Abstract
Photoacoustic tomography (PAT) is an emerging molecular imaging modality. Here, we demonstrate use of semiconductor copper sulfide nanoparticles (CuS NP) for PAT with an Nd:YAG laser at a wavelength of 1064 nm. CuS NP allowed visualization of mouse brain after intracranial injection, rat lymph nodes 12 mm below the skin after interstitial injection, and CuS NP-containing agarose gel embedded in chicken breast muscle at the depth of ~ 5 cm. This imaging approach has great potential for molecular imaging of breast cancer.
Keywords: photoacoustic tomography, CuS nanoparticles, 1064-nm laser, optical imaging
Photoacoustic tomography (PAT), also referred to as optoacoustic tomography, is based on the measurement of ultrasonic waves induced by biological tissues’ absorption of short laser pulses. PAT employs nonionizing laser light to acoustically visualize biological tissues with high optical contrast and high ultrasonic resolution. Photoacoustic effects were demonstrated in turbid medium by Kruger 1 in 1994, in biological tissues by Oraevsky et al. 2 in 1997, and in rats by Wang et al. 3 in 2003. Since then, this imaging modality has been widely researched and advanced toward clinical applications.
The most important chromophores in the human body are oxyhemoglobin and deoxyhemoglobin in red blood cells. The hemoglobins have absorption coefficients of over 100 cm−1 for visible light; hence, they are capable of generating strong photoacoustic signals (~10 bars). Therefore, PAT has been successfully applied to image vascular structures and tumor angiogenesis a few millimeters under the skin. 4–6
Unlike other high-resolution optical imaging modalities, such as confocal microscopy, 2-photon microscopy, and optical coherence tomography, PAT relies on both diffused and ballistic light and thus can be used to image deeper biological tissues. However, because of the overwhelming scattering effect of biological tissues on light, light intensity decreases exponentially with depth with a decay constant that is related to effective penetration depth. The light intensity attenuation can be minimized by choosing an excitation laser wavelength within the near-infrared (NIR) region, in which biological tissues have a relatively low absorption coefficient and a low scattering coefficient.
Wang 7 indicated that the maximum PAT depth was around 50–70 mm, which equaled roughly 10 times the optical effective penetration depth. 6 Oraevsky et al. 8 reported a PAT image of a ductal carcinoma at a depth of 1.1 cm below the breast surface and developed a laser PAT system for breast cancer detection at wavelengths of 757 nm and 1064 nm with an arc-shaped array of 64 acoustic transducers with bandwidth of ~2.5 MHz. Piras et al. 9 developed a PAT system using a 1064-nm laser specifically for breast imaging and successfully used this system for breast imaging in a clinical study. The imaging contrast in these studies arose from differences in the intrinsic properties of blood perfusion owing to angiogenesis and hypoxia between normal tissues and tumors. However, these previous approaches to PAT are not applicable for early cancer detection or detection of deeper tumors.
Successful translation of PAT to the clinic requires a practical laser source that efficiently penetrates biological tissues and a contrast agent whose optical absorption peaks at or near the wavelength of the laser source. Among the pulsed lasers available for NIR, the Q-switched Nd:YAG laser, which emits laser light at 1064 nm, is one of the most reliable and economical. However, at 1064 nm, tumor cells have little specific optical absorption that clearly distinguishes them from normal cells. To enhance the optical absorption of tumor cells and to increase the photoacoustic signal-to-noise ratio, it is imperative that exogenous optical contrast agents with strong absorption at 1064 nm be identified and evaluated. Such contrast agents, when selectively delivered to tumor cells, are expected to increase the specificity and sensitivity of the current PAT techniques for early tumor detection. Although several organic dyes and nanoparticles that absorb light in the NIR region of 564 nm to 824 nm have been evaluated as potential contrast agents for molecular PAT of cancer, 10–14 no contrast agent that absorbs light at 1064 nm has been reported thus far. Here, we report a novel class of semiconductor copper sulfide nanoparticles (CuS NP) with optical absorption tuned to 1064 nm as an excellent PAT contrast agent suitable for deep tissue PAT imaging.
Results and Discussion
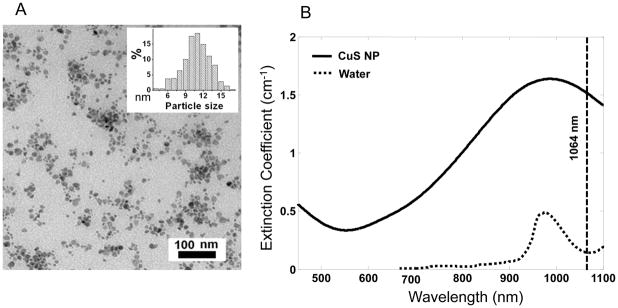
Previously, we demonstrated that CuS NP exhibit quantum size confinement phenomenon and that the interaction of CuS NP with 808-nm NIR light can generate heat for photothermal ablation therapy of cancer cells. 15, 16 Here, we found that the absorption peak could be tuned towards longer wavelengths by simply adjusting the stoichiometric ratio between CuCl2 and Na2S. Figure 1A is a transmission electron microscopy photograph of the type of CuS NP employed in our study. The average diameter of the CuS NP was 11±3 nm. Figure 1B shows the extinction coefficient spectrum of CuS NP in aqueous solution equivalent to 0.5 mM CuS molecules. The absorption peaked at around 990 nm. At the wavelength of 1064 nm, the CuS NP’s extinction measured in a quartz cuvette of a 1.0-cm optical path length was 0.66. Thus, the molar extinction coefficient of CuS molecules was 1.3×103 cm−1M−1. Taking the known values of the average diameter of CuS NP (11 nm) and density (4.76 g/cm3) and assuming that the scattering signal was negligible, it is estimated that the molar absorption coefficient for CuS NP at 1064 nm was 2.6×107 cm−1M−1. The strong absorption at 1064 nm suggests that CuS NP are an excellent candidate for contrast enhancement for PAT. Cell viability assay showed that CuS NP had minimal cytotoxicity at concentrations of up to 96 μg/mL after 24 hr incubation (Supplementary Figure 1).
Fig. 1.
Characterization of CuS NP. (A) Transmission electron microscopy photograph of CuS NP. Inset: size distribution of CuS NP. (B) Extinction coefficient spectra of 0.5 mM CuS NP aqueous solution (solid line) and pure water (dotted line). The vertical line is positioned at 1064 nm.
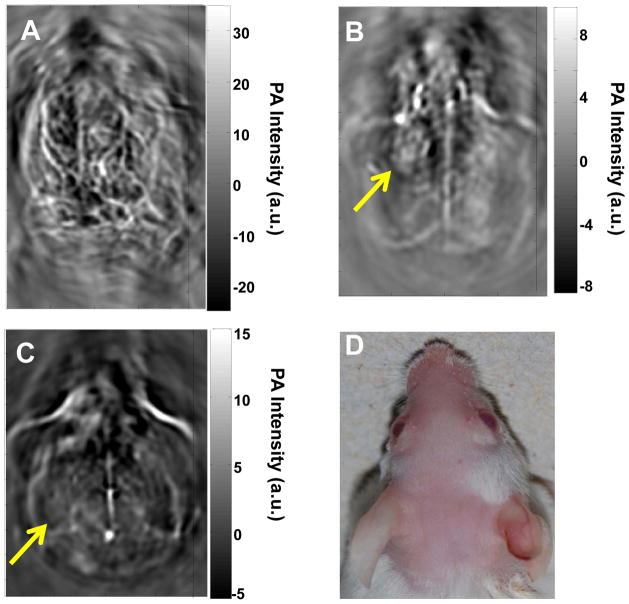
Figure 2 shows in vivo PAT images of a mouse brain acquired with green light at 532 nm without contrast agent (Fig. 2A) and with NIR light at 1064 nm using CuS NP as a contrast agent (Fig. 2B & 2C). Hemoglobin is the major chromophore in biological tissues and has strong absorption of green light at the wavelength of 532 nm. Therefore, green light is ideal for imaging of vascular structures. However, green light cannot penetrate deeply because of strong tissue absorption and scattering at short wavelengths. The superficial vascular structures of the mouse brain, such as the veins and arteries in the cerebral and temporal lobes, were clearly visible with green light (Fig. 2A). In contrast, on PAT images of the mouse brain acquired at 1064 nm, only the sagittal and transverse sinuses were visualized; blood vessels were not discernible (Fig. 2B & 2C). Significantly, a nodule on the left cerebral cortex that was injected intracranially with 15 μl aqueous solution of CuS NP (3×1013 NP/mL, 96 μg NP/mL, 2 OD) 24 h before PAT acquisition was clearly delineated (Fig. 2B). Seven days after CuS NP injection, the nodule in Figure 2B had dissolved, presumably because CuS NP had cleared from the injection site to be below the detection limit (Fig. 2C).
Fig. 2.
Representative in vivo PAT images of a mouse brain. Images were acquired using laser light (A) at a wavelength of 532 nm without contrast agent, (B) at 1064 nm 24 h after intracranial injection of 15 μl of CuS NP solution, and (C) at 1064 nm 7 days after intracranial injection of 15 μl of CuS NP solution. (D) Photograph of the head of the mouse. Laser light was delivered from the top.
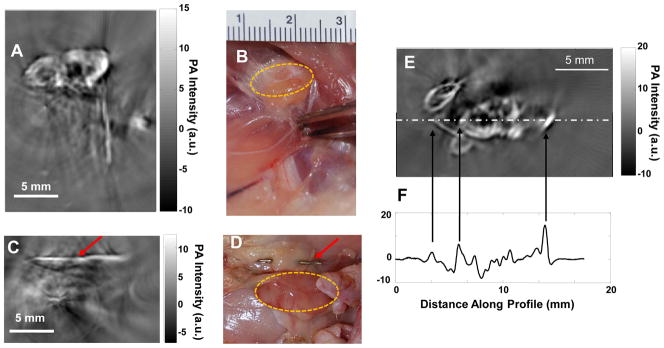
Figure 3 shows PAT images of the axillary and brachial lymph nodes of a rat 24 h after interstitial injection of CuS NP into the front paw pad on one side of the body. The lymph nodes, located 12 mm below the skin surface, were clearly visualized (Fig. 3A). In contrast, the axillary and brachial lymph nodes on the contralateral side, which did not receive an interstitial injection of CuS NP, were not visualized on PAT (Fig. 3C). To assure that the lymph nodes were within the imaging field of view, stainless steel needle tips were placed adjacent to the targets as a reference (red arrows, Fig. 3A & 3C).
Fig. 3.
Representative PAT images of axillary and brachial lymph nodes at depth of 12 mm below the skin of rats. (A, B) PAT image acquired on the right side of a rat 24 h after interstitial injection of 200 μl of CuS NP solution into the right front paw pad (A) and corresponding photograph of exposed rat underarm after imaging experiment (B). (C, D) PAT image acquired on the left side, into which no CuS NP were injected (C), and corresponding photograph of exposed rat underarm after imaging experiment (D). Yellow circles indicate lymph nodes. Red arrows indicate stainless steel needle tips placed adjacent to the lymph nodes to ensure that they were within the imaging field of view. (E) Representative PAT image of axillary and brachial lymph nodes in a different rat. (F) One-dimensional profile showing PA signal intensity along the dashed line in (E).
The PAT experiment on detection of axillary and brachial lymph nodes was conducted in 3 rats. PAT images in all rats depicted uptake of CuS NP by ipsilateral draining lymph nodes after interstitial injection of the nanoparticles. A representative lymph node PAT image and its 1-dimensional photoacoustic signal profile (along the dot-dashed line in Fig. 3E) are shown in Figure 3E & 3F. For quantitative comparison, the absolute photoacoustic signal intensity of each image pixel within the lymph node region of interest) from each rat was obtained and used to calculate the mean signal intensity and standard deviation. The photoacoustic signal intensity was significantly higher in lymph nodes containing CuS NP than in lymph nodes without CuS NP (7.85±3.78 vs. 2.46±0.73 a.u., p = 0.026).
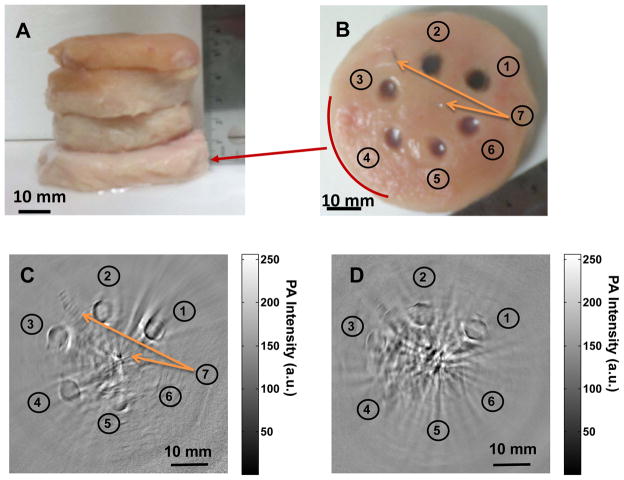
To determine the effective PAT imaging depth at wavelength of 1064 nm, agarose gels containing CuS NP were embedded in a piece of chicken breast muscle, which was then placed under other pieces of chicken breast muscle, each approximately 1.5 cm thick (Fig. 4A). The number of pieces of chicken breast muscle placed on top of the piece with embedded CuS NP was varied so that the depth below the surface of the piece with embedded CuS NP varied from 1 to 5 cm. Six agarose gel objects, containing CuS NP of 100, 50, 25, 12.5, 6.25, and 0 μg/mL, were sequentially embedded in counter clockwise starting at 1 O’clock direction. Two stainless steel needle tips were placed in the same plane as the agarose gels at center and 11 O’clock position, respectively, as references. Figures 4C & 4D show PAT images obtained with the 2.25-MHz transducer when a piece of chicken breast muscle embedded with CuS NP was placed at depths of 2.5 cm and 5 cm, respectively. At 2.5 cm, all the agarose gel objects including plain agarose gel without CuS NP were clearly visualized. These data indicate that CuS NP enhanced photoacoustic contrast between agarose gel and chicken breast tissue. The data also indicate that PAT could detect intrinsic differential optical absorbance between agarose gel and chicken breast tissue at 1064 nm and depths of up to 2.5 cm. At 5 cm, the agarose gel objects containing 100, 50, and 25 μg/mL CuS NP remained detectable. PAT imaging resolution in the central area is estimated to be approximately 800 μm according to the photoacoustic detection bandwidth. 17 On the basis of the visibility at 25 μg/mL and resolved voxel size of 2.56 mm3, the imaging sensitivity to CuS NP at the depth of 5 cm was estimated to be ~0.7 nmol CuS molecules per image voxel. At the depth of 2.5 cm, the sensitivity to CuS NP was estimated to be < 0.2 nmol CuS molecules per image voxel.
Fig. 4.
Comparison of deep embedded objects and their photoacoustic images. Agarose gels containing CuS NP were embedded in a piece of chicken breast muscle, which was then placed under varying numbers of other pieces of chicken breast muscle. Photograph of (A) chicken breast muscle blocks stacked, (B) the cross section of chicken breast muscle where copper sulfide nanoparticles of ➀ 100 μg/mL (2 OD), ➁ 50 μg/mL (1 OD), ➂ 25 μg/mL (0.5 OD), ➃ 12.5 μg/mL (0.25 OD), ➄ 6.25 μg/mL (0.125 OD), ➅ gel without contrast agent, and ➆ two needle tips at center and 11 O’clock position are embedded; Two dimensional photoacoustic image at the depth of (C) ~ 2.5 cm and (D) ~ 5 cm, from laser illuminated surface.
The minimal detectable concentrations of 6.25 μg/mL at 2.5-cm depth and 25 μg/mL at 5-cm depth are quite high. Whether these concentrations in solid tumors are achievable after systemic injection remains to be seen. Assuming that CuS NPs can be safely injected at a dose of 0.042 mmol CuS/kg (4.0 mg/kg), and that 10% of injected dose is deposited to one gram of tumor, the concentration of CuS NPs in the tumor of a 25-gram mouse could reach ~10 μg/g (10 μg/mL). This value is in the same order of magnitude as the minimal detectable concentration of 6.25–25 μg/mL obtained using our prototype photoacoustic imaging device employing a single element transducer. It is expected that by using a more powerful 1064-nm lase and a more sensitive ultrasonic detector array for photoacoustic signal generation and detection, it is possible to significantly increase the detection sensitivity. Therefore, it is feasible to use CuS NPs as the contrast agents in molecular photoacoustic imaging at 1064 nm in vivo setting.
The optical fluence attenuation in chicken breast at 1064 nm can be calculated using photoacoustic data obtained at different depths. The photoacoustic intensities were derived from the reconstructed images and calibrated by the signal amplifications employed in the experiment. Giving the photoacoustic signal intensity generated from stainless steel needle tips and gels containing 100 μg/mL CuS NP, the effective attenuation coefficients were found to be 0.18 and 0.12 mm−1 respectively, by exponential fitting based on Beer’s law. In comparison, the effective attenuation coefficient obtained by measuring light intensities through chicken breast of different thickness using a photodiode detector was 0.17 mm−1. The difference between photoacoustic and photodiode measurements can be partially explained by deviation of light distribution from strict Beer’s law. The fitted effective attenuation coefficients, statistical parameters, and estimated effective penetration depths (multiplicative inverse of effective attenuation coefficient) are summarized in Table 1.
Table 1.
Effective attenuation coefficients and penetration depth of 1064-nm light in chicken breast muscle by exponential fitting of photodiode signals and reconstructed photoacoustic amplitudes at various depths.
| Effective attenuation coefficient (mm−1) | 95% confidence interval | R2 | Effective penetration depth (mm) | |
|---|---|---|---|---|
| Reconstructed needle tip | 0.18 | (0.10, 0.21) | 0.97 | 5.6 |
| Reconstructed CuS NP of 100 mg/L | 0.12 | (0.11, 0.13) | 0.99 | 8.3 |
| Photodiode signal amplitude | 0.17 | (0.14, 0.20) | 0.98 | 5.9 |
The reported optical properties of human breast tissue vary between different studies. Cerussi et al. 18 presented the results of a clinical study performed in 58 patients with stage II/III malignant breast tumors using a noninvasive broadband optical spectroscopy probe. They presented the spectra for breast tumors and contralateral normal breast tissues. According to their data extrapolated from 1000 nm to 1064 nm, the optical penetration depths in normal breast tissues were 11.4 mm at 800 nm and 6.6 mm at 1064 nm. Taroni et. al 19 measured in vivo absorption and scattering spectra of breast tissue from 10 healthy volunteers at wavelengths between 600 nm and 1100 nm. The effect of tissue composition on optical properties was evaluated in terms of blood oxygen level and water and lipid content using a spectrally constrained global fitting procedure. The penetration depths at 800 nm and 1064 nm were estimated to be 7.0 mm and 5.2 mm, respectively.
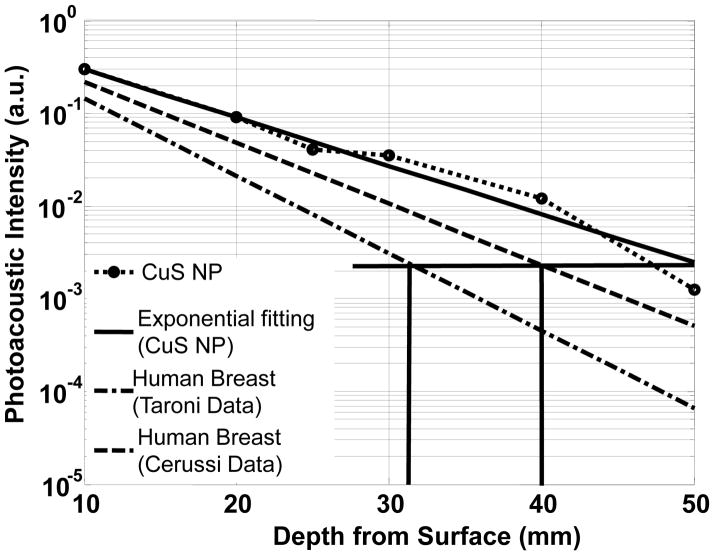
The photoacoustic intensities of CuS NP-containing gels at different depths in chicken breast were derived from the reconstructed images and calibrated according to the signal amplifications employed in the experiment. Figure 5 shows the experimental data and its exponential fitting, revealing the attenuation of 1064-nm laser light in chicken breast as a function of depth. For comparison, the light attenuations in human breast are also plotted in Figure 5 using the data from Cerussi et al. 18 and Taroni et al. 19 The maximum imaging depth of 50 mm achieved in our experiment amounts to approximately 6 times the effective optical penetration depth, corresponding to a 26-dB attenuation of the transmitted light from the surface incident light of 100 mJ/cm2 to ~ 0.24 mJ/cm2. In other words, using an initial light fluence of 100 mJ/cm2 at a surface as excitation, the CuS NP-containing objects can be imaged at 6 times the effective optical penetration depth. By comparing the effective penetration depths of the chicken breast used in our experiments and human breast tissues, an estimation of the imaging depth in human breast tissue can be made. According to the optical effective penetration depths of 6.6 mm and 5.2 mm measured by Cerussi et al. 18 and Taroni et al., 19 respectively, in human breast tissues, the imaging depths equivalent to 6 times the effective optical penetration depth at 1064 nm would be 40 mm and 31 mm, respectively, in human breast tissues.
Fig. 5.
Experimental PA signal intensity of CuS NP contained in agarose gel embedded in chicken breast muscle measured at wavelength of 1064 nm as a function of depth from the laser-illuminated surface. Predicted PA intensities of human breast tissue based on historical data are indicated by the dashed line and the dash-dotted line.
Compared to other NIR lasers, the Q-switched Nd:YAG laser is available at much higher energy level and much lower cost. In fact, its second harmonic radiation at 532 nm is usually employed to pump other laser media, such as Ti:Sapphire, laser dyes, and optical parametric oscillators, to obtain tunable laser output in the NIR region. The laser energy conversion efficiency is characteristically around 10% (~ 50% for second harmonic generation and ~ 20% for pumping other laser media). Typical commercial pulsed Q-switched Nd:YAG laser outputs are ~1 J/pulse at 1064 nm (fundamental oscillation), ~500 mJ/pulse at 532 nm (second harmonic), and ~100 mJ/pulse maximum at 800 nm (pumping Ti:Sapphire laser). With 10 times greater available laser pulse energy, the fluence rate of a 1064-nm laser would be much greater than that of an 800-nm laser at depth ≤40 mm in human breast. This should translate to a stronger photoacoustic signal and higher signal-to-noise ratio in the region necessary for molecular imaging of breast cancer.
Conclusions
Employing a low-cost Nd:YAG laser at the wavelength of 1064 nm for photoacoustic excitation, PAT clearly visualized CuS NP in mouse brain and rat lymph nodes. Moreover, Agarose gel containing CuS NP embedded in chicken breast at the depth of ~ 5 cm could be readily imaged with an in-plane imaging resolution of ~ 800 μm and a sensitivity of ~ 0.7 nmol per imaging voxel. Our study indicates that it should be possible to image lesions in the human breast at a depth of up to 40 mm with imaging resolution and sensitivity similar to that obtained with CuS NP in chicken breast muscles. In addition to breast, lesions located in other anatomic sites such as skin, arm or leg, head and neck, lymph nodes etc. may also be detected with the next generation of photoacoustic imaging device quipped with more powerful 1064-nm lasers and more sensitive ultrasonic detection array. Future studies, in particular in vivo imaging studies of targeted CuS NPs, are warranted to establish the potential utility of 1064-nm laser in molecular photoacoustic imaging.
Methods
Materials
Copper(II) chloride (CuCl2), sodium sulfide (Na2S·9H2O), sodium citrate, and methoxy-PEG-thiol (PEG-SH, molecular weight, 5,000) were purchased from Sigma-Aldrich (St. Louis, MO). Isoflurane was purchased from Baxter (Deerfield, IL).
General procedure for the synthesis of CuS NP
Into 1000 mL of aqueous solution of CuCl2 (1 mmol) and sodium citrate (0.68 mmol) was added 1 mL of sodium sulfide solution (Na2S, 1 M) under stirring at room temperature. Five minutes later, the reaction mixture was heated to 90°C and stirred at 1000 rpm for 15 min until a dark green solution was obtained. Nanoparticles with peak absorption at 1064 nm were obtained by adjusting the stoichiometric ratio between CuCl2 and Na2S. To introduce PEG coating, about 1 mg of PEG-SH was added into the citrate-CuS NP solution (3.0×1013 NP/mL water, 0.096 mg/mL, 2 OD at 1064 nm). The reaction was allowed to proceed overnight at room temperature.
Characterization of CuS NP
For transmission electron microscopy, aqueous solution of CuS NP was deposited on carbon-enhanced copper grids without negative staining. The NP were allowed to adhere on the grid for 1 h, after which they were briefly rinsed with deionized water and air dried. The samples were then examined using a transmission electron microscope (JEM 2010, JEOL Japan) at an accelerating voltage of 200 kV. Digital images were obtained using the AMT Imaging System (Advanced Microscopy Techniques Corp., Danvers, MA). The average diameter of CuS NP was determined by measuring up to 200 individual particles. The extinction spectrum of nanoparticles was measured using a UV-Vis spectrophotometer (DU 800, Beckman Coulter, Inc., Brea, CA). The fraction of the incident light transmitted through a solution of nanoparticles was recorded, and then the extinction coefficient was derived according to energy conservation.
PAT imaging equipment
Our PAT experimental set-up is shown in Supplementary Figure 2. A Q-switched Nd:YAG laser (LS-2137, Symphotic Tii, Camarillo, CA) provides 15-ns, ~1000-mJ NIR and ~500-mJ green laser pulses at wavelengths of 1064 nm and 532 nm, respectively, at a repetition rate of 10 Hz. The laser beam is expanded by a concave lens and homogenized by a ground glass and then directed onto a test sample - e.g., rat head, rat underarm, or the sample made from chicken breast muscle. The incident laser energy density on the tissue surface is controlled at ~100 mJ/cm2 or ~20 mJ/cm2, which are the “maximum permissible exposure” for human skin at the pulse width and wavelength of 1064 nm and 532 nm, respectively. NIR light diffuses inside a tissue sample and induces photoacoustic waves. The waves travel through the tissue and are coupled to the ultrasonic transducer, which converts the photoacoustic pressure into piezoelectric signals. An unfocused ultrasonic transducer (V323/2.25-MHz, Panametrics, Waltham, MA) with a 6-mm-diameter active element and 1.8-MHz detection bandwidth is employed to receive photoacoustic waves. The signals are subsequently amplified by amplifiers (ZFL-500LN, Mini-Circuits and 5072PR, Panametrics, Waltham, MA), bandpass-filtered by our homemade filters, and finally recorded using a digital data acquisition card (CS14100, Gage Applied, Inc., Lockport, IL). The sample and transducers are both immersed in a tank filled with saline for coupling the photoacoustic waves to the transducers. The ultrasonic transducer is driven by a step motor to continuously scan horizontally along a 15-cm-diameter circle around the sample. A personal computer is used to control the scanning and data acquisition.
Imaging mouse brain and sentinel lymph nodes in rats
All experiments involving animals were done in accordance with the guidelines of the Institutional Animal Care and Use Committee. For in vivo photoacoustic imaging experiment on mouse brain, 3 mice (Charles River Laboratories, Wilmington, MA) weighing ~25 g were anesthetized using veterinary anesthesia equipment (Mobile 901807, VetEquip, Pleasanton, CA) that delivered 2% isoflurane in oxygen at a flow rate of 2 L/min. The hairs on each mouse’s head were gently removed by applying hair-remover lotion. Laser light was used to illuminate the animal head from its top, and an ultrasonic detector was used to scan the animal’s head in its horizontal plane. CuS NP (3×1013 NP/mL, 0.096 mg/mL, 2 OD at 1064 nm) in 15 μL of saline were injected intracranially into the left cerebral cortex of each mouse (n = 3) at the depth of ~ 2 mm beneath the mouse skull.
For the ex vivo photoacoustic imaging experiment on rat lymph nodes, five Sprague-Dawley rats (Charles River Laboratories) weighing 250–300 g received subcutaneous injections of 1 mg/site of CpG oligodeoxynucleotides (Alpha NDA 365217, Montreal, Quebec, Canada ) 24 h before the injection of CuS NP (0.096 mg/mL, 300 μL) via the right front paw pad. Twenty-four hours after nanoparticle injection, the animals were euthanized, and PAT imaging experiments were performed.
Imaging gel embedded in chicken breast tissues
To determine maximum imaging depth, various gel cylinders (5 mm in diameter × 4 mm in length) were embedded in chicken breast muscles of ~9 cm in diameter. Six gel objects were obtained by mixing 2% agarose with CuS NP at concentrations of 100, 50, 25, 12.5, 6.25, 0 μg/mL. Two stainless steel needle tips were also placed in the same plane as a reference. When excited by laser light from the top, the embedded objects emit photoacoustic waves when they absorb the light energy. The photoacoustic signals were detected by the circularly scanning transducers and fed into the image reconstruction on the basis of a back-projection algorithm. The tissue’s cross-section containing the embedded objects was imaged when blocks of chicken breast muscles were sequentially stacked up to make the embedded objects at the desired depths from the laser-illuminated tissue surface. The light intensities that transmitted through various tissue depths were detected using a photodiode detector (DET110, Thorlabs, Newton, NJ).
Supplementary Material
Acknowledgments
The authors thank Stephanie Deming for editing the manuscript. We also thank K. Dunner for assistance with the transmission electron microscopy facility. This work was supported in part by National Cancer Institute grant R01CA119387-05S1, a Seed Grant through the Alliance for NanoHealth by the Department of Army Telemedicine and Advanced Technology Research Center (W81XWH-07-2-0101), and the John S. Dunn Foundation. The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA016672.
Footnotes
Supporting Information Available. Cytotoxicity of CuS NP, description and scheme of photoacoustic set-up. This material is available free of charge via the Internet at http://pubs.acs.org.
Conflict of Interest: The authors declare no competing financial interest.
REFERENCES AND NOTES
- 1.Kruger RA. Photoacoustic Ultrasound. Med Phys. 1994;21:127–131. doi: 10.1118/1.597367. [DOI] [PubMed] [Google Scholar]
- 2.Oraevsky AA, Jacques SL, Tittel FK. Measurement of Tissue Optical Properties by Time-resolved Detection of Laser-induced Transient Stress. Appl Opt. 1997;36:402–415. doi: 10.1364/ao.36.000402. [DOI] [PubMed] [Google Scholar]
- 3.Wang XD, Pang YJ, Ku G, Xie XY, Stoica G, Wang LHV. Noninvasive Laser-Induced Photoacoustic Tomography for Structural and Functional in vivo Imaging of the Brain. Nat Biotechnol. 2003;21:803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 4.Jose J, Manohar S, Kolkman RGM, Steenbergen W, van Leeuwen TG. Imaging of Tumor Vasculature Using Twente Photoacoustic Systems. J Biophotonics. 2009;2:701–717. doi: 10.1002/jbio.200910025. [DOI] [PubMed] [Google Scholar]
- 5.Kolkman RGM, Hondebrink E, Steenbergen W, de Mul FFM. In Vivo Photoacoustic Imaging of Blood Vessels Using an Extreme-Narrow Aperture Sensor. IEEE J Sel Top Quant. 2003;9:343–346. [Google Scholar]
- 6.Ku G, Wang XD, Xie XY, Stoica G, Wang LHV. Imaging of Tumor Angiogenesis in Rat Brains in vivo by Photoacoustic Tomography. Appl Opt. 2005;44:770–775. doi: 10.1364/ao.44.000770. [DOI] [PubMed] [Google Scholar]
- 7.Wang LV. Prospects of Photoacoustic Tomography. Med Phys. 2008;35:5758–5767. doi: 10.1118/1.3013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oraevsky AA, Ermilov SA, Conjusteau A, Miller T, Gharieb RR, Lacewell R, Mehta K, Radulescu EG, Herzog D, Thompson S, et al. T. Initial Clinical Evaluation of Laser Optoacoustic Imaging System for Diagnostic Imaging of Breast Cancer. Breast Cancer Res Treat. 2007;106:S47–S47. [Google Scholar]
- 9.Piras D, Xia WF, Steenbergen W, van Leeuwen TG, Manohar S. Photoacoustic Imaging of the Breast Using the Twente Photoacoustic Mammoscope: Present Status and Future Perspectives. IEEE J Sel Top Quant Electron. 2010;16:730–739. [Google Scholar]
- 10.Kim C, Cho EC, Chen J, Song KH, Au L, Favazza C, Zhang Q, Cobley CM, Gao F, Xia Y, et al. In Vivo Molecular Photoacoustic Tomography of Melanomas Targeted by Bioconjugated Gold Nanocages. ACS Nano. 2010;4:4559–4564. doi: 10.1021/nn100736c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, et al. Carbon Nanotubes As Photoacoustic Molecular Imaging Agents in Living Mice. Nat Nanotechnol. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu W, Melancon MP, Xiong C, Huang Q, Elliott A, Song S, Zhang R, Flores LG, Gelovani JG, Wang LV, et al. Effects of Photoacoustic Imaging and Photothermal Ablation Therapy Mediated by Targeted Hollow Gold Nanospheres in an Orthotopic Mouse Xenograft Model of Glioma. Cancer Res. 2011;71:6116–6121. doi: 10.1158/0008-5472.CAN-10-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan D, Pramanik M, Senpan A, Yang X, Song KH, Scott MJ, Zhang H, Gaffney PJ, Wickline SA, Wang LV, et al. Molecular Photoacoustic Tomography with Colloidal Nanobeacons. Angew Chem Int Ed Engl. 2009;48:4170–4173. doi: 10.1002/anie.200805947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li ML, Oh JT, Xie XY, Ku G, Wang W, Li C, Lungu G, Stoica G, Wang LV. Simultaneous Molecular and Hypoxia Imaging of Brain Tumors in vivo Using Spectroscopic Photoacoustic Tomography. Proc IEEE. 2008;96:481–489. [Google Scholar]
- 15.Li YB, Lu W, Huang Q, Huang M, Li C, Chen W. Copper Sulfide Nanoparticles for Photothermal Ablation of Tumor Cells. Nanomed. 2010;5:1161–1171. doi: 10.2217/nnm.10.85. [DOI] [PubMed] [Google Scholar]
- 16.Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, Li C. A Chelator-Free Multifunctional [64Cu]CuS Nanoparticle Platform for Simultaneous Micro-PET/CT Imaging and Photothermal Ablation Therapy. J Am Chem Soc. 2010;132:15351–11358. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku G, Wang X, Stoica G, Wang LV. Multiple-Bandwidth Photoacoustic Tomography. Phys Med Biol. 2004;49:1329–1338. doi: 10.1088/0031-9155/49/7/018. [DOI] [PubMed] [Google Scholar]
- 18.Cerussi A, Shah N, Hsiang D, Durkin A, Butler J, Tromberg BJ. In vivo Absorption, Scattering, and Physiologic Properties of 58 Malignant Breast Tumors Determined by Broadband Diffuse Optical Spectroscopy. J Biomed Opt. 2006;11:044005–044005. doi: 10.1117/1.2337546. [DOI] [PubMed] [Google Scholar]
- 19.Taroni P, Bassi A, Comelli D, Farina A, Cubeddu R, Pifferi A. Diffuse Optical Spectroscopy of Breast Tissue Extended to 1100 nm. J Biomed Opt. 2009;14:054030–054030. doi: 10.1117/1.3251051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.