffold discs were excised using a dermal punch and sterilized under UV light for 10 minutes. Meniscus constructs were created as previously described, but instead of reinserting the central core of tissue, an acellular disc of scaffold was press fit into the defect (Figure 1C). The scaffold repair constructs were cultured in 5 different growth factor regimens: control, continual 50 ng/mL bFGF, 1 week of bFGF, continual 10 ng/mL TGF-β3, or combined 1 week bFGF and continual TGF-β3 (n=8–10/condition). Two replicate experiments were performed for control and TGF-β3 conditions. After 4 weeks, mechanical integration was assessed as described above, with the height (h) of the scaffold disc measured using an OptoNCDT laser measuring device (Micro-Epsilon, Raleigh, NC) after testing. Scaffolds were digested and analyzed for biochemical content as previously described. Similarly, histology was performed by fixing samples in 4% PFA, embedding in Cryo-OCT Compound (Tissue Tek, Fisher, Fairlawn, NJ) with an axial orientation, sectioning and staining as previously described.
2.5 Statistical Analysis
Two-way ANOVA was performed using SYSTAT software (Chicago, IL) to compare time points and conditions, with Tukey's post-hoc testing of differences between groups. Significance was set at p ≤ 0.05.
3 Results
3.1 Evaluation of Meniscus-to-Meniscus Repair with Growth Factor Addition
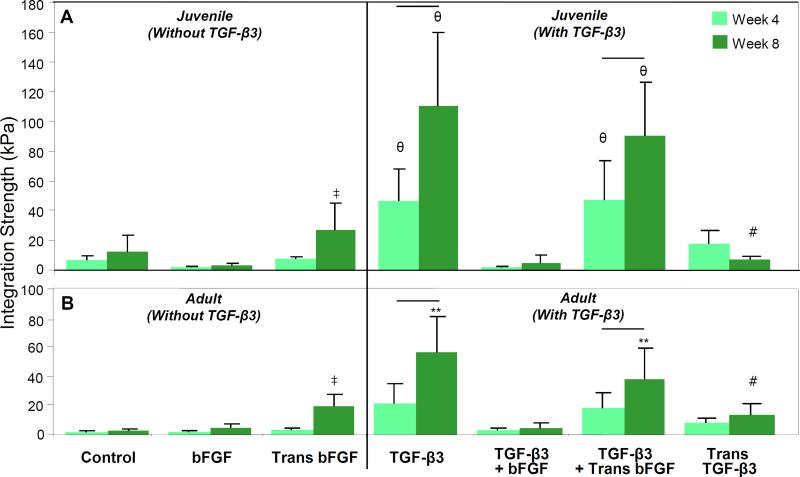
Meniscus-to-meniscus repair constructs were formed and cultured in vitro. Overall, and similar to previous findings [30], juvenile tissue integrated to a greater extent than adult tissue, with 2-to 4-fold differences in integration strength after 8 weeks (Figure 2A,B). After 8 weeks, short-term (1 week) delivery of 50 ng/mL bFGF resulted in better integration compared to control conditions or continual addition of bFGF, for both juvenile and adult tissue (p < 0.05). Exposure to TGF-β3 resulted in greater improvements in integration strength compared to all non-TGF-β3 groups after 4 weeks in juvenile and after 8 weeks in adult meniscus-to-meniscus samples (p < 0.05). In this context, transient bFGF did not further improve repair. Interestingly, continual delivery of bFGF in the context of continual TGF-β3 reduced integration strength to levels comparable to those found in the absence of TGF-β3 (p < 0.05). Transient (1 week) exposure to TGF-β3 resulted in comparable integration strength to continual TGF-β3 exposure at 4 weeks, but integration strength was lower after 8 weeks under this condition. No major changes were seen in explant morphology, except for a slight decrease in diameter for control juvenile explants.
Figure 2. Integration strength of meniscus-to-meniscus repair constructs for (A) juvenile and (B) adult tissue.
Growth factor identity and time course of delivery strongly influenced integration, with TGF-β3 addition eliciting the greatest increases in integration strength for both ages, followed by transient exposure to bFGF. Continual exposure to bFGF or a mixture of bFGF and TGF-β3 did not improve integration. ‡ indicates difference from control and continual bFGF, comparisons made only between non-TGF-β3 groups. θ indicates difference from all non-TGF-β3 groups and bFGF+TGF-β3. Line indicates difference between time points. # indicates difference from TGF-β3 and transient bFGF+TGF-β3. For all comparisons, p < 0.05; n = 10–24/condition.
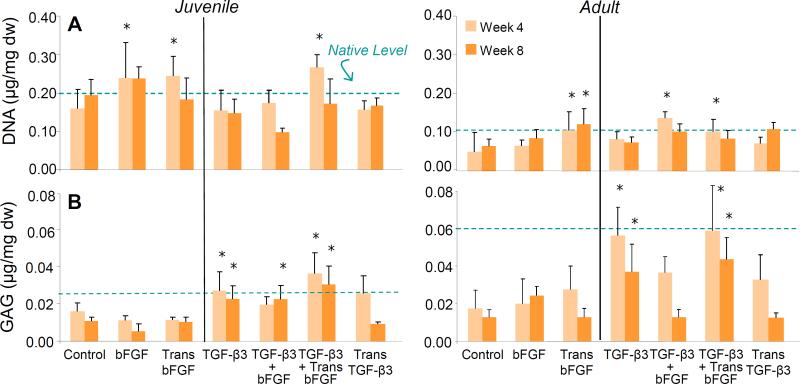
Biochemical analysis revealed only minor fluctuations in DNA content across all groups, with increases after 4 weeks in the continual presence of bFGF and in conditions of transient bFGF (p < 0.05). However, these effects were no longer apparent at 8 weeks (Figure 3B). GAG content was higher and closer to native levels in all samples exposed to TGF-β3 (p < 0.05), with some variations in the group containing TGF-β3 and bFGF (Figure 3C). Juvenile and adult tissue responded in a similar fashion to the different media formulations in terms of biochemical content.
Figure 3. Biochemical content of meniscus-to-meniscus repair constructs.
(A) Short term and continual exposure to bFGF increased DNA content at 4 weeks, but this effect subsided by 8 weeks. (B) Continual delivery of TGF-β3 preserved GAG content in constructs at or near native levels; control, bFGF, and transient TGF exposure did not preserve native GAG levels. Data normalized to dry weight. * indicates difference from control. For all comparisons, p < 0.05.
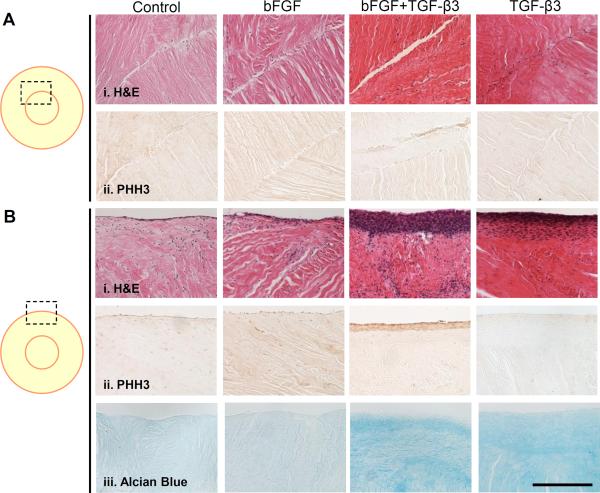
Histologically, cells populated the injury rim in all conditions, with greater amounts of tissue bridging the injury site in repair constructs cultured with continual TGF-β3. No connections formed when TGF-β3 and bFGF where delivered simultaneously (Figure 4Ai). The injury site did not stain strongly for proliferating cells, despite the presence of bFGF (Figure 4Aii). While few significant differences were noted between groups at the injury site, there were marked differences at the exterior edge of the repair construct, where a thick cell sheath formed in the presence of TGF-β3 (Figure 4Bi) that stained strongly for proliferating cells only when bFGF was added (Figure 4Bii). Because continual combined delivery of TGF-β3 and bFGF also resulted in sheath development and exhibited low integration strength, the presence of the sheath alone does not account for the differences observed in integration strength. In contrast, only a very thin layer of cells stained positively for proliferation in the presence of bFGF alone. Overall, and consistent with biochemical measures, the groups exposed to TGF-β3 had the greatest PG staining in the bulk of the tissue (Figure 4Biii) compared to non-TGF-β3 groups. Results were similar between 4 and 8 weeks, with 8 week data shown.
Figure 4. Histology of meniscus-to-meniscus repair constructs.
(A) The injury site and (B) the outer periphery of constructs after 8 weeks in either control media or media supplemented with bFGF, both bFGF and TGF-β3, or TGF-β3 alone. H&E (i) identifies cell nuclei, PHH3 (ii) identifies cells undergoing mitosis, and Alcian Blue (iii) identifies proteoglycans in the extracellular matrix. Scale = 200 μm.
3.2 Evaluation of Meniscus-to-Scaffold Repair with Growth Factor Addition
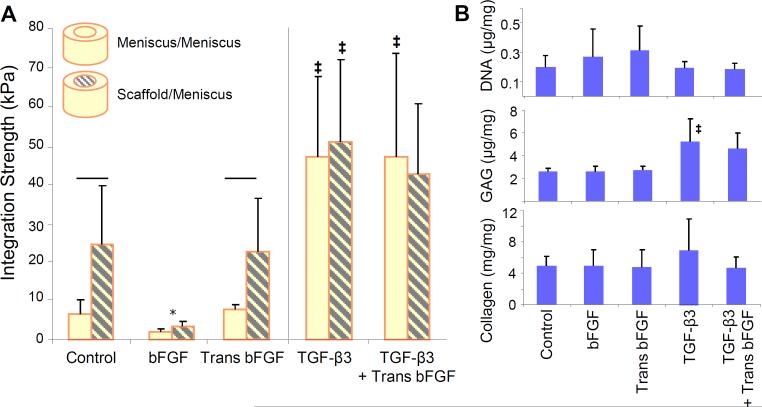
Aligned PCL electrospun scaffolds (Figure 5A) were used to fill meniscus defects in order to form meniscus-to-scaffold repair constructs. Overall, the integration of meniscus-to-scaffold repair constructs in various media conditions followed the same pattern as meniscus-to-meniscus repair constructs (Figure 5A). In control conditions, meniscus-to-scaffold integration strength was 2- to 3-fold greater than meniscus-to-meniscus integration. Unlike meniscus-tomeniscus repair constructs, transient application of bFGF did not further improve integration strength. Similar to meniscus-to-meniscus repair, the continual addition of bFGF significantly decreased integration strength (p < 0.05). The addition of TGF-β3 resulted in 2-fold improvements in integration strength to levels that were comparable to meniscus-meniscus construct integration. Similar to previous findings with native tissue repair constructs, transient bFGF did not improve integration in the presence of TGF-β3. Unlike the native tissue, biochemical content of the scaffolds did not vary significantly, with inclusion of bFGF only slightly improving cellularity and TGF-β3 producing only small increases in GAG and collagen content compared to controls (Figure 5B).
Figure 5. Integration strength and biochemical content of meniscus-to-scaffold repair constructs.
(A) Scaffold-to-meniscus integration strength was higher than meniscus-tomeniscus integration under control conditions after 4 weeks. In the presence of TGF-β3, integration strength was comparable between these groups. (B) DNA and collagen content did not change in scaffold repair constructs exposed to different media conditions; inclusion of TGF-β3 increased scaffold GAG content slightly. Line indicates difference between groups and * indicates difference from control and transient bFGF, considering only non-TGF-β3 groups. ‡ indicates difference from non-TGF-β3 groups. For all comparisons, p < 0.05; n = 8–20/condition.
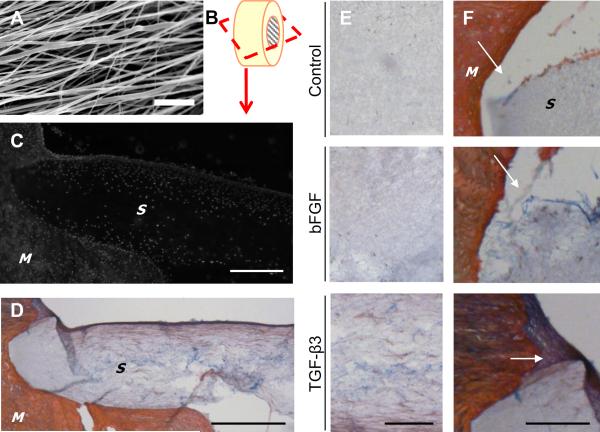
Histological analysis showed that meniscus cells from the tissue colonized the scaffold, populating the outer regions after 4 weeks (Figure 6C). Both collagen (red) and proteoglycan (blue) were distributed throughout the scaffold (Figure 6D), particularly when constructs were cultured in the presence of TGF-β3 (Figure 6E). In the absence of TGF-β3, little tissue was found bridging the scaffold and the native tissue compared to constructs exposed to TGF-β3 (Figure 6F).
Figure 6. Histology of meniscus-to-scaffold repair constructs.
(A) SEM of electrospun scaffolds (scale = 10 μm). (B) Schematic of sectioning plane for histology. (C) DAPI staining demonstrated that cells had migrated into the scaffold (`S ') from the native meniscus (`M ') after 4 weeks of culture (scale = 500 μm, TGF-β3 treated). (D) Collagen (red) and proteoglycans (blue) were present in the scaffold, though to a much lower level than in the native meniscus tissue (M) (scale = 500 μm, TGF-β3 treated). (E) Matrix deposition within the scaffold under different media conditions (scale = 200 μm). (F) Neo-tissue (white arrows) bridges the scaffold and meniscus in TGF-β3 conditions, but not in control or bFGF conditions (scale = 200 μm).
4. Discussion
Clinical techniques to repair or replace the knee meniscus are limited, and as a consequence, a significant number of meniscus injuries are treated by partial meniscectomy (i.e., removal of the damaged segment). The negative sequelae of meniscectomy include the promiscuous development of cartilage erosion in the affected knee compartment. In most instances, surgical removal is the preferred treatment given the poor healing of the avascular regions of the tissue (accounting for greater than 2/3rds of tissue volume) [42]. Indeed, many surgical interventions fail soon after surgery as a result of poor cell-mediated tissue formation at the repair site, necessitating eventual meniscectomy [43]. Methods to improve intrinsic repair of native tissue to itself, or to rapidly integrate engineered materials to the native tissue, would dramatically expand the current state of meniscus treatment options. To that end, this study explored how exposure to growth factors involved in matrix deposition and mitogenesis (TGF-β3 and bFGF), as well as their combination and the duration of their delivery, might modulate meniscus repair in an in vitro model system. Consistent with our previous work, and that of others [38], native meniscus segments cultured in apposition integrated with one another, increasing in mechanical strength as new material was deposited at the wound edge. Media supplementation with bFGF or TGF-β3 led to different outcomes, depending on the timing of administration and the combination in which each growth factor was applied. This work demonstrates clinical delivery of growth factors can improve meniscus repair as well as scaffold integration.
FGF was initially selected for evaluation given its well established pro-mitotic effects on cells in monolayer culture and in constructs [17–20]. Like most dense connective tissues, the adult meniscus, is relatively cell-poor. Thus, agents that increase cellularity at the repair site might increase integration capacity. In the context of meniscus integration, short-term (1 week) exposure to bFGF resulted in a 2- to 4-fold increase in integration strength. However, continual exposure to this factor had negative effects on integration. Continued exposure to bFGF may have promoted cells to adopt a mitotic state, limiting their ability to produce matrix at the wound interface. Alternatively, cells within the meniscus microenvironment may not be as sensitive to mitogenic agents as cells in monolayer. For instance, Kato et al found that chondrocytes, a related cell population, were responsive to bFGF only during the logarithmic cell expansion phase and not after achieving confluency [44]. Supporting this theory, cells within the meniscus or at the injury site did not stain for the proliferation marker PHH3, while cells were positive at the construct outer boundary. Thus, meniscus cells free of associated matrix may respond differently to bFGF than those embedded within a dense tissue construct.
Unlike bFGF, continuous addition of TGF-β3 resulted in greater than 10-fold improvements in integration strength in meniscus-to-meniscus repair constructs. This is consistent with previous findings by McNulty and colleagues, who showed that TGF-β3 could increase integration strength over a two week period [38]. Interestingly, combining the two successful regimens (short-term bFGF and long-term TGF-β3) did not result in further, synergistic improvements in repair. When bFGF was continuously provided along with TGF-β3, a marked reduction in repair strength was observed. While bFGF can foster both cell division and matrix deposition (though its matrix effects are not as robust as TGF-β3 [16, 20, 45]), addition of bFGF in the context of TGF-β3 limited matrix deposition. This was most clearly evidenced in the adult tissue, where integration strength decreased precipitously, and tissue GAG content fell to far below native levels. Of further note, while long-term continuous TGF-β3 exposure was effective at improving integration and maintaining tissue ECM, short-term TGF-β3 exposure did not improve integration past 4 weeks. Previous work using fibrochondrocytes from the temporomandibular joint (cultured in agarose hydrogels) showed that continuous TGF-β3 exposure improved properties to a greater extent than intermittent exposure to this factor [46], consistent with the findings of the present study. Taken together, these results illustrate the importance of delivery duration and growth factor combination in meniscus repair.
In this study, both hypercellular juvenile and hypocellular adult meniscus tissue repair constructs were formed and subjected to the above mentioned growth factor regimens. This was done to account for the fact that meniscus injury occurs in both the younger active population as well as in the aged population. In the younger population, meniscus injury is often acute, and associated with compromise of other structures in the knee, such as the anterior cruciate ligament. In older populations, meniscus damage is more commonly the result of degenerative processes. In both cases, however, the interface is usually rasped to expose fresh tissue before any repair is attempted, and thus operative conditions for both age groups are comparable to the acute incisional wounds made in this study. Healing of these acute injuries showed that tissue age resulted in persistent differences in mechanical integration capacity, where juvenile tissue repair constructs reached integration strengths approximately twice that of adult tissue repairs, consistent with our previous findings [30]. Interestingly, while the magnitude of integration strength differed, the response to specific growth factors and growth factor combinations followed a similar trend in both age groups. These findings suggest that there are no innate differences between juvenile and adult tissues in their response to this set of growth factors, and that clinical procedures based on addition of these growth factors may be equally applicable to the aged and the young population.
In addition to exploring native tissue integration with itself, we also evaluated the integration and maturation of a biomaterial scaffold in the same meniscus defect model. Engineered biomaterial scaffolds will be essential in instances where meniscus damage is so severe that repair cannot be attempted. In these cases, the damaged portion would ideally be replaced with an engineered material or construct matching (or capable of maturing to match) native tissue structure and function. These repair materials, including electrospun scaffolds, can likewise be modified to serve as drug delivery vehicles, focusing biofactor delivery at the wound site. Electrospun scaffolds in particular have been functionalized to deliver growth factors through a number of different mechanisms, including annealing of heparin molecules to enhance growth factor binding, emulsion and coaxial electrospinning, and co-spinning of polymeric microspheres within the fibrous network [33–35, 47].
As a first step in the translation of such novel materials, we evaluated the integration of an aligned PCL scaffold in the meniscus defect model. Results from this study showed that cells from the native tissue colonized the exterior portions of the scaffold within four weeks, and that mechanical integration increased with time. Under control conditions, absent any growth factors, scaffold-to-meniscus repair was superior to meniscus-to-meniscus repair (by a factor of three). This is likely due to the open porous structure of the scaffold, allowing cells to rapidly enter the scaffold and produce contiguous matrix. When these scaffold-based repairs were cultured in the presence of growth factors, a similar integration response was observed as with native tissue repair. Namely, continual exposure to TGF-β3, with or without bFGF, improved integration strength and promoted tissue bridging from the native tissue to the scaffold. While exogenous growth factors modulated integration strength in meniscus-to-scaffold repair constructs, they did not significantly alter tissue content within the scaffold over 4 weeks. This finding is in contrast to a study by Pangborn et al, who showed that exposure to TGF-β1 stimulated increased collagen and proteoglycan production by meniscus fibrochondrocytes in a porous poly(glycolic acid) scaffold compared to bFGF [48]. Also, Gunja et al demonstrated that bFGF increased cell density in PLLA scaffolds compared to control conditions [45]. However, these studies were conducted serum-free media, which may explain the more robust response to the growth factors [49].
Although this work advances our understanding of the role of TGF-β3 and bFGF in augmenting meniscus repair and early integration of engineered materials, some limitations exist that will need to be addressed in future efforts. For instance, the work was performed using bovine rather than human tissue, and the response between species may differ (though most mechanical and biochemical features are similar between the two). Additionally, we only assayed one dose of each growth factor (chosen based on their efficacy in other in vitro studies) and alternative concentrations and dosing schedules may provide interesting differences in the results, and additional factors could be employed in this analysis. Further, we chose to include serum in this study to more closely mimic the variability and complexity expected in the in vivo scenario, but this media formulation does not exactly match the synovial environment of the knee. The culture environment will need to be addressed as the work moves towards large animal in vivo studies. Finally, while the growth factors employed in this study can be quite potent, activity half-life in vivo can be quite short. Here, growth factors were provided through the aqueous environment; clinical translation of these findings will require methods of sustained and localized delivery at the wound edge. Delivery mechanisms will need to be developed to both focus the appropriate dose, as well as the sustained delivery, at the wound site for the most efficacious repair to occur. Genetically modified cells might be delivered to address this limitation, providing a stable and continuous supply of growth factors [21].
5. Conclusions
Few therapeutic options exist for meniscus repair after injury. Local delivery of growth factors may stimulate native tissue repair or create a favorable environment for the rapid integration and maturation of engineered replacement materials. Results from this study demonstrated that both short-term delivery of bFGF and sustained delivery of TGF-β3 increased integration strength for juvenile and adult bovine meniscus defects, with similar findings observed for nanofibrous scaffolds evaluated in the same defect model. While this data suggests that in vivo delivery of bFGF or TGF-β3 can stimulate meniscus repair, the time course of delivery strongly influenced mechanical properties of the repair, as well as native tissue composition. Delivery mechanisms that can address both dosing and duration of growth factor delivery will need to be optimized to bring this work to clinical application. Future work will translate these findings into large animal studies, and may result in a more successful repair strategy of this commonly injured musculoskeletal tissue.
Acknowledgements
The authors gratefully acknowledge Mr. Kilief Zellars for his assistance with the OHP assay, and Dr. Salin Chakkalakal and Mr. Kevin Egan for their advice on IHC staining. This work was supported by the National Institutes of Health (R01 AR056624) and the Penn Center for Musculoskeletal Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures The authors have no conflicts to disclose.
References
- [1].Hsieh HH, Walker PS. Stabilizing mechanisms of the loaded and unloaded knee joint. J Bone Joint Surg Am. 1976;58:87–93. [PubMed] [Google Scholar]
- [2].Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;109:184–92. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- [3].Ghosh P, Taylor TK. The knee joint meniscus: a fibrocartilage of some distinction. Clin Orthop Relat Res. 1987;224:52–63. [PubMed] [Google Scholar]
- [4].Sweigart MA, Athanasiou KA. Toward Tissue Engineering of the Knee Meniscus. Tissue Eng. 2001;7:111–29. doi: 10.1089/107632701300062697. [DOI] [PubMed] [Google Scholar]
- [5].Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–5. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- [6].Arendt EA. Orthopaedic Knowledge Update: Sports Medicine 2: Rosemont, Ill: American Academy of Orthopaedic Surgeons. 1999. [Google Scholar]
- [7].Shapiro F, Glimcher MJ. Induction of osteoarthritus in the rabbit knee joint: Histological changes following meniscectomy and meniscal lesions. Clin Orthop. 1980;147:287. [PubMed] [Google Scholar]
- [8].Lufti AM. Morphological changes in the articular cartilage after meniscectomy. J Bone Joint Surg. 1975;57B:525. [PubMed] [Google Scholar]
- [9].Petersen W, Pufe T, Stärke C, Fuchs T, Kopf S, Raschke M, et al. Locally applied angiogenic factors--a new therapeutic tool for meniscal repair. Ann Anat. 2005;187:509–19. doi: 10.1016/j.aanat.2005.04.010. [DOI] [PubMed] [Google Scholar]
- [10].Kopf S, Birkenfeld F, Becker R, Petersen W, Stärke C, Wruck CJ, et al. Local treatment of meniscal lesions with vascular endothelial growth factor. J Bone Joint Surg Am. 2010;92:2682–91. doi: 10.2106/JBJS.I.01481. [DOI] [PubMed] [Google Scholar]
- [11].Petersen W, Pufe T, Stärke C, Fuchs T, Kopf S, Neumann W, et al. The effect of locally applied vascular endothelial growth factor on meniscus healing: gross and histological findings. Arch Orthop Traum Su. 2007;127:235–40. doi: 10.1007/s00402-005-0024-2. [DOI] [PubMed] [Google Scholar]
- [12].Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr Cartilage. 2006;14:179–89. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [13].Mauck RL, Martinez-Diaz GJ, Yuan X, Tuan RS. Regional multilineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat Rec. 2007;290:48–58. doi: 10.1002/ar.20419. [DOI] [PubMed] [Google Scholar]
- [14].Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [15].Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthr Cartilage. 1995;3:127–38. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- [16].Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthr Cartilage. 2004;12:736–44. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [17].Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–31. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Webber RJ, Harris MG, Hough AJ. Cell culture of rabbit meniscal fibrochondrocytes: proliferative and synthetic response to growth factors and ascorbate. J Orthop Res. 1985;3:36. doi: 10.1002/jor.1100030104. [DOI] [PubMed] [Google Scholar]
- [19].Forriol F. Growth factors in cartilage and meniscus repair. Injury. 2009;40:S12–S6. doi: 10.1016/S0020-1383(09)70005-1. [DOI] [PubMed] [Google Scholar]
- [20].Adesida AB, Grady LM, Khan WS, Hardingham TE. The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Res Ther. 2006;8:R61. doi: 10.1186/ar1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kasemkijwattana C, Menetrey J, Goto H, Niyibizi C, Fu FH, Huard J. The use of growth factors, gene therapy and tissue engineering to improve meniscal healing. Mater Sci Eng C. 2000;13:19–28. [Google Scholar]
- [22].Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- [23].Lieb E, Vogel T, Milz S, Dauner M, Schulz MB. Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: Osteoblastic differentiation. Tissue Eng Part A. 2004;10:1414–25. doi: 10.1089/ten.2004.10.1414. [DOI] [PubMed] [Google Scholar]
- [24].Buma P, Ramrattan NN, van Tienen TG, Veth RPH. Tissue engineering of the meniscus. Biomaterials. 2004;25:1523–32. doi: 10.1016/s0142-9612(03)00499-x. [DOI] [PubMed] [Google Scholar]
- [25].Gulotta LV, Wiznia D, Cunningham M, Fortier L, Maher S, Rodeo SA. What's New in Orthopaedic Research. J Bone Joint Surg Am. 2011;93:2136–41. doi: 10.2106/JBJS.K.00981. [DOI] [PubMed] [Google Scholar]
- [26].Ballyns JJ, Wright TM, Bonassar LJ. Effect of media mixing on ECM assembly and mechanical properties of anatomically-shaped tissue engineered meniscus. Biomaterials. 2010;31:6756–63. doi: 10.1016/j.biomaterials.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cook JL, Fox DB, Malaviya P, Tomlinson JL, Farr J, Kuroki K, et al. Evaluation of small intestinal submucosa grafts for meniscal regeneration in a clinically relevant posterior meniscectomy model in dogs. J Knee Surg. 2006;19:159–67. doi: 10.1055/s-0030-1248100. [DOI] [PubMed] [Google Scholar]
- [28].Chiari C, Koller U, Dorotka R, Eder C, Plasenzotti R, Lang S, et al. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthritis and Cartilage. 2006;14:1056–65. doi: 10.1016/j.joca.2006.04.007. [DOI] [PubMed] [Google Scholar]
- [29].Baker BM, Handorf AM, Ionescu LC, Li WJ, Mauck RL. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Rev Med Devices. 2009;6:515–32. doi: 10.1586/erd.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ionescu LC, Lee GC, Garcia GH, Zachry TL, Shah RP, Sennett BJ, et al. Maturation statedependent alterations in meniscus integration: implications for scaffold design and tissue engineering. Tissue Eng Part A. 2011;14:193–204. doi: 10.1089/ten.tea.2010.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–58. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8:986–92. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim MS, Shin YM, Lee J, Kim SI, Nam YS, Shin CS, et al. Release Kinetics and in vitro Bioactivity of Basic Fibroblast Growth Factor: Effect of the Thickness of Fibrous Matrices. Macromol Biosci. 2011;11:122–30. doi: 10.1002/mabi.201000222. [DOI] [PubMed] [Google Scholar]
- [34].Sahoo S, Ang L-T, Cho-Hong Goh J, Toh S-L. Bioactive nanofibers for fibroblastic differentiation of mesenchymal precursor cells for ligament/tendon tissue engineering applications. Differentiation. 2010;79:102–10. doi: 10.1016/j.diff.2009.11.001. [DOI] [PubMed] [Google Scholar]
- [35].Liao IC, Chew SY, Leong KW. Aligned core-shell nanofibers delivering bioactive proteins. Nanomedicine-UK. 2006;1:465–71. doi: 10.2217/17435889.1.4.465. [DOI] [PubMed] [Google Scholar]
- [36].van de Breevaart Bravenboer J, der Maur CD, Bos PK, Feenstra L, Verhaar JA, Weinans H, et al. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther. 2004;6:R469–76. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Webber RJ, York JL, Vanderschilden JL, Hough AJJ. An organ culture model for assaying wound repair of the fibrocartilaginous knee joint meniscus. Am J Sports Med. 1989;17:393–400. doi: 10.1177/036354658901700314. [DOI] [PubMed] [Google Scholar]
- [38].McNulty AL, Guilak F. Integrative repair of the meniscus: lessons from in vitro studies. Biorheology. 2008;45:487–500. [PMC free article] [PubMed] [Google Scholar]
- [39].Andrish JT. Meniscal Injuries in Children and Adolescents: Diagnosis and Management. J Am Acad Orthop Surg. 1996;4:231–7. doi: 10.5435/00124635-199609000-00001. [DOI] [PubMed] [Google Scholar]
- [40].Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- [41].Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- [42].Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med. 1983;11:131–41. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- [43].Paxton ES, Stock MV, Brophy RH. Meniscal Repair Versus Partial Meniscectomy: A Systematic Review Comparing Reoperation Rates and Clinical Outcomes. Arthroscopy. 2010;27:1275–88. doi: 10.1016/j.arthro.2011.03.088. [DOI] [PubMed] [Google Scholar]
- [44].Kato Y, Gospodarowicz D. Sulfated proteoglycan synthesis by confluent cultures of rabbit costal chondrocytes grown in the presence of fibroblast growth factor. J Cell Biol. 1985;1002:477–85. doi: 10.1083/jcb.100.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gunja NJ, Athanasiou KA. Additive and synergistic effects of bFGF and hypoxia on leporine meniscus cell-seeded PLLA scaffolds. J Tissue Eng Regen M. 2009;4:115–22. doi: 10.1002/term.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kalpakci KN, Kim EJ, Athanasiou KA. Assessment of growth factor treatment on fibrochondrocyte and chondrocyte co-cultures for TMJ fibrocartilage engineering. Acta Biomater. 2011;7:1710–8. doi: 10.1016/j.actbio.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ionescu LC, Lee GC, Sennett BJ, Burdick JA, Mauck RL. An Anisotropic Nanofiber/Microsphere Composite with Controlled Release of Biomolecules For Fibrous Tissue Engineering. Biomaterials. 2010;31:4113–20. doi: 10.1016/j.biomaterials.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23:1184–90. doi: 10.1016/j.orthres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- [49].Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967–77. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]