Abstract
Background and Purpose
Fractalkine (CX3CL1) is a unique chemokine that is constitutively expressed on neurons where it serves as an adhesion molecule for lymphocytes and monocytes. CX3CL1 may also be cleaved from the surface of these cells and enter the circulation to act as a traditional chemokine. CX3CL1 could thus influence the inflammatory response following stroke. We hypothesized that patients with higher plasma CX3CL1 after stroke would have a more robust inflammatory response and experience worse outcome.
Methods
Plasma CX3CL1 concentrations were assessed in 85 patients who were part of a larger study evaluating immune responses following ischemic stroke; CX3CL1 values were available from day 1 to day 180 after stroke onset. CX3CL1 was correlated to measures of inflammation and its effect on outcome assessed.
Results
At 1 day after stroke, CX3CL1 was lower in patients with severe strokes. At 180 days after stroke, CX3CL1 concentrations were lower in patients with poor outcome. The association of CX3CL1 and outcome at 180 days was independent of initial stroke severity. Plasma CX3CL1 at 180 days was inversely associated with systemic markers of inflammation, including white blood cell counts and high sensitivity C reactive protein.
Conclusions
In contrast to our original hypothesis, lower concentrations of CX3CL1 are associated with worse stroke outcome. In light of recent studies suggesting an immunomodulatory and neuroprotective role for CX3CL1 in a variety of neurodegenerative diseases, a therapeutic role for CX3CL1 in stroke recovery should be considered.
Keywords: fractalkine, CX3CL1, stroke, inflammation, outcome
Fractalkine, or CX3CL1, is a unique chemokine that exists in both a membrane bound form and a soluble form. CX3CL1 is expressed constitutively in neurons and its expression is inducible in vascular endothelial cells.1 The CX3CL1 receptor (CX3CR1) is expressed by monocytes, lymphocytes, natural killer (NK) cells, macrophages and microglia.2, 3 In its membrane bound form, CX3CL1 serves as an adhesion molecule for these cells, in its soluble form, it serves as a potent chemoattractant for them.1-3 In the central nervous system (CNS), the CX3CL1/CX3CR1 interaction regulates the communication between neurons, glia and microglia, is important in the response to injury and may contribute to neurogenesis.4-8 There are a number of publications suggesting that the CX3CL1/CX3CR1 pathway is important in the pathogenesis of neurodegenerative diseases like Alzheimer’s and Parkinson’s, but the findings are quite disparate, variably implicating CX3CL1/CX3CR1signaling as neuroprotective or neurotoxic.9-12
The potential contribution of CX3CL1 to ischemic brain injury has been explored in animal models of stroke. These studies show that CX3CL1 expression is upregulated in intact neurons within the penumbra while both CX3CL1 and CX3CR1 expression are upregulated in infarcted brain, the former in neurons and the latter in microglia.13 Mice deficient in CX3CL1 have smaller infarct volumes and improved survival following middle cerebral artery occlusion (MCAO).14 Similarly, CX3CR1 knock out (KO) mice have smaller infarcts and better functional outcome, as well as less inflammatory infiltrate and decreased expression of pro-inflammatory cytokines in the ischemic brain.15
In humans, cerebrospinal fluid (CSF) concentrations of CX3CL1 are increased in a wide range of traumatic and inflammatory neurologic diseases.16-19 In addition, circulating CX3CL1 is elevated in patients with multiple sclerosis and neuropsychiatric lupus.16, 18 To our knowledge, soluble CX3CL1 has not been assessed in the plasma of patients with ischemic stroke. Based on the experimental data, we hypothesized that patients with more severe strokes would have higher concentrations of plasma CX3CL1, more evidence of inflammation and worse stroke outcome. The plasma concentrations of the traditional leukocyte adhesion molecules vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 were also assessed since both are known to be elevated in plasma following ischemic stroke.20-23
Materials and Methods
Research Subjects
The patients in this study were part of a longitudinal study of immune responses after ischemic stroke. Patients were admitted to Harborview Medical Center from 9/2005 through 5/2009 and had to be at least 18 years and enrolled within 72 hours of symptom onset. Individuals with ongoing therapy for malignancy, known history of HIV, hepatitis B or C, history of brain tumor, anemia (hematocrit<35 on admission), and those taking immunomodulatory drugs were excluded. Blood was drawn as soon as possible after stroke onset and at 72 hours, 7 days, 90 days, and 180 days after stroke onset. Blood was also obtained from 40 volunteers to determine normative data for CX3CL1, VCAM-1 and ICAM-1 concentrations. The study was approved by the Institutional Review Board; all patients or their surrogates as well as control subjects provided informed consent.
Clinical Data
Clinical and demographic data were collected on all patients; information about therapeutic interventions for the treatment of stroke was collected. Stroke severity was determined by the National Institutes of Health Stroke Scale (NIHSS) score and outcome by the modified Rankin Scale (mRS), Glasgow Outcome Scale-extended (GOSE), and the Stroke Impact Scale (SIS).24-26 In hospital infection was defined as clinical symptoms of an infection (fever and/or pyruia for urinary tract infection [UTI] and fever and/or productive cough and radiographic evidence of consolidation for pneumonia [PNA]) and positive culture data (for both PNA and UTI). Total infarct volume on initial diffusion weighted MRI imaging was calculated by the ABC/2 method.27
Laboratory Studies
White blood cell (WBC) counts were determined by the clinical hematology laboratory. The concentration of high sensitivity C reactive protein (hsCRP) was determined by the hospital laboratory using standard methods. Additional plasma was immediately frozen at −80°; the concentrations of ICAM-1, VCAM-1, and CX3CL1 were determined by enzyme linked immunoassay (ELISA; R& D systems). The sensitivity of the assays was 0.096 ng/mL, 0.6 ng/mL and 0.018 ng/mL, respectively. Interleukin (IL)-6 was measured using a cytometric bead-based system (Fluorokine MAP; R&D Systems); the lower limit of detection was 1.1 pg/mL.
Statistics
Descriptive data are presented as median and interquartile range (IQR) for continuous variables; group comparisons were performed using the Kruskal-Wallis H test and the Mann-Whitney U test as appropriate. For correlations, parametric data was log transformed and the data presented as the Pearson Correlation Coefficient. Logistic regression was used to test the association between admission clinical and laboratory measures and poor outcome (GOSE <5) at 180 days, both unadjusted and adjusted for stroke severity as measured by the highest NIHSS in the first 72 hours or the total infarct volume on MRI. The odds ratio (OR) is used as the measure of association. Significance was set at P<0.05. No formal adjustment is made to P values to account for the fact that many predictor variables are tested; results should therefore be interpreted cautiously in light of the multiple comparisons performed.
Results
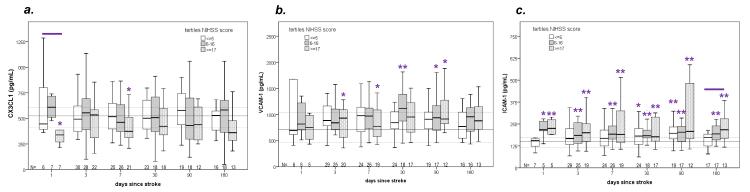
A total of 114 patients were enrolled in the parent study; plasma CX3CL1 concentrations were available for 85 of these 114 patients. The median age of these 85 patients was 56 years (46, 67), the median NIHSS score was 10 (4, 18), the median infarct volume was 10.7 cc (1.3, 68.5), and 33 (39%) were women. The highest plasma CX3CL1 by 72 hours in these 85 patients was 514 ng/mL (367, 650). In keeping with our prior publications on the larger patient population from the parent study, we categorized patients into tertiles based on stroke severity.28,29 Important clinical and demographic data from these tertiles are presented in the supplemental Table. Patients with more severe strokes were more likely to evidence systemic inflammation than patients with less severe strokes. Changes in plasma CX3CL1, VCAM-1 and ICAM-1 over time are depicted in Figure 1. Patients with the most severe strokes (NIHSS score ≥17) have lower plasma CX3CL1at 24 hours after stroke onset when compared to patients with less severe strokes. In comparison to the control population (N=40), patients with more severe strokes had lower concentrations of plasma CX3CL1 at days 1 and 7 (a) while the concentrations of circulating VCAM-1 (b) and ICAM-1 (c) were higher among patients with severe stroke at multiple time points after stroke.
Figure 1.
Concentrations of circulating CX3CL1 (a), VCAM-1 (b) and ICAM-1 (c) over the course of 180 days after stroke. The solid horizontal line represents the median value of the control population and the dashed lines the interquartile range. The solid line above the boxes indicates that the values among patients with different tertiles of stroke severity differ from each other at P<0.05 (Kruskal-Wallis H test); * indicates that the tertile of stroke severity differs from controls at P<0.05 and ** indicates that the tertile of stroke severity differs from controls at P<0.01 (Mann-Whitney U test).
The distribution of clinical outcomes at 180 days is presented in the upper portion of Table 1. Higher circulating concentrations of CX3CL1 at this time point are associated with better clinical outcome (higher scores on the GOSE and SIS and lower scores on the mRS). The correlation between outcome (mRS and SIS) and CX3CL1 persists even after controlling for initial stroke severity. There was no relationship between VCAM-1at 180 days and stroke outcome, and the relationship between ICAM-1 and outcome was explained by initial stroke severity.
Table 1.
Neurological outcomes at 180 days. Correlations between circulating concentrations of CX3CL1, VCAM-1 and ICAM-1 at 180 days and neurologic outcome at this time point are also presented using Pearson’s r. Data are normalized and analyses are both unadjusted and adjusted for initial stroke severity (using the NIHSS score or initial infarct volume). Poor outcome is arbitrarily defined as an outcome worse than 75% of the entire cohort.
| outcome measure : |
mRS | GOSE | SIS | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
median
(IQR) |
2 (1, 3) | 6 (5, 8) | 94 (69, 100) | ||||||
| correlati ons |
unadjust ed |
adjust ed for |
adjust ed for |
unadjust ed |
adjust ed for |
adjust ed for |
unadjust ed |
adjust ed |
adjust ed for |
| initial NIHS S |
initial infarct volum e |
initial NIHS S |
initial infarct volum e |
initial infarct volum e |
|||||
|
CX3CL
1 |
-0.397
P=0.005 |
-0.348
P=0.0 16 |
-0.415
P=0.0 04 |
0.421
P=0.005 |
0.171 NS |
0.278 P=0.0 62 |
0.421
P=0.005 |
0.337
P=0.0 29 |
0.374
P=0.0 15 |
|
VCAM- 1 |
−0.023 NS |
−0.095 NS |
−0.071 NS |
0.115 NS |
0.219 P=0.1 49 |
0.213 P=0.1 60 |
0.115 NS |
−0.164 NS |
−0.185 NS |
|
ICAM- 1 |
0.125 NS |
−0.135 NS |
0.024 NS |
-0.316
P=0.032 |
0.170 NS |
0.030 NS |
-0.316
P=0.032 |
−0.131 NS |
−0.251 P=0.0 97 |
|
poor
outcome : |
>3 | <5 | <70 | ||||||
mRS = modified Rankin Scale, GOSE = Glasgow Outcome Score - extended, SIS = Stroke Impact Scale, IQR = interquartile range, CX3CL1= fractalkine, VCAM-1 = vascular cell adhesion molecule-1, ICAM-1 = intercellular adhesion molecule-1, NS=not significant (P≥0.200)
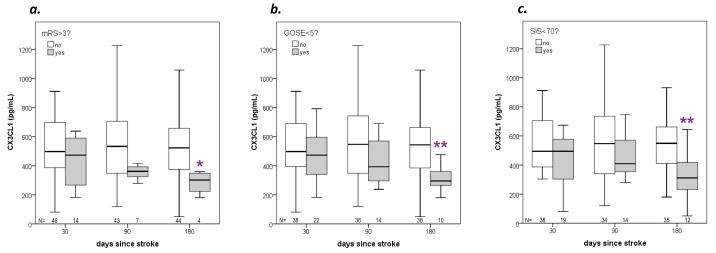
For the purpose of further analyses, poor outcome was considered to be an outcome worse than 75% of the entire cohort (values lower than the lower IQR for the SIS and GOSE and values higher than the upper IQR for the mRS). Initial values (at 1 day, 3 days or 7 days after stroke onset) of CX3CL1, VCAM-1 and ICAM-1 were not independently predictive of 180 day outcome (data not shown). Figure 2 depicts the changes in CX3CL1 over time based on outcomes at those time points. Even after controlling for initial stroke severity, at 180 days after stroke the concentration of CX3CL1 is lower in those patients with poor outcome than in those with better outcomes; there is also a trend towards decreasing CX3CL1 values over the course of time in patients with poor outcome compared to those with better outcomes. The concentrations of VCAM-1 and ICAM-1 were similar in patients with poor outcome and in those with better outcomes (data not shown). The difference in plasma CX3CL1 between patients with poor outcome and those without was most robust for the GOSE and the SIS (Figure 2). Using the GOSE as the indicator of outcome, important differences between those with a poor outcome and those without are presented in Table 2; data are adjusted for initial stroke severity using either the initial NIHSS score or infarct volume. The most consistent independent predictor of outcome (apart from NIHSS score or infarct volume) was patient age.
Figure 2.
Concentrations of CX3CL1 over the course of 180 days after stroke onset in patients with poor outcome compared to those without poor outcome (based on mRS, GOSE and SIS). *P<0.05 and **P<0.01 unadjusted for initial stroke severity (Mann-Whitney U test). (After controlling for initial stroke severity using the NIHSS score, the P value for CX3CL1 and mRS = 0.155, GOSE = 0.046 and SIS = 0.022; controlling for initial stroke severity using infarct volume, the P value for CX3CL1 and mRS = 0.132, GOSE = 0.015 and SIS = 0.014.)
Table 2.
Predictors of poor outcome (GOSE<5) at 180 days after stroke.
| at 180 days: | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
|---|---|---|---|---|---|---|
| unadjusted | adjusted for NIHSS |
adjusted for infarct volume |
||||
| baseline demographics (n=99) | ||||||
| age (per 10 years) |
1.684 (1.085- 2.614) |
0.020 |
4.304 (1.52- 812.12) |
0.006 |
2.489 (1.269- 4.884) |
0.008 |
| caucasian | 1.286 (0.126- 13.085) |
NS | 0.450 (0.031- 6.598) |
NS | 3.800 (0.082- 175.8) |
NS |
| male | 0.903 (0.326- 2.496) |
NS | 1.219 (0.255- 5.837) |
NS | 0.889 (0.243- 3.252) |
NS |
| HTN | 1.111 (0.409- 3.021) |
NS | 1.046 (0.224- 4.893) |
NS | 1.588 (0.433- 1.301) |
NS |
| DM | 2.778 (0.964- 8.006) |
0.059 | 1.673 (0.319- 8.784) |
NS | 3.470 (0.907- 13.27) |
0.069 |
| CAD | 1.714 (0.587- 5.006) |
NS | 2.290 (0.384- 13.65) |
NS | 3.522 (0.875- 14.17) |
0.076 |
| AF | 0.868 (0.207- 3.636) |
NS | 1.344 (0.122- 14.82) |
NS | 1.172 (0.188- 7.317) |
NS |
| smoker | 2.471 (0.891- 6.850) |
0.082 | 1.837 (0.381- 8.861) |
NS | 2.433 (0.664- 8.916) |
0.180 |
| old stroke on imaging* | 2.050 (0.655- 6.414) |
NS |
60.50 (2.120- 1726) |
0.016 | 4.361 (0.992- 19.16) |
0.051 |
| temperature (per °C) |
3.920 (1.560- 9.855) |
0.004 | 1.423 (0.459- 4.408) |
NS | 2.126 (0.805- 5.619) |
0.128 |
| initial stroke characteristics and treatment (n=99, except for infarct volume, where n=97) | ||||||
| NIHSS (per point) |
1.364 (1.188- 1.567) |
<0.001 | --- | --- | 1.043 (0.956- 1.138) |
NS |
| infarct volume (per 10 cc)* |
1.182 (1.079- 1.295) |
<0.001 | 1.043 (0.956- 1.138) |
NS | --- | --- |
| IV tPA | 1.267 (0.427- 3.754) |
NS | 1.240 (0.249- 6.188) |
NS | 1.496 (0.367- 6.089) |
NS |
| endovascular therapy | 3.630 (0.739- 17.824) |
0.112 | 1.681 (0.177- 15.948) |
NS | 1.874 (0.243- 14.451) |
NS |
| hemicraniectomy |
11.333 (1.187- 108.2) |
0.035 | 1.479 (0.099- 21.99) |
NS | 0.015 (0.000- 1.028) |
0.052 |
| initial laboratory values (highest value by 72 hours; n≥65 for all variables) | ||||||
| WBCs (per thou/mL) |
1.176 (1.034- 1.337) |
0.014 | 0.890 (0.718- 1.103) |
NS | 1.078 (0.916- 1.269) |
NS |
| PMNs (per thou/mL) |
1.438 (1.139- 1.815) |
0.002 | 1.162 (0.809- 1.668) |
NS |
1.321 (1.017- 1.716) |
0.037 |
| lymphs (per thou/mL) | 0.699 (0.261- 1.869) |
NS | 0.986 (0.247- 3.944) |
NS | 0.505 (0.119- 2.152) |
NS |
| monos (per thou/mL) | 15.760 (2.023- 122.7) |
0.008 | 2.736 (0.081- 92.20) |
NS | 8.691 (0.617- 122.4) |
0.109 |
| hsCRP (per 10 mg/L) |
1.236 (1.082- 1.411) |
0.002 | 1.339 (1.158- 1.548) |
NS |
1.161 (1.016- 1.328) |
0.029 |
| IL-6 (per pg/mL) |
1.067 (1.010- 1.126) |
0.020 | 1.038 (0.951- 1.133) |
NS |
1.061 (1.007- 1.119) |
0.026 |
| glucose (per 10 /dL) |
1.090 (1.001- 1.189) |
0.049 | 1.069 (0.925- 1.235) |
NS | 1.085 (0.979- 1.202) |
0.119 |
|
CX3CL1 (per 100 - pg/mL) |
0.894 (0.272- 1.100) |
NS | 1.037 (0.814- 1.322) |
NS | 1.011 (0.814- 1.257) |
NS |
|
VCAM-1 (per 100
pg/mL) |
0.820 (0.681- 0.987) |
0.036 | 0.830 (0.585- 1.178) |
NS | 0.905 (0.733- 1.118) |
NS |
|
ICAM-1 (per 100
pg/mL) |
1.034 (0.625- 1.711) |
NS | 0.723 (0.343- 1.524) |
NS | 0.998 (0.521- 1.912) |
NS |
| medical complications (n=99) | ||||||
| any infection |
9.200 (2.785- 30.39) |
<0.001 | 3.277 (0.553- 19.42) |
0.191 |
9.840 (2.149- 45.04) |
0.003 |
| stroke etiology (n=99) | ||||||
| lacunar | NC | --- | NC | --- | NC | --- |
| cardioembolic | 1.096 (0.331- 3.635) |
NS | 1.298 (0.187- 9.025) |
NS | 1.320 (0.281- 6.208) |
NS |
|
large vessel
atherosclerosis |
5.371 (1.513- 19.06) |
0.009 | 4.539 (0.641- 32.16) |
0.130 |
6.090 (1.280- 28.97) |
0.023 |
| other | 0.606 (0.191- 1.919) |
NS | 0.406 (0.073- 2.258) |
NS | 0.487 (0.112- 2.116) |
NS |
| unknown | 0.603 (0.174- 2.094) |
NS | 0.587 (0.087- 3.949) |
NS | 0.454 (1.081- 1.300) |
NS |
GOSE=Glasgow Outcome Scale - extended, OR=odds ratio, CI=confidence interval; HTN=hypertension, DM=diabetes mellitus, CAD=coronary artery disease, AF=atrial fibrillation, IV tPA=intravenous tissue plasminogen activator, WBCs=white blood cells, PMNs=polymorphonuclear cells, lymphs=lymphocytes, monos=monocytes, hsCRP=high sensitivity C reactive protein, IL=interleukin. CX3CL1=fractalkine, VCAM=vascular cell adhesion molecule, ICAM=intercellular adhesion molecule.
there were 2 patients who did not have an admission MRI; information regarding infarct volume and old strokes was therefore not available. NS=not significant (P>0.200), NC=not calculable
Table 3 shows the correlation between CX3CL1 and initial stroke severity as well as between CX3CL1 and circulating leukocytes, hsCRP and IL-6 at various time points after stroke. There is a trend towards an inverse relationship between CX3CL1 and peripheral markers of inflammation at multiple time points after stroke, but the relationship is most marked at 180 days after stroke.
Table 3.
Correlations between plasma CX3CL1, initial stroke severity (as determined by NIHSS score and infarct volume) and inflammatory markers at various time points after stroke. Data are presented as Pearson’s r. The correlation of CX3CL1 with inflammatory markers is adjusted for initial stroke severity using the NIHSS score or the infarct volume.
| day from strok e |
NIHS S |
infarc t volum e |
WBCs | PMNs | lymphs | monos | hsCRP | IL-6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| adjuste d for NIHS S |
adjuste d for infarct volum e |
adjuste d for NIHS S |
adjuste d for infarct volum e |
adjust ed for NIHS S |
adjust ed for infarct volum e |
adjuste d for NIHS S |
adjuste d for infarct volum e |
adjuste d for NIHS S |
adjuste d for infarct volum e |
adjust ed for NIHS S |
adjust ed for infarct volum e |
|||
| 1 | −0.343 P=0.1 50 |
−0.283 NS |
−0.230 NS |
−0.292 NS |
−0.121 NS |
−0.210 NS |
−0.104 NS |
−0.073 NS |
0.045 NS |
0.024 NS |
−0.439 P=0.1 77 |
−0.508 P=0.1 10 |
0.085 NS |
−0.034 NS |
| 3 | −0.122 NS |
−0.140 NS |
−0.114 NS |
−0.106 NS |
−0.017 NS |
−0.028 NS |
−0.157 P=0.1 97 |
−0.130 NS |
−0.105 NS |
−0.096 NS |
−0.016 NS |
−0.004 NS |
0.002 NS |
0.006 NS |
| 7 |
-0.196
P=0.0 09 |
-0.261
P=0.0 29 |
−0.056 NS |
−0.065 NS |
0.023 NS |
−0.050 NS |
−0.117 NS |
-.093 NS |
−0.209 0.103 |
−0.239 P=0.0 66 |
−0.019 NS |
0.015 NS |
0.054 NS |
0.066 NS |
| 30 | −0.147 NS |
−0.119 NS |
−0.174 P=0.1 91 |
−0.184 P=0.1 70 |
−0.150 NS |
−0.162 NS |
−0.116 NS |
−0.118 NS |
−0.349 P=0.0 07 |
−0.352 P=0.0 07 |
−0.097 NS |
−0.118 NS |
−0.128 NS |
−0.128 NS |
| 90 | −0.232 P=0.1 09 |
−0.091 NS |
−0.250 P=0.0 94 |
−0.270 P=0.0 73 |
−0.251 P=0.1 00 |
−0.283 P=0.0 66 |
−0.118 NS |
−0.133 NS |
−0.152 NS |
−0.165 NS |
−0.101 NS |
−0.146 NS |
0.100 NS |
0.101 NS |
| 180 | −0.209 P=0.1 58 |
−0.095 NS |
−0.423 P=0.0 03 |
−0.467 P=0.0 01 |
−0.483 P=0.0 01 |
−0.517 P<0.0 01 |
0.030 NS |
0.041 NS |
−0.271 0.072 |
−0.302
P=0.0 44 |
−0.332
P=0.0 24 |
−0.384
P=0.0 08 |
−0.224 P=0.1 39 |
−0.254 P=0.0 92 |
CX3CL1= fractalkine, NIHSS = National Institutes of Health Stroke Scale, WBCs = white blood cells, PMNs = polymorphonuclear cells, lymphs = lymphocytes, monos=monocytes, hsCRP= high sensitivity C reactive protein, IL = interleukin, NS=not significant (P≥0.200).
Discussion
In contrast to our original hypothesis, we found that patients with more severe strokes (NIHSS score ≥17) had decreased concentrations of plasma CX3CL1 in comparison to patients with less severe strokes and control subjects at 1 day after stroke onset. This relative decrease in CX3CL1 in comparison to controls appeared to persist to 180 days after stroke, but was only significant at 7 days after stroke. Most striking was the finding that patients with poor neurological outcome at 180 days after stroke (defined by the mRS, GOSE and SIS) had significantly lower plasma CX3CL1 than patients with better outcomes. Importantly, plasma concentrations of CX3CL1 early after stroke onset were not predictive of long term outcome.
To our knowledge, this study is the first to address the role of CX3CL1 in patients with stroke. In vitro, neuronal injury leads to cleavage of CX3CL1 from its membrane bound form.30,31 Whether this cleaved CX3CL1 enters the circulation or remains within the local microenvironment is unknown. Published animal data provide information only about the expression of CX3CL1 in the brain following stroke.13 In review of our own animal data, however, we find that the concentration of plasma CX3CL1 is lower in animals undergoing transient middle cerebral artery occlusion as compared to those undergoing a sham surgery (P=0.071) one month after the surgical procedure (unpublished data). Our clinical data are in keeping with this observation.
CX3CL1 acts as both an adhesion molecule and chemotactic agent for lymphocytes, monocytes, NK cells and microglia, suggesting that it should help to mediate the inflammatory response. There are accumulating data, however, that suggest the situation is a bit more complex than initially appreciated. For instance, CX3CL1 can suppress microglial activation and decrease the release of pro-inflammatory cytokines.8,11,32,33 In fact, the enhanced microglial activation in the brains of aged animals is associated with (and may be due to) decreased CX3CL1 expression in these older animals.34,35 CX3CL1 also appears to modulate the response to systemic inflammatory stimuli since CX3CL1 deficient mice display enhanced “sickness” behavior following injection of lipopolysaccharide (LPS).36 CX3CL1 may also protect neurons against excitotoxic injury.37 Based on these observations, a recent study was undertaken to address the potential neuroprotective properties of CX3CL1 and found that intracerebroventricular administration of the chemokine improved outcome from stroke through an apparent inhibition of microglial activation.38 To explore whether CX3CL1 affected the inflammatory response in our patient cohort, we evaluated systemic markers of inflammation, including the numbers of circulating WBCs, plasma hsCRP and IL-6. Higher CX3CL1 was associated with a trend towards decreased WBCs, polymorphonuclear cells (PMNs), monocytes and hsCRP at multiple time points after stroke; these associations were significant at 180 days after stroke.
The potential role of CX3CL1 and its interactions with CXC3R in neurodegenerative diseases is actively being studied, although the data are somewhat contradictory.9-12 In addition to its potential to modulate the inflammatory response in the brain, CX3CL1 appears to be important in neurogenesis.8 Neuroprotective properties of CX3CL1 have been demonstrated both in vitro and in vivo.11, 39-41 Presuming that plasma CX3CL1 has access to brain, it is possible that a relative deficiency of CX3CL1 at later time points after stroke (ie. 180 days) hinders important recovery processes. It is interesting to note that patients with mild cognitive impairment have higher circulating CX3CL1 than patients with Alzheimer’s disease, and patients with mild/moderate Alzheimer’s disease have higher circulating CX3CL1 than patients with severe Alzheimer’s disease.42
There are several limitations to this study, including the relatively small number of patients for which CX3CL1 data were available (N=85), and the very small number with CX3CL1 measured at 24 hours. The wide variation in stroke severity and the quality of outcome measures, however, allow for a full description of the changes in CX3CL1 and its association with the clinical course after stroke. And as already mentioned, circulating CX3CL1 was assessed given that direct access to the CNS compartment is not available in clinical studies; evaluation of the local expression of CX3CL1 may have yielded different results. And because CX3CL1 is expressed by both neurons and endothelial cells, we have no way of knowing whether increased plasma CX3CL1 in patients with good outcome reflects increases in neuronal CX3CL1, endothelial CX3CL1 or both.
In summary, our data suggest that decreased CX3CL1 may be associated with more systemic inflammation and worse long-term outcome form stroke. Based on these data as well as emerging experimental data that suggest a neuroprotective effect of exogenous CX3CL1 administration, further studies to address the potential neuroprotective properties of CX3CL1 in stroke are warranted. Perhaps most exciting is the possibility that CX3CL1 could in some way modulate stroke recovery given its potential role in neurogenesis and that differences in CX3CL1 were apparent at 180 days in patients with poor outcome compared to those with better outcome. Additional studies with this chemokine in experimental stroke to assess both its neuroprotective and neurorestorative roles are warranted.
Supplementary Material
Acknowledgements
We thank the individuals who volunteered to participate in this study.
Funding
This study was funded by NINDS 5R01NS049197.
Acknowledgements/Funding: This work was funded by National Institute of Neurological Disorders and Stroke R01NS049197.
Footnotes
Disclosures
The authors have no other disclosures.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a cx3c motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 2.Fong S, Jones S, Renz ME, Chiu HH, Ryan AM, Presta LG, et al. Mucosal addressin cell adhesion molecule-1 (madcam-1). Its binding motif for alpha 4 beta 7 and role in experimental colitis. Immunol Res. 1997;16:299–311. doi: 10.1007/BF02786396. [DOI] [PubMed] [Google Scholar]
- 3.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, et al. Identification and molecular characterization of fractalkine receptor cx3cr1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 4.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and cx3cr1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruitenberg MJ, Vukovic J, Blomster L, Hall JM, Jung S, Filgueira L, et al. Cx3cl1/fractalkine regulates branching and migration of monocyte-derived cells in the mouse olfactory epithelium. J Neuroimmunol. 2008;205:80–85. doi: 10.1016/j.jneuroim.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- 7.Maggi L, Scianni M, Branchi I, D’Andrea I, Lauro C, Limatola C. Cx(3)cr1 deficiency alters hippocampal-dependent plasticity phenomena blunting the effects of enriched environment. Front Cell Neurosci. 2011;5:22. doi: 10.3389/fncel.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, et al. Fractalkine and cx 3 cr1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, et al. Cx3cr1 deficiency alters microglial activation and reduces beta-amyloid deposition in two alzheimer’s disease mouse models. Am J Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, et al. Cx3cr1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of alzheimer disease. J Biol Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pabon MM, Bachstetter AD, Hudson CE, Gemma C, Bickford PC. Cx3cl1 reduces neurotoxicity and microglial activation in a rat model of parkinson’s disease. J Neuroinflammation. 2011;8:9. doi: 10.1186/1742-2094-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shan S, Hong-Min T, Yi F, Jun-Peng G, Yue F, Yan-Hong T, et al. New evidences for fractalkine/cx3cl1 involved in substantia nigral microglial activation and behavioral changes in a rat model of parkinson’s disease. Neurobiol Aging. 2011;32:443–458. doi: 10.1016/j.neurobiolaging.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Tarozzo G, Campanella M, Ghiani M, Bulfone A, Beltramo M. Expression of fractalkine and its receptor, cx3cr1, in response to ischaemia-reperfusion brain injury in the rat. Eur J Neurosci. 2002;15:1663–1668. doi: 10.1046/j.1460-9568.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 14.Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 15.Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of cx3cr1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- 16.Kastenbauer S, Koedel U, Wick M, Kieseier BC, Hartung HP, Pfister HW. Csf and serum levels of soluble fractalkine (cx3cl1) in inflammatory diseases of the nervous system. J Neuroimmunol. 2003;137:210–217. doi: 10.1016/s0165-5728(03)00085-7. [DOI] [PubMed] [Google Scholar]
- 17.Rancan M, Bye N, Otto VI, Trentz O, Kossmann T, Frentzel S, et al. The chemokine fractalkine in patients with severe traumatic brain injury and a mouse model of closed head injury. J Cereb Blood Flow Metab. 2004;24:1110–1118. doi: 10.1097/01.WCB.0000133470.91843.72. [DOI] [PubMed] [Google Scholar]
- 18.Yajima N, Kasama T, Isozaki T, Odai T, Matsunawa M, Negishi M, et al. Elevated levels of soluble fractalkine in active systemic lupus erythematosus: Potential involvement in neuropsychiatric manifestations. Arthritis Rheum. 2005;52:1670–1675. doi: 10.1002/art.21042. [DOI] [PubMed] [Google Scholar]
- 19.Kasama T, Odai T, Wakabayashi K, Yajima N, Miwa Y. Chemokines in systemic lupus erythematosus involving the central nervous system. Front Biosci. 2008;13:2527–2536. doi: 10.2741/2864. [DOI] [PubMed] [Google Scholar]
- 20.Fassbender K, Mossner R, Motsch L, Kischka U, Grau A, Hennerici M. Circulating selectin- and immunoglobulin-type adhesion molecules in acute ischemic stroke. Stroke. 1995;26:1361–1364. doi: 10.1161/01.str.26.8.1361. [DOI] [PubMed] [Google Scholar]
- 21.Blann A, Kumar P, Krupinski J, McCollum C, Beevers DG, Lip GY. Soluble intercelluar adhesion molecule-1, e-selectin, vascular cell adhesion molecule-1 and von willebrand factor in stroke. Blood Coagul Fibrinolysis. 1999;10:277–284. doi: 10.1097/00001721-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Shyu KG, Chang H, Lin CC. Serum levels of intercellular adhesion molecule-1 and e-selectin in patients with acute ischaemic stroke. J Neurol. 1997;244:90–93. doi: 10.1007/s004150050055. [DOI] [PubMed] [Google Scholar]
- 23.Simundic AM, Basic V, Topic E, Demarin V, Vrkic N, Kunovic B, et al. Soluble adhesion molecules in acute ischemic stroke. Clin Invest Med. 2004;27:86–92. [PubMed] [Google Scholar]
- 24.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, et al. Ninds tpa stroke trial investigators Underlying structure of the national institutes of health stroke scale: Results of a factor analysis. Stroke. 1999;30:2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 25.van Swieten J, Koudstaal P, Visser M, Schouten H, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 26.Levin HS, Boake C, Song J, McCauley S, Contant C, Diaz-Marchan P, et al. Validity and sensitivity to change of the extended glasgow outcome scale in mild to moderate traumatic brain injury. J Neurotrauma. 2001;18:575–584. doi: 10.1089/089771501750291819. [DOI] [PubMed] [Google Scholar]
- 27.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. Abc/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zierath D, Tanzi P, Cain K, Shibata D, Becker K. Plasma {alpha}-melanocyte stimulating hormone predicts outcome in ischemic stroke. Stroke. 2011;42:3415–3420. doi: 10.1161/STROKEAHA.111.627331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanzi P, Cain K, Kalil A, Zierath D, Savos A, Gee JM, et al. Post-stroke infection: A role for il-1ra? Neurocrit Care. 2011;14:244–252. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatori K, Nagai A, Heisel R, Ryu JK, Kim SU. Fractalkine and fractalkine receptors in human neurons and glial cells. J Neurosci Res. 2002;69:418–426. doi: 10.1002/jnr.10304. [DOI] [PubMed] [Google Scholar]
- 31.Chapman GA, Moores K, Harrison D, Campbell CA, Stewart BR, Strijbos PJ. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20:RC87. doi: 10.1523/JNEUROSCI.20-15-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates tnf-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]
- 33.Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- 34.Duan RS, Yang X, Chen ZG, Lu MO, Morris C, Winblad B, et al. Decreased fractalkine and increased ip-10 expression in aged brain of app(swe) transgenic mice. Neurochem Res. 2008;33:1085–1089. doi: 10.1007/s11064-007-9554-z. [DOI] [PubMed] [Google Scholar]
- 35.Lyons A, Lynch AM, Downer EJ, Hanley R, O’Sullivan JB, Smith A, et al. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110:1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- 36.Corona AW, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, et al. Fractalkine receptor (cx3cr1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauro C, Cipriani R, Catalano M, Trettel F, Chece G, Brusadin V, et al. Adenosine a1 receptors and microglial cells mediate cx3cl1-induced protection of hippocampal neurons against glu-induced death. Neuropsychopharmacology. 2010;35:1550–1559. doi: 10.1038/npp.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, et al. Cx3cl1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci. 2011;31:16327–16335. doi: 10.1523/JNEUROSCI.3611-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of cx3cr1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Limatola C, Lauro C, Catalano M, Ciotti MT, Bertollini C, Di Angelantonio S, et al. Chemokine cx3cl1 protects rat hippocampal neurons against glutamate-mediated excitotoxicity. J Neuroimmunol. 2005;166:19–28. doi: 10.1016/j.jneuroim.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Deiva K, Geeraerts T, Salim H, Leclerc P, Hery C, Hugel B, et al. Fractalkine reduces n-methyl-d-aspartate-induced calcium flux and apoptosis in human neurons through extracellular signal-regulated kinase activation. Eur J Neurosci. 2004;20:3222–3232. doi: 10.1111/j.1460-9568.2004.03800.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim TS, Lim HK, Lee JY, Kim DJ, Park S, Lee C, et al. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and alzheimer’s disease. Neurosci Lett. 2008;436:196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.