Abstract
Background and Purpose
Enhanced angiogenesis facilitates neurovascular remodeling processes and promotes brain functional recovery after stroke. Previous studies from our laboratory demonstrated that valproate (VPA), a histone deacetylase (HDAC) inhibitor, protects against experimental brain ischemia. The present study investigated whether VPA could enhance angiogenesis and promote long-term functional recovery after ischemic stroke.
Methods
Male rats underwent middle cerebral artery occlusion (MCAO) for 60 minutes followed by reperfusion for up to 14 days. Assessed parameters were: locomotor function via rotarod test; infarct volume via T2-weighted magnetic resonance imaging; microvessel density via immunohistochemistry; relative cerebral blood flow (rCBF) via perfusion-weighted imaging; protein levels of pro-angiogenic factors via Western blotting; and matrix metalloproteinase (MMP)-2/9 activities via gelatin zymography.
Results
Post-ischemic VPA treatment robustly improved the rotarod performance of MCAO rats on days 7 and 14 after ischemia, and significantly reduced brain infarction on day 14. Concurrently, VPA markedly enhanced microvessel density, facilitated endothelial cell proliferation, and increased rCBF in the ipsilateral cortex. The transcription factor hypoxia-inducible factor (HIF)-1α and its downstream pro-angiogenic factors, vascular endothelial growth factor (VEGF) and MMP-2/9, were upregulated after MCAO and significantly potentiated by VPA in the ipsilateral cortex. Acetylation of histone-H3 and H4 was robustly increased by chronic VPA treatment. The beneficial effects of VPA on rotarod performance and microvessel density were abolished by HIF-1α inhibition.
Conclusions
Chronic VPA treatment enhances angiogenesis and promotes functional recovery after brain ischemia. These effects may involve HDAC inhibition and upregulation of HIF-1α and its downstream pro-angiogenic factors VEGF and MMP-2/9.
Keywords: angiogenesis, cerebral ischemia, hypoxia-inducible factor-1, matrix metalloproteinase, MRI, valproate, vascular endothelial growth factor
INTRODUCTION
Angiogenesis is a key component of post-stroke neurovascular remodeling processes, in which new capillaries are formed through directed proliferation and migration of endothelial progenitor cells from pre-existing blood vessels. Post-mortem studies reveal that angiogenesis can be observed several days after cerebral ischemic stroke; notably, higher microvessel density correlates with longer patient survival.1 In the rodent brain, endothelial cell proliferation and capillary sprouting begin as early as 24–48 hours after ischemic injury.2–3 Post-stroke angiogenesis increases collateral circulation and restores oxygen and nutrient supply to the injured tissue. Moreover, newly generated vessels provide neurotrophic support to concurrent neurogenesis and synaptogenesis, and these ultimately lead to functional recovery.4 Therefore, strategies that enhance post-stroke angiogenesis hold great promise for the treatment of stroke.
Vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) are key pro-angiogenic factors that increase after ischemia in both rodent and human brains.5–8 VEGF induces endothelial cell proliferation and mediates the effects of other pro-angiogenic factors.9–10 MMPs set the stage for endothelial cell migration by degrading the extracellular matrix.11 VEGF and MMPs are regulated by the transcription factor hypoxia-inducible factor-1 (HIF-1),12 which regulates gene transcription to facilitate adaptation and survival after hypoxia/ischemia. Late inhibition of the HIF-1α subunit appears to worsen ischemic outcomes.13 Therefore, enhanced HIF-1 activation and HIF-1-dependent transcription could be advantageous in ischemic stroke.
Valproate (VPA), a histone deacetylase (HDAC) inhibitor, is commonly used to treat seizures and bipolar disorder. Studies have shown that VPA has protective properties in cellular and animal models of neurodegenerative diseases, including stroke.14–15 We have recently demonstrated that post-ischemic VPA treatment markedly attenuates blood-brain barrier (BBB) disruption and brain edema in a rat model of middle cerebral artery occlusion (MCAO), and this protection persists at least three days after ischemia.16 However, the effects of VPA on later-phase recovery remain unclear. The present study investigated whether chronic VPA treatment can enhance post-ischemic angiogenesis and promote functional recovery in a rat MCAO model.
MATERIALS AND METHODS
MCAO and drug administration
All animal experiments were performed according to protocols approved by the National Institute of Mental Health Animal Care and Use Committee. Male Sprague-Dawley rats (200–220 g, Charles River Laboratories, Wilmington, MA) underwent right MCAO under inhalational anesthesia (1.5% isoflurane in 70% N2O and 30% O2) as previously described.16–17 Detailed procedures are available online (Supplemental Methods, http://stroke.ahajournals.org).
VPA (200 mg/kg, i.p., Sigma, St. Louis, MO) was administrated immediately after ischemic onset, 12 hours later and then once daily for up to 14 days. 2-methoxyestradiol (2ME2, 5 mg/kg, i.p., Sigma) was dissolved in 1% DMSO and co-injected with VPA once daily for 10 days, beginning on day 4 after reperfusion.
Accelerating rotarod test
An accelerating rotarod apparatus (San Diego Instruments, San Diego, CA) was used to measure motor skill learning and coordination in the MCAO rats; the speed was accelerated from 0 to 40 rpm over 4 minutes.17 Rats received once daily training sessions of three trials separated by 30-minute intervals for three consecutive days before MCAO. The longest amount of time each rat remained on the rod was recorded as baseline. Seven and 14 days after MCAO, rats underwent three trials on the rotarod, and the best performance of each rat was recorded for that day.
Magnetic Resonance Imaging (MRI)
All MRI experiments were performed on a 7-T (Bruker Avance, Billerica, MA), 210 mm horizontal scanner. Infarct volume and relative cerebral blood flow (rCBF) on day 14 after MCAO were evaluated using T2-weighted MRI and perfusion-weighted imaging (PWI), respectively. Detailed procedures are available online (Supplemental Methods).
Immunohistochemistry
Microvessel density in the ipsilateral cortex was assessed by CD31 immunostaining on days 7 and 14. Endothelial cell proliferation was evaluated by double immunofluorescent staining with RECA-1 (an endothelial cell marker) and Ki67 (a cell proliferation marker) on day 14. Detailed procedures are available online (Supplemental Methods).
Western blotting
The protein levels of HIF-1α, VEGF and acetylated histone-H3 and H4 in the ipsilateral cortex were detected by Western blotting on days 7 and 14. Detailed procedures are available online (Supplemental Methods).
Gelatin zymography
The activities of MMP-2 and MMP-9 were measured by gelatin zymography, and the pro- and active forms of MMP-2/9 were identified by reference to their respective standards as previously described.16 Detailed procedures are available online (Supplemental Methods).
Statistical Analyses
Data are expressed as mean±SEM. For rotarod data, two-way repeated measures ANOVA were performed to analyze the overall difference between treatment groups over time. Bonferroni corrected post-hoc comparisons were then used to analyze the difference between treatment groups at each time point. Comparisons between two groups and multiple groups were evaluated by Student's t-test and one-way ANOVA followed by Tukey’s post-hoc comparisons, respectively. Differences were considered statistically significant at P<0.05.
RESULTS
VPA reduced brain infarct volume and promoted functional recovery
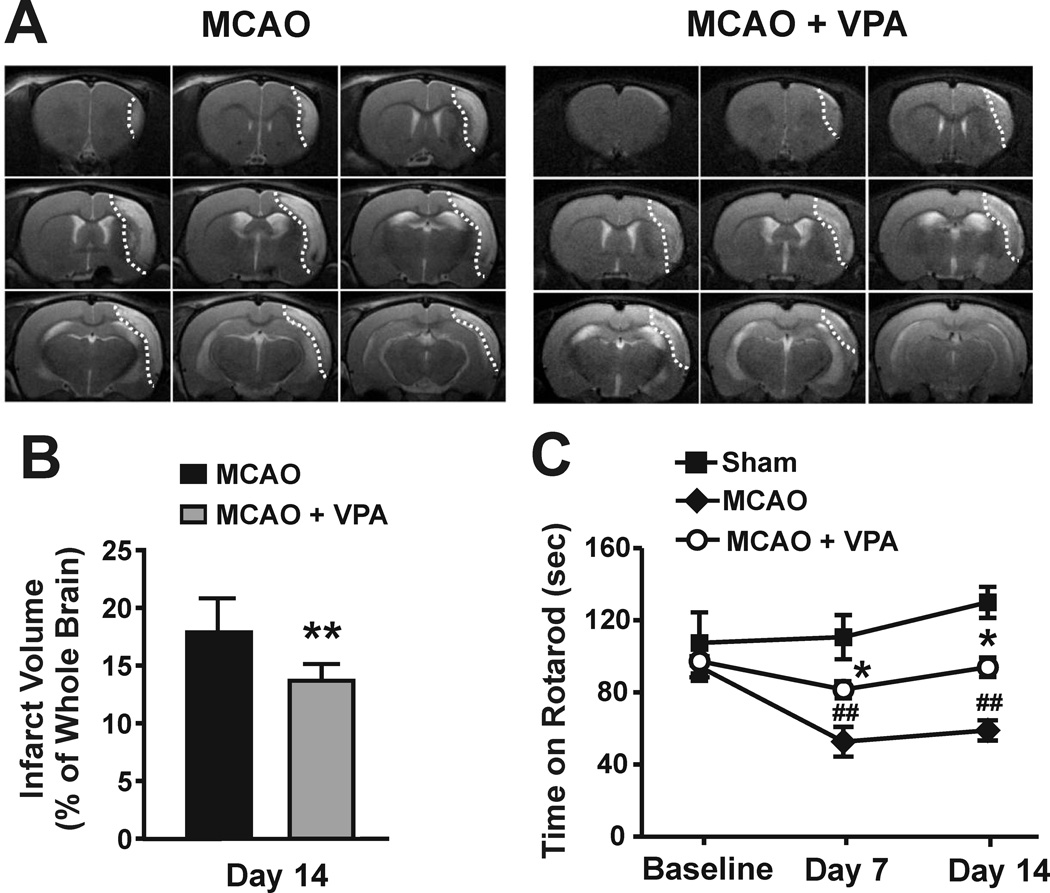
On day 14, T2-weighted images showed that infarction comprised approximately 18% of the whole brain in MCAO rats. VPA treatment significantly reduced the infarct size to 13% (Figure 1A and B). To confirm the effect of VPA on MCAO-induced brain infarction, we further measured the infarct area on day 14 using hematoxylin and eosin staining. The infarct area in the VPA-treated group was significantly smaller compared with the untreated MCAO rats (11.2±1.3 versus 17.3±1.4 mm2; Supplemental Figure S1). The length of time that rats were able to stay on the accelerating rotarod was markedly reduced on days 7 and 14 after MCAO. Post-ischemic treatment with VPA robustly ameliorated this motor function deficit by prolonging rotarod retention time (Figure 1C).
Figure 1. Post-ischemic chronic treatment with VPA reduced brain infarct volume and improved functional recovery in MCAO rats.
(A and B) Brain infarction was detected by T2-weighted MRI. VPA significantly reduced the brain infarct volume in MCAO rats on day 14. N = 5 per group. The infarction is shown in the dotted areas. (C) Behavioral deficits were evaluated by rotarod test. VPA markedly increased the retention time of MCAO rats on an accelerating rotarod on days 7 and 14 after ischemia. N = 8 per group. ##P<0.01 compared with sham control; *P<0.05, **P<0.01 compared with MCAO group.
VPA enhanced post-ischemic angiogenesis
To investigate whether VPA enhanced post-ischemic angiogenesis, microvessel density was first analyzed by immunostaining for CD31, an endothelial cell marker, in the ipsilateral cortex of MCAO rats. On days 7 and 14 after MCAO, microvessel density in the ipsilateral cortex was increased to 136.1±4.0% and 155.3±4.0% of the corresponding contralateral side, respectively, compared with sham-operated rats (100±1.5% for day 7 and 100±2.4% for day 14; Figure 2A). VPA treatment further enhanced microvessel density to 148.4±3.9% and 182.6±6.4% on days 7 and 14, respectively. Microvessel density in the contralateral cortex was not affected by MCAO alone or with VPA treatment (data not shown). Endothelial cell proliferation was examined by double staining Ki67 and RECA-1. VPA markedly increased Ki67 and RECA-1 double positive cells in the ipsilateral cortex of MCAO rats from 5.3±0.8% to 8.8±0.9% of Ki76-positive cells on day 14 (Figure 2B), indicating that VPA promoted endothelial cell proliferation.
Figure 2. VPA enhanced post-ischemic angiogenesis in the ipsilateral cortex.
(A) CD31 microvessel staining in the boxed area. MCAO increased microvessel density on days 7 and 14, and VPA markedly enhanced this increase. N = 10 per group. (B) VPA significantly increased endothelial cell proliferation on day 14. N = 7 per group. ##P<0.01 compared with the sham control, *P<0.05, **P<0.01 compared with MCAO group.
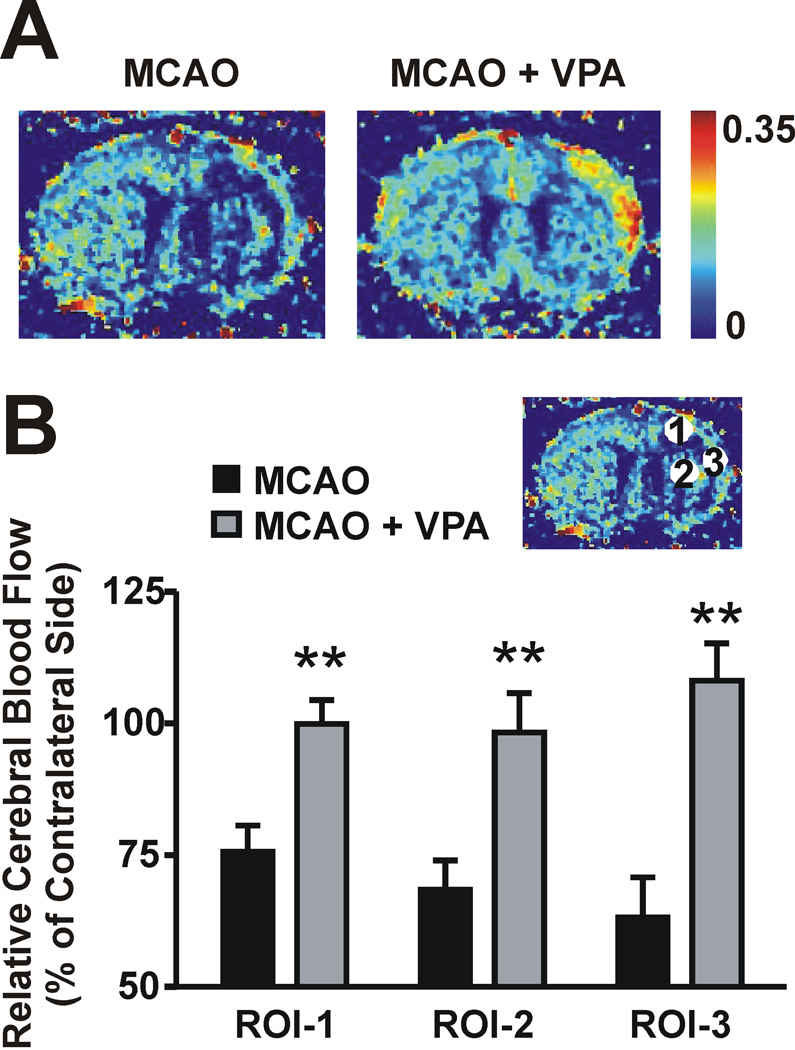
The effects of VPA on enhanced microvessel density were more pronounced on day 14 than 7 after MCAO. Therefore, PWI maps were quantitatively analyzed for the hemodynamic changes on day 14. Figure 3A shows representative PWI maps from an untreated and a VPA-treated MCAO rat (corresponding T2 maps are presented in Figure 1A). PWI analysis was carried out in three regions of interest (ROI) indicated by the circled numbers on the lesioned side of the brain in Figure 3B. ROI-1 and 3 were located in the cortex and ROI-2 was in the striatum. The T2 value was higher in ROI-3, compared with the values in ROI-1 and 2 (Supplemental Figure S2), indicating that the severity of the lesion was greatest in ROI-3. The rCBF ratio of the ipsilateral hemisphere to the contralateral hemisphere was calculated at each ROI. On day 14 after MCAO, the rCBF ratios in all three ROIs declined to 60–70% of their respective contralateral areas, and ROI-3 had the lowest rCBF ratio. Notably, VPA treatment robustly increased the rCBF ratio in all three ROIs (99.9±4.6% versus 75.7±5.0% for ROI-1, 108.1±7.1% versus 68.5±5.6% for ROI-2, and 98.3±7.5% versus 63.1±7.6% for ROI-3), suggesting that VPA enhanced post-ischemic angiogenesis in both the ipsilateral cortex and striatum.
Figure 3. VPA increased relative cerebral blood flow (rCBF) in the ipsilateral hemisphere on day 14.
rCBF was assessed by perfusion-weighted imaging. (A) Representative images. (B) Quantification of rCBF in three regions of interest (ROI). N = 5 per group. **P<0.01 compared with MCAO group.
VPA upregulated pro-angiogenic factors and inhibited HDACs
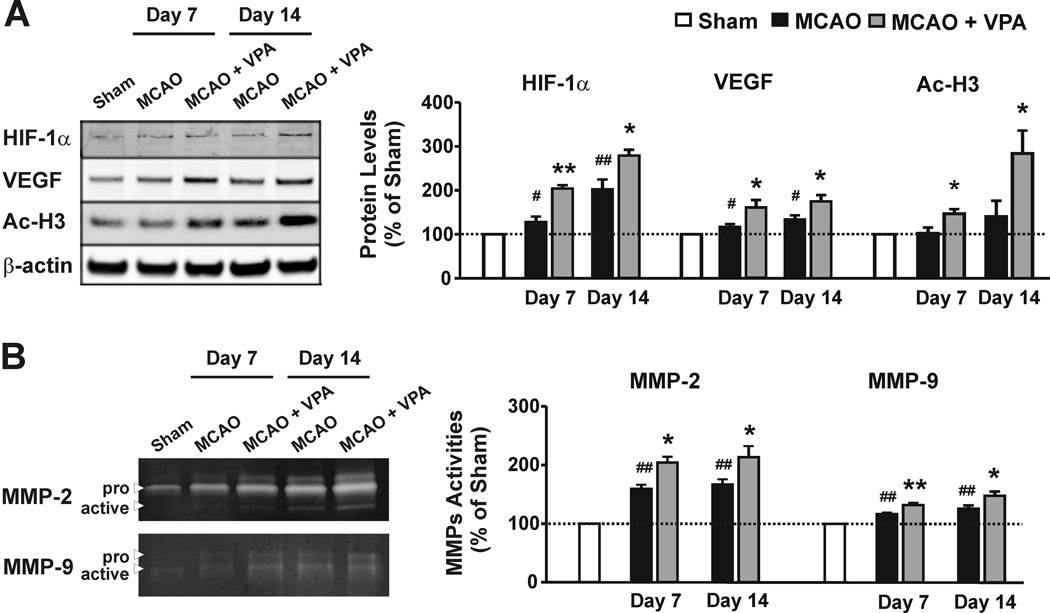
As an essential part of the HIF-1 complex, HIF-1α is rapidly degraded under normoxia, and becomes stabilized after hypoxia/ischemia.12 As expected, the protein levels of HIF-1α gradually increased in the ipsilateral cortex of MCAO rats on days 7 and 14. This increase was further augmented by VPA treatment (201.3±22.7% versus 127.5±12.4% for day 7, and 278.4±13.3% versus 203.5±7.5% for day 14, compared with sham-operated rats; Figure 4A).
Figure 4. VPA upregulated pro-angiogenic factors in the ipsilateral cortex.
(A) Western blotting showed upregulation of HIF-1α and VEGF protein levels in MCAO rats on days 7 and 14, and VPA markedly enhanced this upregulation. Concurrently, VPA robustly increased acetylated histone-H3 (Ac-H3) levels. N = 6 per group. (B) MCAO increased MMP-2/9 activities on days 7 and 14, as assessed by gelatin zymography. VPA significantly enhanced this increase. N = 8 per group. #P<0.05, ##P<0.01 compared with the sham control; *P<0.05, **P<0.01 compared with MCAO group.
To study the effects of VPA on pro-angiogenic factors after brain ischemia, the protein levels of VEGF and activities of MMP-2/9 were examined using Western blotting and gelatin zymography, respectively. VEGF protein levels gradually increased in the ipsilateral cortex of MCAO rats on days 7 and 14, compared with sham-operated rats (Figure 4A). VPA treatment further enhanced the MCAO-induced upregulation of VEGF protein levels (134.2±9.4% versus 115.6±6.2% on day 7, and 175.1±14.4% versus 159.9±16.8% on day 14, compared with sham-operated rats). In the zymography gel, MMP-2 had a much stronger signal than MMP-9. Total MMP-2 activity in MCAO rats sustained a 1.6-fold increase on days 7 and 14 compared with sham-operated rats, while MCAO induced a relatively mild increase in total MMP-9 activity (Figure 4B). VPA treatment more than doubled total MMP-2 activity, and also significantly upregulated total MMP-9 activity.
Acetylated histone-H3 and/or H4 (Ac-H3 and/or Ac-H4) is an index of HDAC inhibition. In MCAO rats, a gradual increase in Ac-H3 protein levels was noted from days 7 to 14 (1-fold and 1.5-fold compared with sham-operated rats, respectively), although this increase did not reach statistical significance. VPA treatment robustly amplified Ac-H3 expression to 1.4-fold and 2.8-fold on days 7 and 14, respectively (Figure 4A). Ac-H4 was also mildly increased on day 7 and returned to baseline on day 14. VPA treatment markedly potentiated Ac-H4 levels on day 14 (1.5-fold for day 7 and 2.3-fold for day 14; Supplemental Figure S3).
HIF-1α inhibition abolished the beneficial effects of VPA on post-ischemic angiogenesis and functional recovery
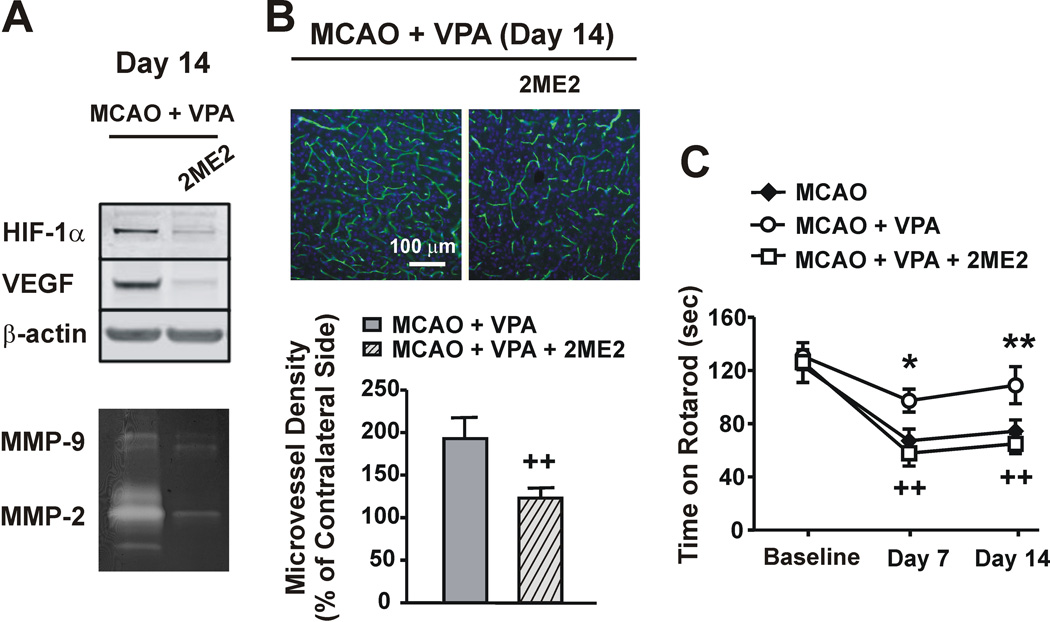
2ME2 is a natural metabolite of estradiol and has been shown to inhibit hypoxia-dependent HIF-1α stabilization and transcriptional activation.18–19 When co-injected with VPA, 2ME2 robustly inhibited VPA-induced upregulation of HIF-1α and VEGF protein levels, and total MMP-2/9 activities on day 14 after MCAO (Figure 5A). In addition, as determined via RECA-1 staining, 2ME2 co-administration substantially reversed VPA-enhanced microvessel density from 193.1±22.4% to 122.9±10.4% on day 14 (Figure 5B), which was even lower than that of the untreated MCAO group (155.3±4.0% in Figure 2A). Furthermore, 2ME2 co-treatment completely blocked the beneficial effects of VPA on the rotarod performance of MCAO rats, making their retention time similar to the untreated MCAO rats on both days 7 and 14 (Figure 5C). 2ME2 itself did not affect the rotarod performance of MCAO rats (Supplemental Figure S4).
Figure 5. HIF-1α inhibition abolished the beneficial effects of VPA on post-ischemic angiogenesis and functional recovery.
(A) 2-methoxyestradiol (2ME2), an effective HIF-1α inhibitor, robustly inhibited the VPA-induced elevation of HIF-1α and VEGF protein levels and MMP-2/9 activities in the ipsilateral cortex on day 14. (B) RECA-1 staining shows that 2ME2 completely abolished the VPA-enhanced increase in microvessel density on day 14. Nuclei were counterstained with DAPI (blue). N = 12 per group. (C) 2ME2 completely reversed the beneficial effects of VPA on the rotarod performance of MCAO rats. N = 8 per group. *P<0.05, **P<0.01 compared with MCAO group; ++P<0.01 compared with VPA-treated MCAO group.
DISCUSSION
In this study, VPA markedly reduced infarct volume and improved functional recovery on day 14 after MCAO in rats. Concurrently, VPA treatment enhanced post-ischemic angiogenesis by increasing microvessel density, facilitating endothelial cell proliferation, and upregulating rCBF in the ipsilateral cortex. In addition to enhancing HDAC inhibition, VPA potentiated MCAO-induced HIF-1α accumulation and upregulated VEGF protein levels and MMP-2/9 activities in the ipsilateral cortex on days 7 and 14 post-MCAO. Furthermore, HIF-1α inhibition reversed the enhanced post-ischemic angiogenesis and functional recovery observed after VPA treatment. Our findings suggest that (1) chronic VPA treatment enhances post-ischemic angiogenesis and promotes long-term functional recovery in an experimental model of ischemic stroke, and (2) the pro-angiogenic effects of VPA likely involve HDAC inhibition and upregulation of HIF-1α and pro-angiogenic factors VEGF and MMP-2/9.
Accumulating evidence has established that angiogenesis naturally occurs after brain ischemia in humans and animals, potentially functioning as an endogenous mechanism to restore oxygen and nutrient supply to affected brain tissue.4 However, post-ischemic angiogenesis is often insufficient to improve clinical outcomes. Our results demonstrated that despite higher microvessel density, rCBF was significantly lower in the ipsilateral versus contralateral hemisphere on day 14 after MCAO. This finding echoes a clinical study demonstrating that the infarct region of post-mortem stroke patients contains a higher proportion of empty microvessels than normal brain tissue.1 Additionally, a rat MCAO study indicated that ischemia induces a transient population of leaky microvessels.20 Taken together, these observations suggest that the newly-formed microvessels may not be perfused or functional. In the current study, VPA treatment strongly potentiated both microvessel density and rCBF in the ischemic hemisphere, suggesting that VPA may facilitate microvessel formation as well as perfusion. Furthermore, it has been proposed that ischemia-triggered angiogenesis requires additional factors, such as brain-derived neurotrophic factor (BDNF), for long-term stabilization.21 VPA can induce BDNF in rat cortical neurons by activating BDNF promoter IV through HDAC inhibition,22 suggesting that VPA may also stabilize post-ischemic angiogenesis by inducing BDNF expression.
VEGF expression rapidly increases within hours and remains elevated for weeks after ischemia in rodent and human brains.6–7 Late administration of exogenous VEGF in the ischemic penumbra of rats enhances angiogenesis and improves neurological recovery.23 MMP-9 activity is elevated 2–4 days after stroke in the post-mortem human brain, whereas MMP-2 activity is elevated at later intervals.5 Delayed MMP inhibition in the peri-infarct cortex suppresses neurovascular remodeling and functional recovery on day 14 in ischemic rats.8 Consistent with these studies, we found that VEGF protein levels and MMP-2/9 activities were significantly increased on days 7 and 14 after MCAO. VPA treatment markedly enhanced the upregulation of VEGF and MMP-2/9, suggesting that VEGF and MMP-2/9 contribute to VPA’s ability to enhance post-ischemic angiogenesis.
Both VEGF and MMPs increase BBB permeability in the acute phase and subsequently facilitate neurovascular remodeling after stroke.11, 23 In the present study, VPA enhanced post-ischemic angiogenesis by upregulating VEGF protein expression and MMP-2/9 activities on day 14 after MCAO. We recently demonstrated that VPA attenuates BBB disruption and brain edema by suppressing MMP-9 induction and tight junction degradation 24 hours after MCAO.16 In a swine hemorrhagic shock model, VPA mitigates pathologic endothelial cell function by attenuating the overexpression of VEGF and its receptor 6 hours after resuscitation.24 Therefore, VPA appears to have a dual role in preserving post-ischemic endothelial cell function: it limits cell damage by inhibiting MMP-9 and VEGF in the early phase, whereas it enhances angiogenesis by upregulating VEGF and MMP-2/9 in the later recovery phase.
HIF-1 regulates pro-anigogenic genes after hypoxia/ischemia, and its activation is predominantly controlled by α subunit stabilization.12 In this study, VPA treatment significantly enhanced MCAO-induced HIF-1α protein accumulation in the ipsilateral cortex on days 7 and 14 after MCAO. 2ME2, a HIF-1α inhibitor, completely abolished the ability of VPA to increase microvessel density and improve rotarod performance. These findings suggest that the pro-angiogenic effects of VPA involve regulation of HIF-1α and are critical for the ability of VPA to improve long-term functional recovery after ischemia. It is suggested that VEGF and MMP-2/9 are under transcriptional regulation of HIF-1.12 In support of this notion, we found that HIF-1α inhibition suppressed VEGF and MMP-2/9 upregulation induced by VPA treatment in MCAO rats. To confirm that HIF-1α can directly upregulate VEGF and MMP-2/9, HIF-1α was induced in primary rat brain microvascular endothelial cells (RBMVECs), and VEGF and MMP-2/9 mRNA levels were measured by quantitative real-time PCR. Cobalt chloride (CoCl2) is widely used to mimic hypoxia by stabilizing HIF-1α.25–26 CoCl2 treatment increased HIF-1α protein levels with a peak observed at 6 hours (Supplemental Figure S5A). At this time point there was also a significant increase in VEGF and MMP-2/9 mRNA levels in RBMVECs (Supplemental Figure S5C). Pretreatment with 2ME2 significantly suppressed HIF-1α upregulation (Supplemental Figure S5B). Consequently, 2ME2 almost completely inhibited CoCl2-induced VEGF mRNA increase, partially reduced the MMP-9 mRNA increase, but did not affect MMP-2 mRNA levels (Supplemental Figure S5C). Together with the in vivo data, these findings indicate that upregulation of HIF-1α results in the elevation of VEGF and MMP-2/9, although it is possible that HIF-1α differentially regulates the transcription of these three genes.
The precise mechanisms underlying VPA-enhanced HIF-1α accumulation and angiogenesis in ischemic brain remain unclear. VPA is a pan-inhibitor of HDAC class I (1, 2, 3, 8 isoforms) and IIa (4, 5, 7, 9 isoforms).14 VPA-induced HDAC inhibition results in histone hyperacetylation, chromatin relaxation and gene transcription. As an index of HDAC inhibition, acetylation of histone-H3 and H4 was robustly increased by VPA treatment, especially on day 14 after MCAO, suggesting that HDAC inhibition may participate in the pro-angiogenic effects of VPA following ischemia. In support of this notion, a recent in vitro study shows that HDAC inhibitors, VPA and suberoylanilide hydroxamic acid (SAHA), greatly enhance VEGF-induced spheroid sprout formation in endothelial cells, and that VPA displays a trend toward increasing endothelial cell migration.27 Additionally, VPA potentiates extracellular signal-regulated kinase 1/2 activation in endothelial cells, which is known to promote cell survival and angiogenesis.27 Furthermore, HDAC inhibition can induce the differentiation of multipotent adult progenitor cells into endothelial cells, with or without VEGF co-stimulation.28 Besides endothelial cells, microvascular pericytes also play an important role in optimizing post-insult angiogensis. In human microvascular pericytes, a qPCR angiogenesis array showed that VPA leads to a general increase in genes associated with vessel stabilization and maturation, such as endothelial survival, endothelial tube formation/stabilization/branching, and maintenance of direct cell-cell contacts between endothelial cells and pericytes.29 Taken together, these findings suggest that VPA-induced HDAC inhibition may modulate pro-angiogenic gene expression and contribute to post-ischemic angiogenesis.
Interestingly, HDAC inhibitors are currently undergoing clinical evaluation as potential anti-cancer therapies because of their anti-angiogenic effects in tumors.30 VPA has been shown to inhibit HIF-1α stabilization and tumor angiogenesis in diverse cancer cell lines.31–32 However, there is no evidence showing the effects of VPA on HIF-1α and angiogenesis in noncancerous brain cells, especially after ischemia. In line with the in vivo findings, in an in vitro oxygen-glucose deprivation (OGD) model, we found that 3 hours OGD and 24 hours reperfusion increased HIF-1α protein levels in RBMVECs, and this increase was significantly augmented by treatment with 1 mmol/L VPA (Supplemental Figure S6A). In addition, VPA also significantly increased cell viability after OGD insult (Supplemental Figure S6B). VPA alone did not affect HIF-1α protein levels or cell viability under normoxic conditions. These findings provide complementary evidence supporting the pro-angiogenic effects of VPA after brain ischemia, conditions that likely differ significantly from those in cancer models.
Notably, existing studies suggest the likelihood that each HDAC isoform interacts differently with angiogenic pathways under specific conditions. There are also controversial findings regarding specific HDAC isoforms associated with HIF-1α in different cancer cell lines. For instance, one study found that HDAC7 directly interacts with HIF-1α and increases its transcriptional activity in HEK293 cells.33 Another study in C2 cells showed that VPA inhibits HIF-1α only at high concentrations that are effective against class II HDACs, and further demonstrated that HDAC4 and 6, instead of HDAC7, are involved.32 In contrast, protein kinase D-dependent phosphorylation and nuclear export of HDAC5 and 7 mediates VEGF-induced angiogenesis in endothelial cells.34–35 Additionally, HDAC5 silencing has been shown to increase endothelial cell migration, sprouting, and tube formation, whereas HDAC5 overexpression decreases sprout formation.36 An electron microscopy study has demonstrated that the pattern of new blood vessels in the ischemic brain is similar to that in normal brain, but differs from that in growing tumors.37 Therefore, it is conceivable that interactions between HDAC isoforms and HIF-1α may be dissimilar in endothelial cells and cancer cells under different oxygen tension conditions, and that the pan-inhibition of HDACs by VPA would affect the post-ischemic angiogenesis differently than it would affect angiogenesis in cancer.
To our knowledge, this study is the first to demonstrate that VPA enhances post-ischemic angiogenesis in vivo. This may contribute to its observed effects in improving long-term functional outcome after ischemic stroke. Our findings lead to a better understanding of the beneficial effects of VPA against ischemic stroke, and pave the way for potential clinical trials.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Mr. Daryl Despres and Ms. Vivian Diaz of NINDS, NIH for their technical assistance in MRI procedures, Dr. Fengshan Yu for his assistance in brain slicing, Drs. Joshua Hunsberger and Chi-Tso Chiu for their comments, and Ms. Ioline Henter for her editorial assistance.
SOURCES OF FUNDING
This research was supported by the Intramural Research Program of the NIMH, NIH, and by the Hsu family gift fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 5.Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/s0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 7.Slevin M, Krupinski J, Slowik A, Rubio F, Szczudlik A, Gaffney J. Activation of MAP kinase (ERK-1/ERK-2), tyrosine kinase and VEGF in the human brain following acute ischaemic stroke. Neuroreport. 2000;11:2759–2764. doi: 10.1097/00001756-200008210-00030. [DOI] [PubMed] [Google Scholar]
- 8.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 9.Shimotake J, Derugin N, Wendland M, Vexler ZS, Ferriero DM. Vascular endothelial growth factor receptor-2 inhibition promotes cell death and limits endothelial cell proliferation in a neonatal rodent model of stroke. Stroke. 2010;41:343–349. doi: 10.1161/STROKEAHA.109.564229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009;29:1620–1643. doi: 10.1038/jcbfm.2009.100. [DOI] [PubMed] [Google Scholar]
- 11.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 13.Yeh SH, Ou LC, Gean PW, Hung JJ, Chang WC. Selective inhibition of early--but not late--expressed HIF-1alpha is neuroprotective in rats after focal ischemic brain damage. Brain Pathol. 2011;21:249–262. doi: 10.1111/j.1750-3639.2010.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of hdac inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZF, Fessler EB, Chuang DM. Beneficial effects of mood stabilizers lithium, valproate and lamotrigine in experimental stroke models. Acta Pharmacol Sin. 2011;32:1433–1445. doi: 10.1038/aps.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: The roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42:2932–2939. doi: 10.1161/STROKEAHA.110.612788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating hif. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 19.Hagen T, D'Amico G, Quintero M, Palacios-Callender M, Hollis V, Lam F, et al. Inhibition of mitochondrial respiration by the anticancer agent 2-methoxyestradiol. Biochem Biophys Res Commun. 2004;322:923–929. doi: 10.1016/j.bbrc.2004.07.204. [DOI] [PubMed] [Google Scholar]
- 20.Yu SW, Friedman B, Cheng Q, Lyden PD. Stroke-evoked angiogenesis results in a transient population of microvessels. J Cereb Blood Flow Metab. 2007;27:755–763. doi: 10.1038/sj.jcbfm.9600378. [DOI] [PubMed] [Google Scholar]
- 21.Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115:653–663. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Causey MW, Salgar S, Singh N, Martin M, Stallings JD. Valproic acid reversed pathologic endothelial cell gene expression profile associated with ischemia-reperfusion injury in a swine hemorrhagic shock model. J Vasc Surg. 2012;55:1096–1103. e1051. doi: 10.1016/j.jvs.2011.08.060. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki E, Matsunaga T, Aonuma A, Sasaki T, Nagata K, Ohmori S. Effects of hypoxia-inducible factor-1alpha chemical stabilizer, CoCl(2) and hypoxia on gene expression of cyp3as in human fetal liver cells. Drug Metab Pharmacokinet. 2012 doi: 10.2133/dmpk.dmpk-11-rg-074. published ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin G, Bausch D, Knightly T, Liu Z, Li Y, Liu B, et al. Histone deacetylase inhibitors enhance endothelial cell sprouting angiogenesis in vitro. Surgery. 2011;150:429–435. doi: 10.1016/j.surg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahpatra S, Firpo MT, Bacanamwo M. Inhibition of DNA methyltransferases and histone deacetylases induces bone marrow-derived multipotent adult progenitor cells to differentiate into endothelial cells. Ethn Dis. 2010;20:S1-60–S1-64. [PMC free article] [PubMed] [Google Scholar]
- 29.Karen J, Rodriguez A, Friman T, Dencker L, Sundberg C, Scholz B. Effects of the histone deacetylase inhibitor valproic acid on human pericytes in vitro. PLoS One. 2011;6:e24954. doi: 10.1371/journal.pone.0024954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mottet D, Castronovo V. Histone deacetylases: Anti-angiogenic targets in cancer therapy. Curr Cancer Drug Targets. 2010;10:898–913. doi: 10.2174/156800910793358014. [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Jeong JW, Park JA, Lee JW, Seo JH, Jung BK, et al. Regulation of the HIF-1alpha stability by histone deacetylases. Oncol Rep. 2007;17:647–651. [PubMed] [Google Scholar]
- 32.Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, et al. Class II histone deacetylases are associated with vhl-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 33.Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem. 2004;279:41966–41974. doi: 10.1074/jbc.M406320200. [DOI] [PubMed] [Google Scholar]
- 34.Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, et al. Protein kinase d-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem. 2008;283:14590–14599. doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008;105:7738–7743. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbich C, Rossig L, Kaluza D, Potente M, Boeckel JN, Knau A, et al. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood. 2009;113:5669–5679. doi: 10.1182/blood-2009-01-196485. [DOI] [PubMed] [Google Scholar]
- 37.Krupinski J, Stroemer P, Slevin M, Marti E, Kumar P, Rubio F. Three-dimensional structure and survival of newly formed blood vessels after focal cerebral ischemia. Neuroreport. 2003;14:1171–1176. doi: 10.1097/01.wnr.0000075304.76650.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.