Abstract
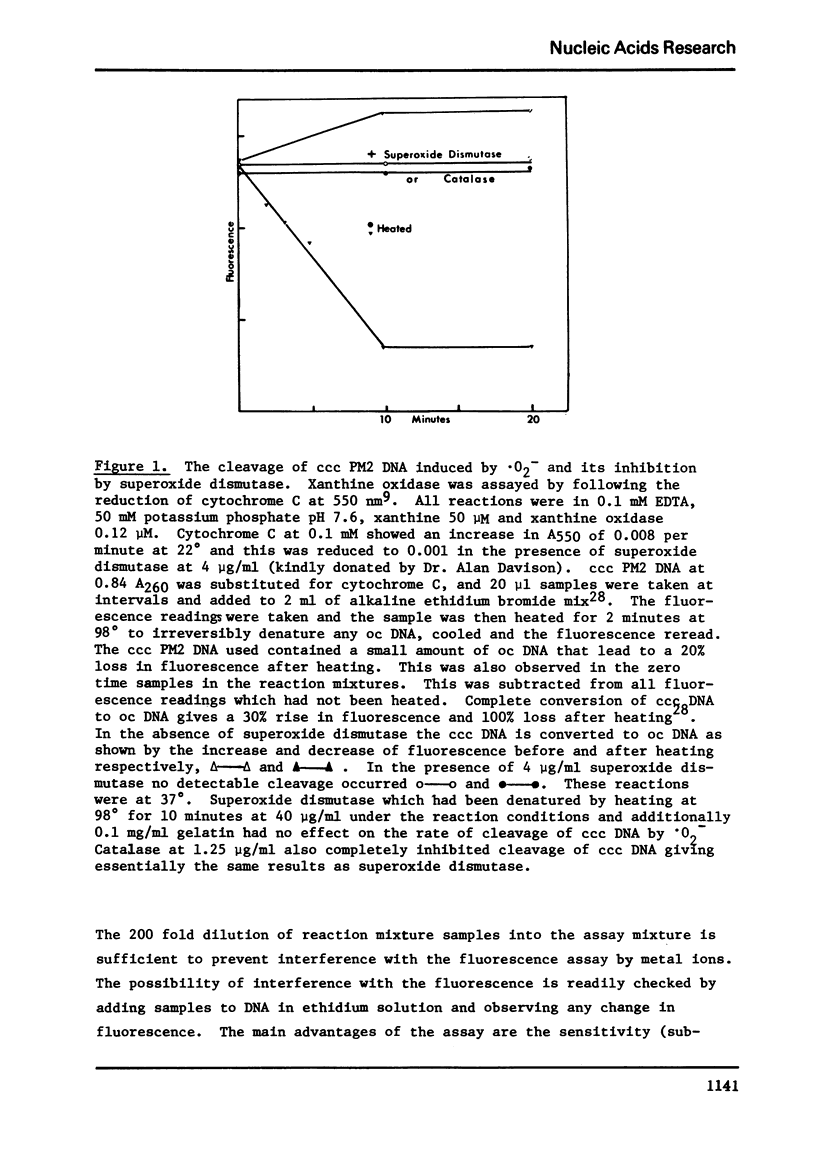
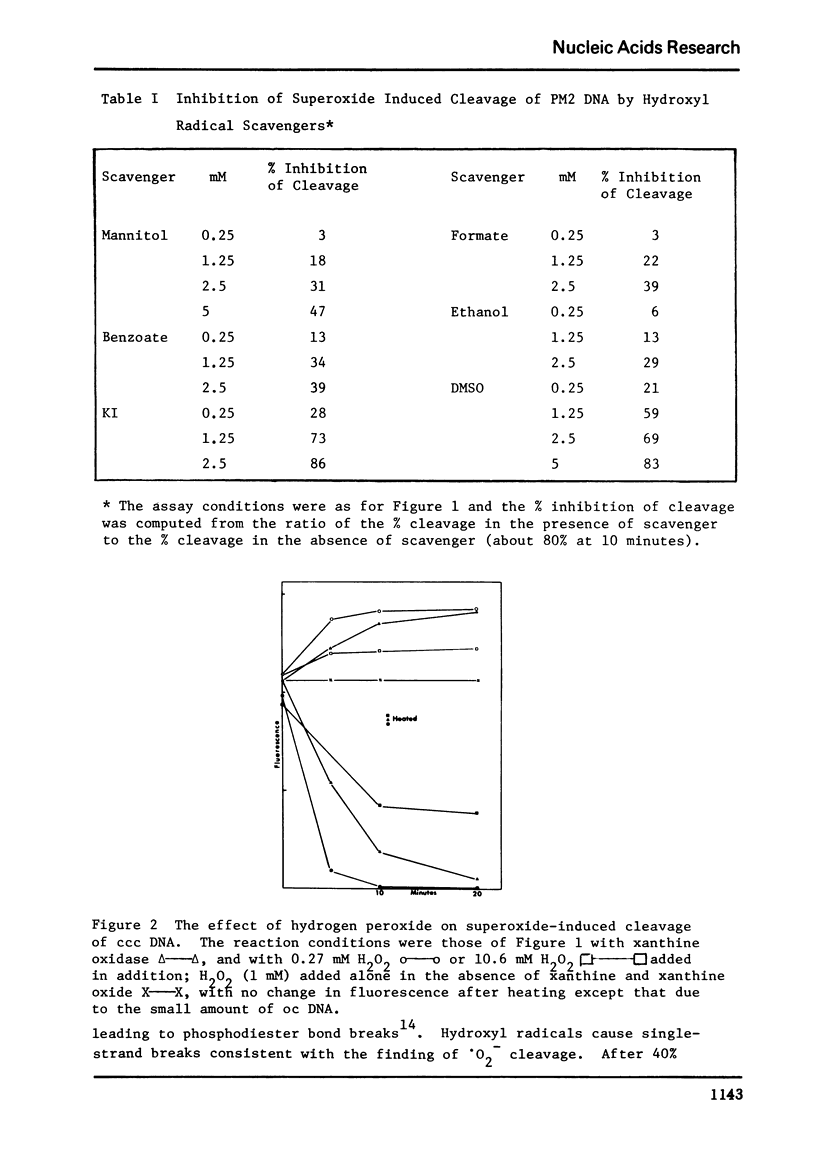
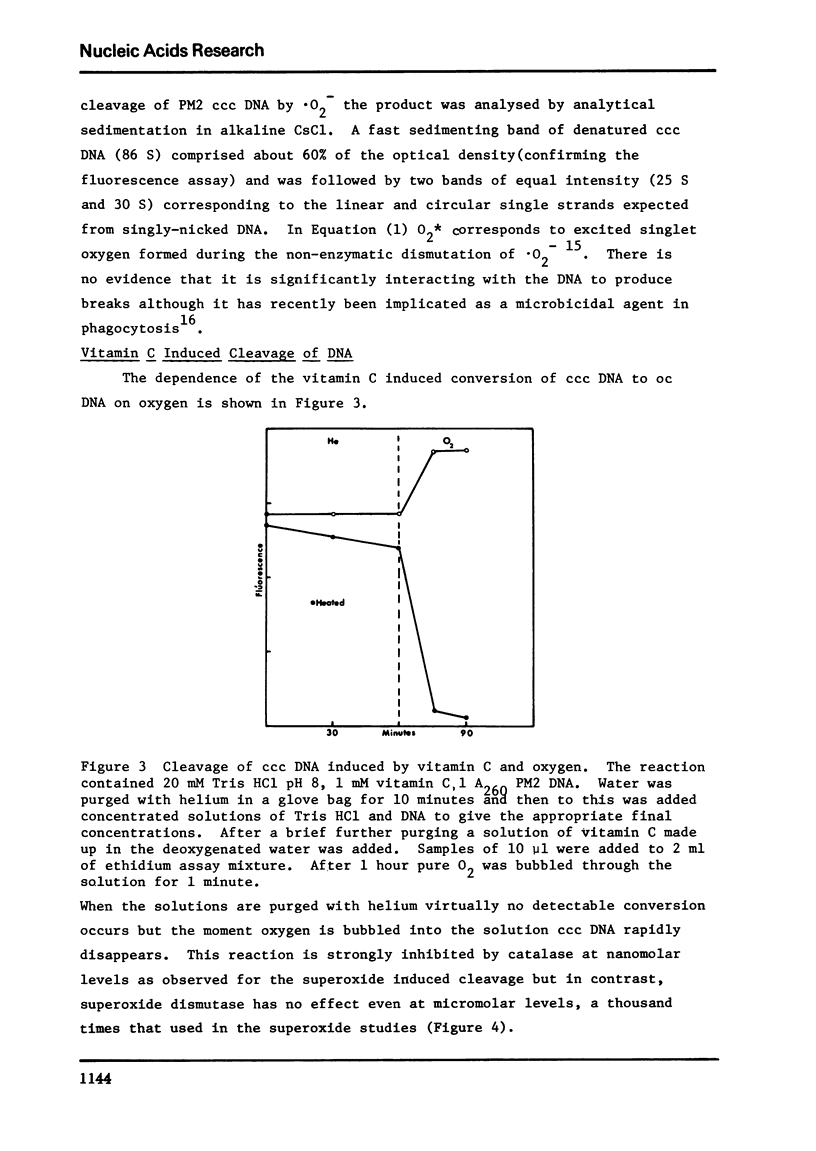
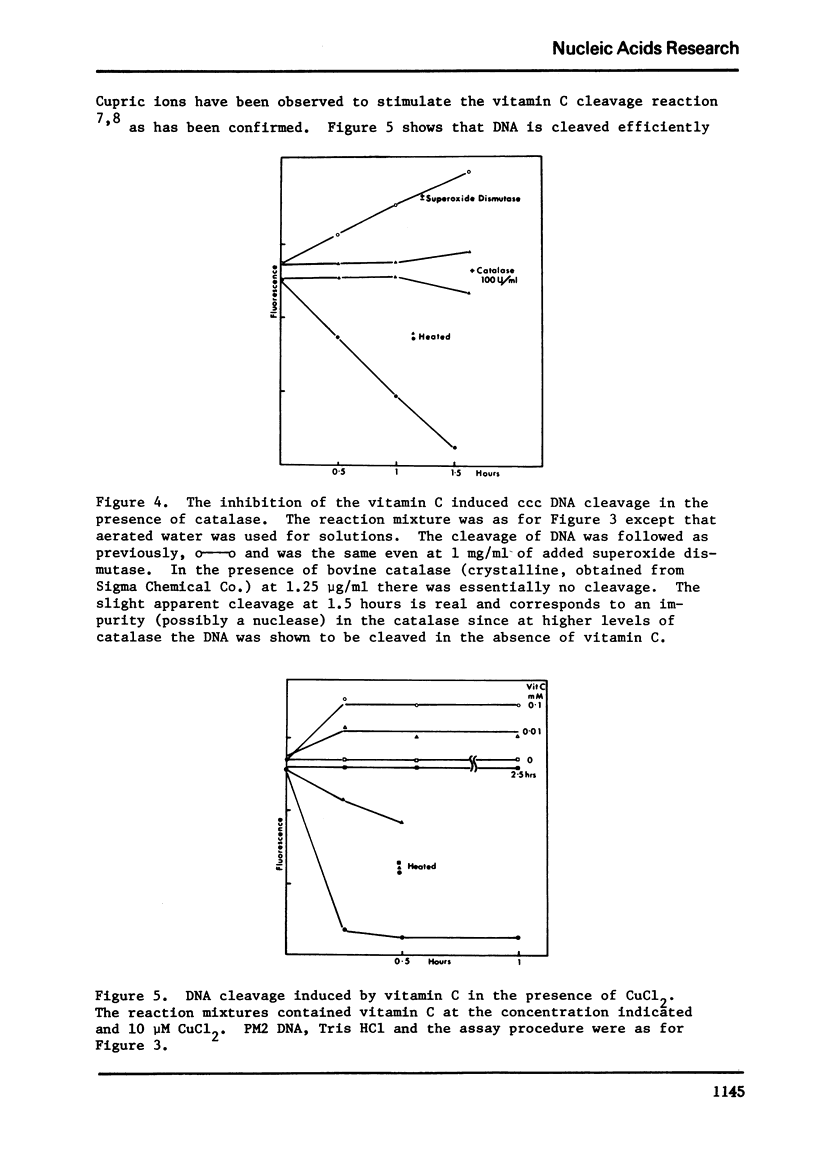
Vitamin C breaks DNA only in the presence of oxygen. Superoxide dismutase has no effect on the reaction but catalase suppresses it. Superoxide also gives rise to breaks in DNA suppressible by both superoxide dismutase and catalase. The hydroxyl radical seems to be the agent responsible for strand cleavage itself.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Blok J., Luthjens L. H., Roos A. L. The radiosensitivity of bacteriophage DNA in aqueous solution. Radiat Res. 1967 Mar;30(3):468–482. [PubMed] [Google Scholar]
- Bode V. C. Single-strand scissions induced in circular and linear lambda DNA by the presence of dithiothreitol and other reducing agents. J Mol Biol. 1967 May 28;26(1):125–129. doi: 10.1016/0022-2836(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Cameron E., Pauling L. Ascorbic acid and the glycosaminoglycans. An orthomolecular approach to cancer and other diseases. Oncology. 1973;27(2):181–192. doi: 10.1159/000224733. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hayatsu H., Tanooka H. Concentration effect of bisulfite on the inactivation of transforming activity of DNA. Chem Biol Interact. 1972 Jul;5(2):85–95. doi: 10.1016/0009-2797(72)90035-x. [DOI] [PubMed] [Google Scholar]
- Lange C. S. The organization and repair of mammalian DNA. FEBS Lett. 1974 Aug 25;44(2):153–156. doi: 10.1016/0014-5793(74)80714-3. [DOI] [PubMed] [Google Scholar]
- Mayeda E. A., Bard A. J. Singlet oxygen. The suppression of its production in dismutation of superoxide ion by superoxide dismutase. J Am Chem Soc. 1974 Jun 12;96(12):4023–4024. doi: 10.1021/ja00819a054. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969 Nov 25;244(22):6056–6063. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- Misra H. P. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974 Apr 10;249(7):2151–2155. [PubMed] [Google Scholar]
- Morgan A. R., Pulleyblank D. E. Native and denatured DNA, cross-linked and palindromic DNA and circular covalently-closed DNA analysed by a sensitive fluorometric procedure. Biochem Biophys Res Commun. 1974 Nov 27;61(2):396–403. doi: 10.1016/0006-291x(74)90970-x. [DOI] [PubMed] [Google Scholar]
- Pouwels P. H., van Rotterdam J., Cohen J. A. Structure of the replicative form of bacteriophage phi-X-174. VII. Renaturation of denatured double-stranded phi-X DNA. J Mol Biol. 1969 Mar 28;40(3):379–390. doi: 10.1016/0022-2836(69)90160-0. [DOI] [PubMed] [Google Scholar]
- Raleigh J. A., Greenstock C. L., Kremers W. Chemical radiosensitization of phosphate ester cleavage. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 May;23(5):457–467. doi: 10.1080/09553007314550531. [DOI] [PubMed] [Google Scholar]
- Robinson A. B., Irving K., McCrea M. Acceleration of the rate of deamidation of GlyArgAsnArgGly and of human transferrin by addition of L-ascorbic acid. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2122–2123. doi: 10.1073/pnas.70.7.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. F., Kuo I. Deoxynucleotides--models for studying mechanisms of strand breakage in DNA. II. Thymidine 3'5'-diphosphate. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Jun;23(6):543–557. doi: 10.1080/09553007314550651. [DOI] [PubMed] [Google Scholar]
- White H. L., White J. R. Lethal action and metabolic effects of streptonigrin on Escherichia coli. Mol Pharmacol. 1968 Nov;4(6):549–565. [PubMed] [Google Scholar]
- Wong K., Morgan A. R., Paranchych W. Controlled cleavage of phage R17 RNA within the virion by treatment with ascorbate and copper (II). Can J Biochem. 1974 Nov;52(11):950–958. doi: 10.1139/o74-133. [DOI] [PubMed] [Google Scholar]
- Yew M. L. "Recommended daily allowances" for vitamin C. Proc Natl Acad Sci U S A. 1973 Apr;70(4):969–972. doi: 10.1073/pnas.70.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]



