Abstract
Poly(ADP-ribose) polymerase (PARP) is an enzyme involved in DNA repair. PARP inhibitors can act as chemosensitizers, or operate on the principle of synthetic lethality when used as single agent. Clinical trials have shown drugs in this class to be promising for BRCA mutation carriers. We postulated that inability to demonstrate response in non-BRCA carriers in which BRCA is inactivated by other mechanisms or with deficiency in homologous recombination for DNA repair is due to lack of molecular markers that define a responding subpopulation. We identified candidate markers for this purpose for olaparib (AstraZeneca) by measuring inhibitory effects of nine concentrations of olaparib in 22 breast cancer cell lines and identifying features in transcriptional and genome copy number profiles that were significantly correlated with response. We emphasized in this discovery process genes involved in DNA repair. We found that the cell lines that were sensitive to olaparib had a significant lower copy number of BRCA1 compared to the resistant cell lines (p value 0.012). In addition, we discovered seven genes from DNA repair pathways whose transcriptional levels were associated with response. These included five genes (BRCA1, MRE11A, NBS1, TDG, and XPA) whose transcript levels were associated with resistance and two genes (CHEK2 and MK2) whose transcript levels were associated with sensitivity. We developed an algorithm to predict response using the seven-gene transcription levels and applied it to 1,846 invasive breast cancer samples from 8 U133A/plus 2 (Affymetrix) data sets and found that 8–21 % of patients would be predicted to be responsive to olaparib. A similar response frequency was predicted in 536 samples analyzed on an Agilent platform. Importantly, tumors predicted to respond were enriched in basal subtype tumors. Our studies support clinical evaluation of the utility of our seven-gene signature as a predictor of response to olaparib.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-012-2188-0) contains supplementary material, which is available to authorized users.
Keywords: PARP, Olaparib, Breast cancer, Biomarker, Bioinformatics
Introduction
Several mechanisms have been identified in mammalian cells that function to maintain genome integrity [1, 2]. Mechanisms for the repair of single strand breaks (SSB) include base excision repair (BER), mismatch repair (MMR), and nucleotide excision repair (NER). DNA damage due to double strand breaks (DSB) is repaired via the homologous recombination (HR) pathway or with non-homologous end joining (NHEJ). The Fanconi anemia (FA)/BRCA pathway is involved in repair of DSBs or DNA interstrand cross links, and the DNA damage response (DDR) pathway is a network of DDRs for the regulation of many steps in the DNA repair process [3].
These repair pathways are frequently deregulated in cancer cells, motivating efforts to develop drugs that are preferentially effective in cells with defective repair. One emerging class of therapeutic agents for this purpose is based on the principle of synthetic lethality. Synthetic lethality is defined as cell death that results from complete inactivation of two genes in cells where inactivation of either alone does not result in death [4, 5]. The first therapy to reach the clinic was based on the concept that synthetic lethality would result from coordinate inactivation of HR repair (due to a genomic aberration) and poly(ADP-ribose) polymerase (PARP). This approach was based on studies showing that cells with HR repair deficiency caused by BRCA1/2 mutation [6, 7], PTEN mutation [8, 9], RAD51D loss of function [10], and PALB2 loss of function [11] exhibited synthetic lethality with an inhibitor of one or more PARP family proteins, differentiating cancerous from non-cancerous cells by only targeting cells with complete loss of the former genes. The PARP family consists of 18 PARP domain enzymes [12], with the most well-studied members being PARP1, PARP2, VPARP (PARP4), tankyrase 1 (TNKS, PARP5a), and TNKS2 (PARP5b). PARP1 is the most ubiquitous member. This protein rapidly binds to sites of damaged DNA to modulate a variety of proteins involved in DNA repair and other cellular processes, making it a key protein in the BER pathway for SSB repair.
PARP is involved in SSB DNA repair and PARP inhibitors cause some of them to be converted into DSBs at replication forks [13, 14]. In HR competent cells, DSBs are repaired so that the cells can survive. However, in HR-deficient cells, DSBs are repaired via the less accurate NHEJ pathway or the single strand annealing subpathway of HR, resulting in chromatid aberrations that usually lead to cell death. These conditions therefore make cells with BRCA mutations or other HR defects [11, 15, 16] preferentially sensitive to (i.e., to show synthetic lethality with) PARP inhibitors. PARP inhibitors also have been proposed as possibly useful for treatment of triple negative breast cancers that exhibit “BRCAness” [7, 17]. BRCAness is defined as the spectrum of phenotypes that some sporadic tumors share with BRCA mutated cancers, reflecting the underlying distinctive DNA repair defects arising from loss of HR; for example, by epigenomic down regulation of BRCA1 and FANCF [17].
PARP inhibitors in clinical studies for BRCA-associated, triple negative and/or basal-like breast cancer include olaparib (AstraZeneca, London), ABT-888 (also known as Veliparib; Abbott Laboratories, IL), and PF-01367338 (AG014699; Pfizer Inc., NY) [14, 18, 19]. These agents are licensed for monotherapy in DNA repair deficient patients or as chemo-potentiating agents after SSBs are created by common anticancer treatments such as radiotherapy and DNA damaging agents [19]. Results obtained from clinical trials so far, however, appear to vary depending on the specific breast cancer patient population, the specificity of the PARP inhibitor, and the nature of the therapeutic agent used in combination with the PARP inhibitor [20, 21]. A multicenter phase 2 trial showed that olaparib as monotherapy led to objective response rates in 41 % of BRCA1/2 mutation carriers who had previously received several courses of chemotherapy [22]. Results for triple negative breast cancer patients without known BRCA1/2 mutations have been inconsistent. Preclinical studies and phase 1 trials suggested that PARP inhibitors can increase cell death in these patients when combined with paclitaxel [23], whilst triple negative breast cancer patients largely did not respond to olaparib monotherapy in a phase 2 trial [24]. Thus, our aim in this study was to identify candidate biomarkers that can be tested for their ability to better identify subsets of sporadic cancers with defects in HR-directed repair that will respond to PARP inhibitors.
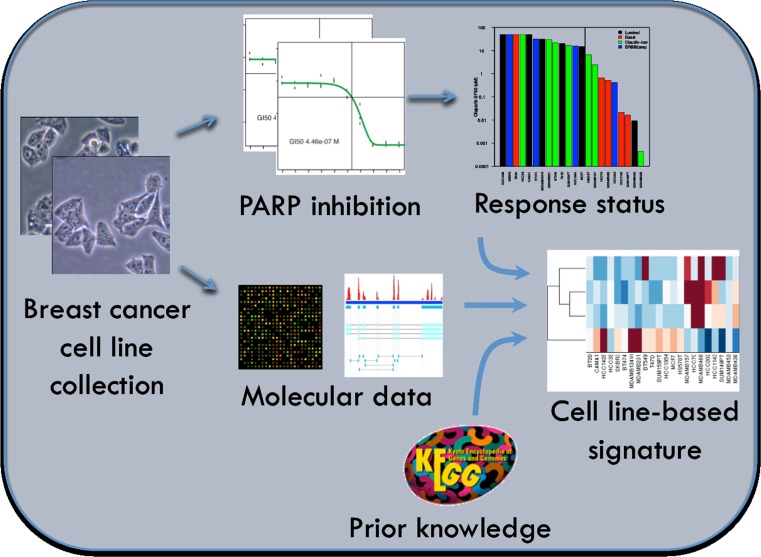
We focused in this study on olaparib, a small-molecule, reversible, oral inhibitor of both PARP1 and PARP2 [25]. We identified candidate biomarkers associated with response to olaparib by correlating responses to nine concentrations of olaparib in a panel of well-characterized breast cancer cell lines with the transcription levels of genes involved in aspects of DNA repair. Genes tested for correlation with olaparib response included those reported in the literature to be directly relevant to PARP inhibitor response or involved more generally in some aspect of DNA repair (Fig. 1). We applied this signature to primary tumor data to identify the frequency and characteristics of tumors that might be expected to respond to olaparib. These studies set the stage for a clinical test of the sensitivity and specificity of this predictor and indicate known subtypes of breast cancers that might be preferentially sensitive to olaparib.
Fig. 1.
Approach for the development of a predictor of olaparib response in a breast cancer cell line panel with inclusion of prior knowledge of DNA repair pathways. For 22 breast cancer cell lines, growth inhibition assays were used to measure their sensitivity to olaparib, expressed as the surviving fraction at 50 % (SF50). For these cell lines, expression data were obtained with three different platforms (Affymetrix U133A, Affymetrix Exon 1.0 ST, and whole transcriptome shotgun sequencing). The bottom-up approach was used for biomarker selection, incorporating prior knowledge of the principal DNA repair pathways BER, NER, MMR, HR/FA, NHEJ, and DDR. Biomarkers from [31] were systematically expanded with genes assigned to any of these pathways in the KEGG database, resulting in 118 genes. For each DNA repair pathway and expression data set the most important markers were obtained with LR in combination with forward feature selection, followed by reduction to those selected with consistent pattern of sensitivity for all three platforms
Materials and methods
Breast cancer cell lines, assay, and molecular data
The sensitivity of a panel of 22 breast cancer cell lines to KU0058948 (olaparib; KuDOS Pharmaceuticals/AstraZeneca) was measured with a growth inhibition assay as described in Supplementary Material and [7, 26]. The following molecular data were collected for the panel: copy number (Affymetrix SNP6), gene expression (Affymetrix U133A, Affymetrix Exon 1.0 ST), transcriptome sequencing (Illumina GAII), methylation (Illumina Methylation27), protein abundance (reverse protein lysate array), and mutation status (COSMIC, [27]). A detailed description of the availability and preprocessing of all molecular data sets is provided in Supplementary Material and [28].
Statistical analyses
The Wilcoxon rank sum test was used to test the association of drug response with individual biomarkers. Drug response was associated with subtype, triple negativity, and mutation status with use of the Fisher’s exact test. Due to the small sample size, a p < 0.05 was deemed significant, whilst a p < 0.1 was considered a trend. Logistic regression (LR) with forward feature selection (fivefold CV) was used to identify candidate biomarkers and was applied to each considered DNA repair pathway separately. The resulting biomarkers were combined into a predictor using a weighted voting algorithm [29]. The Matlab code used for signature development and validation is provided in Supplementary Material. A Chi-square test was used to test for associations of breast cancer subtype with response to olaparib. We refer to Supplementary Material for a detailed description of the statistical methods.
Results
Olaparib response in a panel of 22 breast cancer cell lines
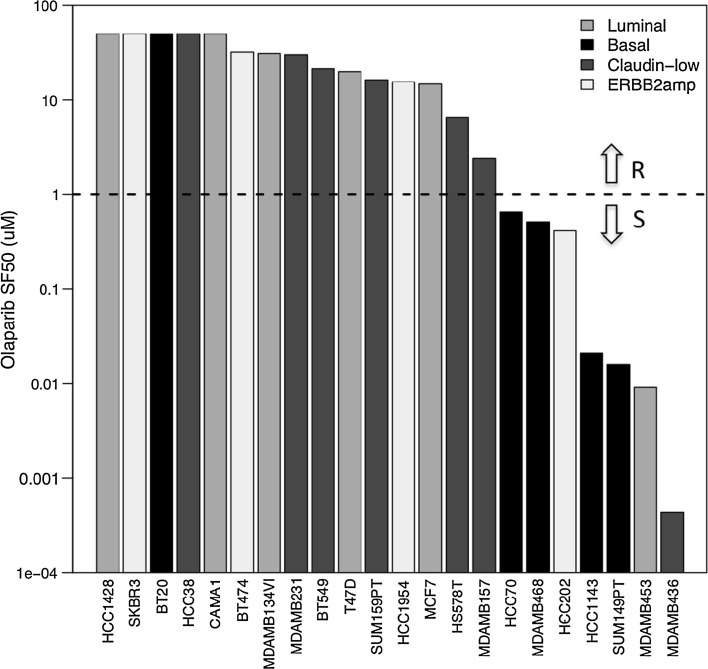
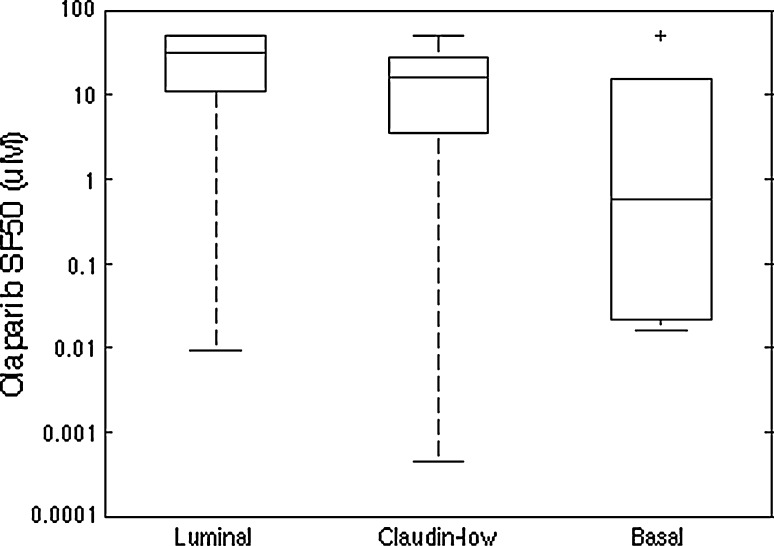
Twenty-two breast cancer cell lines previously profiled for RNA transcript levels were tested for response to nine concentrations of olaparib (see Table 1). These cells mirror many of the transcriptional and genomic characteristics of primary breast tumors and have been used to model responses to a large number of experimental and approved therapeutic compounds [28, 30]. The concentration of olaparib needed to reduce survival to 50 % (SF50) was used as a quantitative measure of sensitivity and ranged from 0.44 nM to 32 µM. The SF50 was not reached for five cell lines at the maximum treatment concentration of 50 µM olaparib. Olaparib response obtained with the growth inhibition assay was not influenced by growth rate assessed as doubling time (Spearman correlation coefficient −0.036, p value 0.874). Figure 2 shows the waterfall plot of SF50 with cell lines ordered from most resistant at the left to most sensitive at the right. Cell lines were divided into a group of 15 resistant and 7 sensitive cell lines, based on an SF50 threshold of 1 µM. Drug response was not significantly associated with breast cancer subtype (p value luminal vs. basal 0.136; Fig. 3), and did not differ between ERBB2-amplified and non-ERBB2-amplified cell lines (p value 1), with transcriptional subtypes assigned to cell lines as previously reported [28]. Four of the seven sensitive cell lines (57 %) were triple negative, compared to 5 of 15 (33 %) resistant cell lines (p value 0.376). Table 1 summarizes characteristics for the 22 cell lines, with SF50, doubling time, transcriptional ER, PR, and ERBB2 status, and the molecular data available for each of them.
Table 1.
Overview of the breast cancer cell line panel with response to olaparib expressed as SF50 (µM); ER, PR, and ERBB2 expression with + indicating up-regulation relative to the other cell lines, − down-regulation, and NC no change in expression; and availability of the different molecular data sets indicated with N for unavailability and Y for availability
| Cell line | Olaparib SF50 (µM) | Doubling time (h) | ER a | PR a | ERBB2 a | COSMIC | SNP6 | RPPA | Methylation | RNA-seq | Exon array | U133A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC1428 | 50 | 88.5 | + | + | − | N | Y | Y | Y | Y | Y | Y |
| SKBR3 | 50 | 56.2 | − | + | + | Y | Y | Y | Y | Y | Y | Y |
| BT20 | 50 | 66.1 | − | NC | − | Y | Y | Y | Y | Y | Y | Y |
| HCC38 | 50 | 51.0 | − | − | − | Y | Y | Y | Y | Y | Y | Y |
| CAMA1 | 50 | 72.9 | + | NC | NC | Y | Y | Y | Y | Y | Y | Y |
| BT474 | 31.99 | 92.5 | − | − | − | Y | Y | Y | Y | Y | Y | Y |
| MDAMB134VI | 30.90 | 82.7 | + | + | − | Y | N | N | Y | Y | Y | Y |
| MDAMB231 | 29.96 | 25.0 | − | − | − | Y | Y | Y | Y | Y | Y | Y |
| BT549 | 21.43 | 25.5 | − | − | + | Y | Y | Y | Y | Y | Y | Y |
| T47D | 19.95 | 55.8 | + | + | NC | Y | Y | Y | Y | Y | Y | Y |
| SUM159PT | 16.29 | 21.7 | − | + | − | Y | Y | Y | Y | Y | Y | Y |
| HCC1954 | 15.49 | 43.8 | − | − | − | Y | Y | Y | Y | Y | Y | Y |
| MCF7 | 14.69 | 56.5 | − | − | − | Y | Y | Y | Y | Y | Y | Y |
| HS578T | 6.55 | 32.3 | − | − | − | Y | Y | Y | Y | Y | Y | Y |
| MDAMB157 | 2.41 | 67.0 | − | + | + | Y | Y | Y | Y | Y | Y | Y |
| HCC70 | 0.655 | 67.8 | − | − | NC | Y | Y | Y | Y | Y | Y | Y |
| MDAMB468 | 0.514 | 79.8 | − | − | − | Y | Y | Y | Y | N | Y | Y |
| HCC202 | 0.413 | 212.5 | − | NC | NC | N | Y | Y | Y | Y | Y | Y |
| HCC1143 | 0.0211 | 54.6 | − | − | − | Y | Y | Y | Y | Y | Y | Y |
| SUM149PT | 0.0161 | 33.9 | + | + | − | Y | Y | Y | Y | Y | Y | Y |
| MDAMB453 | 0.00915 | 62.5 | − | + | + | Y | Y | Y | Y | Y | Y | Y |
| MDAMB436 | 0.00044 | 89.3 | − | NC | − | Y | Y | Y | Y | N | Y | Y |
| # cell lines | 20 | 21 | 21 | 22 | 20 | 22 | 22 |
Doubling times were estimated for each cell line from measurements of the number of doublings of untreated cells that occurred in 72 h during the course of assessing responses to 123 therapeutic compounds (Heiser et al. [28], PNAS 2012)
aFor ER, probe 205225_at on the Affymetrix U133A array was investigated; for PR, probe 208305_at; and for ERBB2 probes 210930_s_at and 216836_s_at
Fig. 2.
Waterfall plot of the response to olaparib (expressed as SF50 in µM) for 22 breast cancer cell lines, ordered from most resistant at the left to most sensitive at the right, with bars colored according to subtype (luminal in light grey, basal in black, claudin-low in dark grey, and ERBB2 amplified in white). The threshold of 1 µM used to divide the cell lines into a group of 15 resistant cell lines (indicated with R) and a group of 7 sensitive cell lines (indicated with S) is represented with a horizontal dashed line
Fig. 3.
Boxplot of SF50 for the cell lines divided according to breast cancer subtype (9 luminal, 7 claudin-low, and 6 basal lines). No association was found between breast cancer subtype and response to olaparib in the cell line panel (Fisher’s exact test for basal vs. luminal, p value 0.136)
Molecular features involved in DNA repair associate with olaparib response
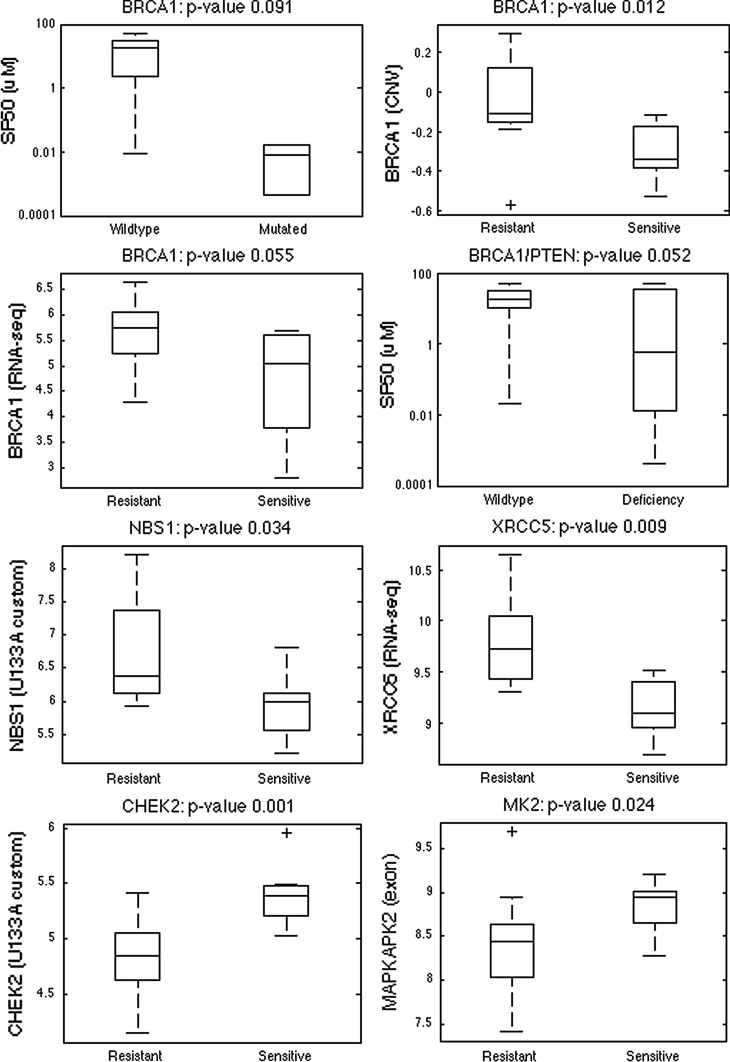
We selected candidate molecular features that might be developed as biomarkers for prediction of response to olaparib as those features involved in DNA repair activities that were associated with quantitative response to olaparib in the cell line panel. Molecular features included pretreatment RNA transcript levels, mutation status, copy number variation, and promoter methylation status. Specific genes tested involved aspects of DNA repair listed by Wang and Weaver [31]; ER, PR and ERBB2 due to the importance of PARP inhibition for triple negative breast cancer [17, 19]; and PARP family members PARP1, PARP2, VPARP, TNKS, and TNKS2. This approach is based on observations that in vitro models showing high sensitivity to PARP inhibitors often have BRCA and PTEN deficiencies [7, 8], copy number variations involving BRCA1 and PARP1 [32], and/or hypermethylation of the promoter regions of genes BRCA1 and FANCF [20]. Molecular features showing statistically significant associations with SF50 values are summarized in Supplementary Table 1 and illustrated in Fig. 4.
Fig. 4.
Overview of individual DNA repair-associated markers that are significantly associated with or do trend towards an association with response to olaparib in the 22 breast cancer cell lines, based on mutation, copy number, and expression data (see Supplementary Table 1 for the complete list of markers). The four boxplots at the top show the association results for BRCA1. The BRCA1-mutated cell lines MDAMB436 and SUM149PT tend to be more sensitive to olaparib compared to the wild-type cell lines (p value 0.091). The sensitive cell lines are also characterized by a significant lower copy number of BRCA1 (p value 0.012) and by BRCA1 down-regulation (RNA-seq, p value 0.055). Cell lines with a deficiency in BRCA1 and/or PTEN tend to be more sensitive to olaparib than cell lines with functional BRCA1 and PTEN (p value 0.052). The boxplots at the bottom show the association for genes NBS1 and XRCC5 that are significantly down-regulated and for genes CHEK2 and MK2 that are significantly up-regulated in the sensitive compared to the resistant cell lines
The transcription levels of MRE11A, NBS1, TNKS, TNKS2, XPA, and XRCC5 were significantly lower (p < 0.05; fold-change >2) in the sensitive compared to the resistant cell lines for at least one expression platform (U133A, exon array and RNA-seq), whilst transcription levels for BRCA1, ERCC4, FANCD2, and PR tended to be lower in sensitive lines (p < 0.1). We refer to Supplementary Table 1a for the list of significant associations per platform. PR protein levels measured using reverse phase protein lysate arrays [33] were also significantly reduced in the sensitive cell lines (p < 0.05). Transcript levels for CHEK2 and MK2 were significantly higher in the sensitive compared to the resistant lines (p < 0.05), with a similar trend for PARP2 and XRCC3 (p < 0.1). Although PARP1 has been shown to be overexpressed in 58 % of invasive breast cancer samples [34] and upregulated at protein level in 82 % of BRCA1-associated breast cancer samples [35], there is no consensus on its importance as a biomarker of response to PARP inhibitors [36, 37]. In our cell line panel, expression of PARP1 mRNA levels were not significantly higher in the sensitive lines compared to the resistant lines (median p value 0.277) (Supplementary Table 1a).
The BRCA1-mutated cell lines MDAMB436 and SUM149PT had a trend to be more sensitive to olaparib compared to the wild-type cell lines (p value 0.091) (Supplementary Table 1b). Likewise, cells with reduced BRCA1 copy number were significantly more sensitive to olaparib than cells with normal copy number at this locus (p value 0.012) (Supplementary Table 1c). PTEN loss of function, which was defined as mutation and/or lack of expression, was not significantly associated with olaparib SF50 response (p value 0.145), even though previous studies from our group suggested that PTEN deficiency can cause olaparib sensitivity [8, 9]. Lack of association in the cell line panel could be ascribed to the small sample size and/or to the possibility that the univariate associations do not take into account important multigene effects. Since BRCA1 mutations have been associated with reduced PTEN expression [38], we tested for association of either BRCA1 mutation or PTEN deficiency with olaparib sensitivity. We found that cell lines with a deficiency in either gene tended to be more sensitive to olaparib than cell lines with functional BRCA1 and PTEN (p value 0.052) (Supplementary Table 1b). No association was found between TP53 mutation status and drug response (p value 0.376).
Cell line-based seven-transcript signature predicts response to olaparib
We used a breast cancer cell line panel comprised luminal, basal, and claudin-low cell lines to develop a multi-transcript predictor of sensitivity to olaparib according to the REMARK recommendations [39]. We limited the predictor to transcript levels to facilitate clinical application. We considered all breast cancer subtypes for the development of the predictor based on a study of RAD51 focus formation in cells responding to a PARP inhibitor. That study showed that 30–40 % of triple negative breast cancers appeared not to have defective HR and therefore might not benefit from a PARP inhibitor whilst ~20 % of non-triple negative breast cancers appeared to have defective HR and therefore might respond to a PARP inhibitor [40]. Thus, we reasoned that a predictor developed using the complete cell line panel might be applicable to the full spectrum of breast cancer covered by the cell line panel. As shown in Fig. 1, the molecular features tested as candidate biomarkers were limited to genes involved in DNA repair pathways BER, NER, MMR, HR/FA, NHEJ, and DDR as defined by Wang and Weaver [31] and in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database release 55.1 [41]. This led to the selection of 118 genes (see Supplementary Table 2) that were tested for association between transcript levels and response to olaparib. These transcript levels were measured using three different mRNA analysis platforms (Affymetrix U133A arrays, Affymetrix exon arrays and Illumina RNA-seq).
We identified the most important transcripts by applying LR with forward feature selection (fivefold CV) 100 times. Markers significantly associated with olaparib response in over half of the iterations are shown in Table 2. These were further reduced to seven gene transcripts that were significantly associated with olaparib response in at least 2 out of 3 mRNA analysis platforms. Five transcript levels (candidate resistance markers BRCA1, MRE11A, NBS1, TDG, and XPA) were inversely associated with predicted probability of response and two transcript levels (candidate sensitivity markers CHEK2 and MK2) were positively associated with predicted probability of response (see Table 3). BRCA1 is involved in DSB repair via RAD51-mediated HR [42, 43]. CHEK2 is a kinase with signal transduction function in cell-cycle regulation and checkpoint responses [2], and is involved in the major parallel DDR pathway ATM-CHEK2 [31]. CHEK2 has also been reported as an intermediate-level breast cancer risk gene, regardless of family history [44, 45]. Besides the standard DDR pathways, the cell-cycle checkpoint pathway p38MAPK/MK2 is additionally activated in TP53 mutant cells [46]. MK2 activity is critical for prolonged checkpoint maintenance through a process of posttranscriptional regulation of gene expression [47]. MRE11A and NBS1 are part of the MRN complex, a multifaceted molecular machine for DSB recognition [48]. Finally, TDG is part of the BER pathway, whilst XPA encodes a zinc finger protein that is part of the NER complex.
Table 2.
Overview per expression platform of genes from six principal DNA repair pathways that are selected with the LR approach in over half of the iterations
| Biomarker source | Platform | # Genes | Genes selected in >250/500 iterationsa | Avg. AUC (std)b |
|---|---|---|---|---|
| DNA repair biomarkers (Wang and Weaver [31]) | U133A (standard) | 11/29 | BRCA1, BRCA2, CHEK2, DSS1, MRE11A, NBS1, PALB2, PARP2, PTEN, TP53, XPA | 0.793 (0.083) |
| U133A (custom) | 7/29 | BRCA1, BRCA2, CHEK2, DSS1, NBS1, RAD51, XPA | 0.945 (0.059) | |
| Exon array | 12/29 | BRCA2, CHEK2, DSS1, ERCC1, ERCC4, FANCD2, MK2, MRE11A, NBS1, USP11, XPA, XRCC5 | 0.717 (0.084) | |
| RNA-seq | 14/29 | ATM, BRCA1, DSS1, FANCD2, JTB, MK2, MRE11A, NBS1, PALB2, PARP1, PARP2, XPA, XRCC5, XRCC6 | 0.715 (0.132) | |
| KEGG | U133A (standard) | 5/103 | DNTT, MUTYH, POLM, RPA2, TOP3B | 0.745 (0.075) |
| U133A (custom) | 9/103 | DNTT, FEN1, MUTYH, NBS1, POLD1, POLM, RAD51, RAD51C, XRCC5 | 0.725 (0.092) | |
| Exon array | 4/103 | DNTT, MRE11A, TDG, UNG | 0.753 (0.083) | |
| RNA-seq | 5/103 | DCLRE1C, FEN1, RPA4, TDG, XRCC5 | 0.839 (0.054) |
aGenes with consistent pattern of sensitivity for all three platforms (U133A, exon array, RNA-seq) and for both measures of class comparison (mean, median) are shown in bold
bAverage fivefold CV area under the receiver operating characteristics curve (AUC) (standard deviation) across 100 randomizations for a LR model with optimized coefficients and inclusion of the platform-specific genes selected in >1/2 of the iterations
Table 3.
Overview of the seven genes selected for prediction of response to treatment with olaparib based on breast cancer cell line expression data
| Gene symbol | Gene name | Pathway | Entrez gene ID | Marker | Probe | Weight (w g) | Decision boundary (b g) |
|---|---|---|---|---|---|---|---|
| BRCA1 | Breast cancer 1, early onset | HR | 672 | Resistance | 204531_s_at | −0.5320 | −0.0153 |
| CHEK2 | CHK2 checkpoint homolog | DDR | 11200 | Sensitivity | 210416_s_at | 0.5806 | −0.0060 |
| MK2 | Mitogen-activated protein kinase-activated protein kinase 2 | DDR | 9261 | Sensitivity | 201461_s_at | 0.0713 | 0.0031 |
| MRE11A | MRE11 meiotic recombination 11 homolog A | DDR/HR | 4361 | Resistance | 205395_s_at | −0.1396 | −0.0044 |
| NBS1 | Nibrin | DDR | 4683 | Resistance | 202906_s_at | −0.1976 | 0.0014 |
| TDG | Thymine-DNA glycosylase | BER | 6996 | Resistance | 203743_s_at | −0.3937 | −0.0165 |
| XPA | Xeroderma pigmen-tosum, complemen-tation group A | NER | 7507 | Resistance | 205672_at | −0.2335 | −0.0126 |
The weights and decision boundaries were determined with data from the U133A expression array platform measured for the 22 cell lines used to assess response to olaparib. For each of the five resistance and two sensitivity markers, gene symbol is shown together with gene name, the DNA repair pathway the gene belongs to, entrez gene identifier, corresponding probe set from the Affymetrix U133A array, and weight and decision boundary obtained with the weighted voting algorithm
We combined information on the seven-transcript levels to form a predictive signature using a weighted voting algorithm (Supplementary Material and [28]). This algorithm assigns a weight and decision boundary to each of the seven genes, based on their expression distribution for the class of sensitive versus resistant cell lines (see Table 3). For this signature to work on external samples, the transcript levels were normalized to the geometric mean of seven control genes, followed by median normalization across the cell lines (see Supplementary Material). The larger the weight for a gene transcript level, the more influence this gene has on predicted probability of response. Positive weights were assigned for sensitivity markers and negative weights were assigned for resistance markers.
Prevalence of 8–21 % of predicted responding patients, with trend towards the basal subtype
We analyzed expression profiles measured for breast cancer patients not treated with PARP inhibitors to understand which patients would have a likelihood of response to olaparib according to our seven-transcript predictor. We used seven U133A and one U133 plus two data sets on 1,846 primary breast tumors with or without metastasis, heterogeneous in treatment and ER/PR/LN status. Our seven-transcript response algorithm predicted that 8–21 % of patients in the eight data sets would be responsive to olaparib (Table 4), using threshold 0.0372 obtained from the cell lines to distinguish sensitive from resistant (see Supplementary Material). The fraction predicted to respond was inversely related to the fraction of ER-positive patients in each data set (Pearson correlation coefficient −0.614, p value 0.1). We also tested the seven-transcript predictor in Agilent mRNA transcript profiles measured for 536 breast invasive carcinoma samples collected by The Cancer Genome Atlas (TCGA) [49]. This required that an Agilent-specific threshold distinguishing sensitive from resistant be established. We accomplished this using a set of Affymetrix and Agilent mRNA transcript profiles measured for 80 I-SPY 1 samples [50, 51]. The Agilent threshold was set so that the fraction of I-SPY 1 samples in the Agilent data set predicted to be sensitive was the same as that predicted to be sensitive using the Affymetrix data (see Supplementary Materials). The fraction of samples predicted to be sensitive in the TCGA data set was 12 % (Table 4). We assessed the transcriptional subtypes of the patient populations predicted to respond to olaparib in 464 samples from GSE25066 and in 528 TCGA tumor samples after exclusion of the normal-like samples. The tumors predicted to respond were enriched in samples classified as basal-like compared to samples classified as luminal A, luminal B or HER2 (p value 0.002 and 2.6 × 10−28 for GSE25066 and TCGA, respectively; Table 5).
Table 4.
Prevalence of the seven-gene signature in tumor samples from 9 different studies on primary breast tumors with or without metastasis, heterogeneous in treatment and ER/PR/LN status
| Data set | Platform | # Samples | Characteristics | Treatment | Event rate (%) | # Predicted responders (%)a |
|---|---|---|---|---|---|---|
| GSE2034 | U133A | 286 | 73.1 % ER+ | Untreated | 37.4 Distant metastasis | 55 (19.2) |
| 58 % PR+ | ||||||
| 18.2 % ERBB2+ | ||||||
| 0 % LN+ | ||||||
| GSE20271 | U133A | 177 | 55.7 % ER+ |
49.2 % FAC; 50.8 % T/FAC |
14.1 pCR | 26 (14.7) |
| 46.9 % PR+ | ||||||
| 14.2 % ERBB2+ | ||||||
| GSE23988 | U133A | 61 | 52.5 % ER+ | FEC/wTx | 32.8 pCR | 9 (14.8) |
| 0 % ERBB2+ | ||||||
| 65.6 % LN+ | ||||||
| Median tumor size 6 cm (2–17.5) | ||||||
| GSE4922 | U133A + B | 289 | 86.1 % ER+ | 37.7 % Systematic adjuvant therapy | 35.7 Local/distant recurrence or death | 24 (8.3) |
| 33.7 % LN+ | ||||||
| Median tumor size 2 cm (0.2–13) | ||||||
| GSE25066 | U133A | 508 | 58.9 % ER+ | Neoadj. taxane & anthra-cycline-based regimen | 19.5 pCR | 94 (18.5) |
| 69.1 % LN+ | ||||||
| 31.5 % LumA | ||||||
| 15.3 % LumB | ||||||
| 37.2 % Basal-like | ||||||
| 7.3 % HER2-enr | ||||||
| 8.7 % Normal-like | ||||||
| GSE7390 | U133A | 198 | 67.7 % ER+ | Untreated | 31.3 Distant metastasis | 33 (16.7) |
| 14.1 % ERBB2+ | ||||||
| 0 % LN+ | ||||||
| Median tumor size 2 cm (0.6–5) | ||||||
| GSE11121 | U133A | 200 | 78 % ER+ | Untreated | 23 Distant metastasis | 20 (10.0) |
| 65 % PR+ | ||||||
| 12.3 % ERBB2+ | ||||||
| 0 % LN+ | ||||||
| Median tumor size 2 cm (0.1–6.0) | ||||||
| GSE5460 | U133 plus 2 | 127 | 58.3 % ER+ | Untreated | – | 27 (21.3) |
| 23.6 % ERBB2+ | ||||||
| 49.6 % LN+ | ||||||
| Median tumor size 2.2 cm (0.8–8.5) | ||||||
| TCGA | Agilent G4502A | 536 | 44.0 % LumA | Heterogeneous | – | 67 (12.5) |
| 25.2 % LumB | ||||||
| 18.5 % Basal-like | ||||||
| 10.8 % HER2-enr | ||||||
| 1.5 % Normal-like |
FAC neoadjuvant chemotherapy regimen with 5-fluorouracil, docorubicin, and cyclophosphamide; T/FAC neoadjuvant chemotherapy regimen with paclitaxel and 5-fluorouracil, docorubicin, and cyclophosphamide; FEC/wTx neoadjuvant chemotherapy regimen with four courses of 5-fluorouracil, docorubicin, and cyclophosphamide, followed by four additional courses of weekly docetaxel and capecitabine
aNumber and percentage of patients predicted to respond to treatment with a PARP inhibitor according to the seven-gene predictor with use of threshold 0.0372 for response assignment for Affymetrix data, and threshold 0.174 for Agilent data
Table 5.
Association of breast cancer subtype with predicted response to olaparib in 464 GSE25066 and 528 TCGA tumor samples, after exclusion of the normal-like samples
| GSE25066 | Non-responders N (%) |
Responders N (%) |
TCGA | Non-responders N (%) |
Responders N (%) |
|---|---|---|---|---|---|
| Luminal A | 120 (75.0) | 40 (25.0) | Luminal A | 233 (98.7) | 3 (1.3) |
| Luminal B | 72 (92.3) | 6 (7.7) | Luminal B | 126 (93.3) | 9 (6.7) |
| Basal-like | 155 (82.0) | 34 (18.0) | Basal-like | 54 (54.5) | 45 (45.5) |
| HER2-enriched | 35 (94.6) | 2 (5.4) | HER2-enriched | 50 (86.2) | 8 (13.8) |
|
p value Chi-square test |
0.002 |
p value Chi-square test |
2.6 × 10−28 | ||
Discussion
In this hypothesis generating study, our overall aim was to use quantitative measurements of response to olaparib in 22 breast cancer cell lines to identify molecular features associated with response as a first step towards development of a molecular signature to predict clinical responses. We limited our search for features associated with olaparib response to copy number, DNA sequence abnormalities or transcription levels for 42 genes suggested in [31] for their association with DNA repair. Molecular features associated with 15 of these 42 genes were found to be significantly associated or to show a trend of association with olaparib response. Specifically, cell lines that were sensitive to olaparib were enriched in BRCA1 mutations or deletions, PARP1 amplification, reduced expression of BRCA1, ERCC4, FANCD2, MRE11A, NBS1, PR, TNKS, TNKS2, XPA, and XRCC5 and increased expression of CHEK2, MK2, PARP2, and XRCC3.
Since multiple mechanisms may contribute to olaparib sensitivity, we developed a weighted voting signature to combine influences from multiple markers. We included only transcript levels in our algorithm since most molecular features associated with response were apparent at the transcript level. We limited the search space to molecular features of 118 genes from six principal DNA repair pathways in order to increase statistical power. Associations of transcript levels for 118 genes and responses to olaparib for 22 breast cancer cell lines resulted in a seven-gene predictive signature that included five resistance markers (BRCA1, MRE11A, NBS1, TDG, and XPA) and two response markers (CHEK2 and MK2).
The transcript levels of the seven genes in the predictor were consistent with expectations from the literature. Mutations in BRCA1, loss of heterozygosity at the BRCA1 locus and deregulated expression have been described in the literature as potential markers for prediction of response to PARP inhibitors [17]. These studies are consistent with our finding that reduced BRCA1 transcript levels are associated with olaparib sensitivity. PARP1 is required for rapid accumulation of MRE11A at DSB sites. Due to the direct interaction between PARP1 and MRE11A, deficiency in MRE11A has been suggested as a mechanism of sensitizing cells to PARP1 inhibition based on the concept of synthetic lethality [52]. Moreover, a dominant negative mutation in MRE11A in MMR deficient cancers has been shown to sensitize cells to agents causing replication fork stress [53]. These reports are consistent with our finding that reduced MRE11A transcription is associated with olaparib sensitivity. Experimental disruption of the HR pathway protein NBS1 by RNAi has been reported to increase sensitivity to PARP inhibitors [15]. This is consistent with our finding that reduced transcription of NBS1 is associated with olaparib sensitivity. Cells with defective NER have been shown to be hypersensitive to platinum agents, with low XPA protein levels in testis tumor cell lines explaining the low capacity to repair cisplatin-induced DNA damage [54]. PARP inhibitors also enhance lethality in XPA-deficient cells after UV irradiation [55]. Tumor cells with deficiency of the DDR pathway have been suggested to be hypersensitive to PARP inhibitors, with the DNA repair biomarker CHEK1 shown to be overexpressed in BRCA1-like versus non-BRCA1-like triple negative breast cancer [56]. This is consistent with our finding that increased CHEK2 transcription is associated with olaparib sensitivity.
Our seven-gene transcript algorithm suggests that 8–21 % of patients with primary breast cancers may respond to olaparib and that the responsive tumors are enriched in basal-like breast cancers. This represents a hypothesis that can now be tested in clinical trials. Since the signature has not yet been tested clinically, it is inappropriate to use it to select patients for treatment with olaparib or other PARP inhibitors or to use it in any way to manage breast cancer treatment. However, it does present a signature that can be tested in planned translational analyses of ongoing clinical trials of PARP inhibitors and that can be used to determine whether clinical trials are properly sized to detect a response of the magnitude predicted by this signature.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary File The supplementary file contains a detailed description of materials and methods, Supplementary Table 1, Supplementary Table 2 and the matlab code used for signature development and signature validation. Supplementary Table 1 shows the association of individual DNA repair biomarkers with response to olaparib in the breast cancer cell line panel with use of the non-parametric Wilcoxon rank sum test. Supplementary Table 2 lists the 118 unique DNA repair biomarkers from [Wang X, Weaver D (2011) The ups and downs of DNA repair biomarkers for PARP inhibitor therapies. Am J Cancer Res 1 (3):301–327] and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, divided according to the principal DNA repair pathways BER, NER, MMR, HR/FA, NHEJ and DDR (DOCX 159 kb)
Supplementary Data 1 Processed exon array data for the 22 breast cancer cell lines (TXT 2885 kb)
Supplementary Data 2 Log-transformed RNA-seq data for 20/22 breast cancer cell lines (TXT 4577 kb)
Acknowledgments
AD is partly supported by a BAEF Fellowship of the Belgian American Educational Foundation for postdoctoral research, OLG is supported by a Fellowship from the Canadian Institutes of Health Research. This study was supported by the Director, Office of Science, Office of Biological & Environmental Research, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231; by the National Institutes of Health, National Cancer Institute Grants P50 CA 58207 Breast SPORE (LJV), U54 CA 112970, SU2C-AACR-DT0409, and the Pfizer Corporation Grant to JWG; Stand Up to Cancer/AACR Breakthrough Breast Cancer, Cancer Research UK and the Breast Cancer Research Foundation. The content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
Conflict of interest
AA and CJL may benefit financially from the development of PARP inhibitors through patents held jointly with AstraZeneca through the Institute of Cancer Research “rewards to inventors” scheme. All other authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Contributor Information
Anneleen Daemen, Email: anneleen.daemen@gmail.com.
Laura J. van’t Veer, Email: vantveerl@cc.ucsf.edu
References
- 1.Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature. 2000;407(6805):777–783. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- 2.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 3.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iglehart JD, Silver DP. Synthetic lethality—a new direction in cancer-drug development. N Engl J Med. 2009;361(2):189–191. doi: 10.1056/NEJMe0903044. [DOI] [PubMed] [Google Scholar]
- 5.Ashworth A, Lord CJ, Reis-Filho JS. Genetic interactions in cancer progression and treatment. Cell. 2011;145(1):30–38. doi: 10.1016/j.cell.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 7.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 8.Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, Waldman T, Lord CJ, Ashworth A. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1(6–7):315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, Vatcheva R, Savage K, Mackay A, Lord CJ, Ashworth A, Reis-Filho JS (2010) PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med 2(53):53ra75. doi:10.1126/scitranslmed.3001538 [DOI] [PubMed]
- 10.Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, Bowden G, Kalmyrzaev B, Warren-Perry M, Snape K, Adlard JW, Barwell J, Berg J, Brady AF, Brewer C, Brice G, Chapman C, Cook J, Davidson R, Donaldson A, Douglas F, Greenhalgh L, Henderson A, Izatt L, Kumar A, Lalloo F, Miedzybrodzka Z, Morrison PJ, Paterson J, Porteous M, Rogers MT, Shanley S, Walker L, Eccles D, Evans DG, Renwick A, Seal S, Lord CJ, Ashworth A, Reis-Filho JS, Antoniou AC, Rahman N. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43(9):879–882. doi: 10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buisson R, Dion-Cote AM, Coulombe Y, Launay H, Cai H, Stasiak AZ, Stasiak A, Xia B, Masson JY. Cooperation of breast cancer proteins PALB2 and piccolo BRCA2 in stimulating homologous recombination. Nat Struct Mol Biol. 2010;17(10):1247–1254. doi: 10.1038/nsmb.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldecott KW. Mammalian single-strand break repair: mechanisms and links with chromatin. DNA Repair. 2007;6(4):443–453. doi: 10.1016/j.dnarep.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Liang H, Tan A. PARP inhibitors. Curr Breast Cancer Rep. 2011;3:44–54. doi: 10.1007/s12609-010-0036-y. [DOI] [Google Scholar]
- 15.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O’Connor MJ, Tutt AN, Zdzienicka MZ, Smith GC, Ashworth A. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66(16):8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 16.Williamson CT, Muzik H, Turhan AG, Zamo A, O’Connor MJ, Bebb DG, Lees-Miller SP. ATM deficiency sensitizes mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1 inhibitors. Mol Cancer Ther. 2010;9(2):347–357. doi: 10.1158/1535-7163.MCT-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 18.Vinayak S, Ford J. PARP inhibitors for the treatment and prevention of breast cancer. Curr Breast Cancer Rep. 2010;2:190–197. doi: 10.1007/s12609-010-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plummer R. Poly(ADP-ribose) polymerase inhibition: a new direction for BRCA and triple-negative breast cancer? Breast Cancer Res. 2011;13(4):218. doi: 10.1186/bcr2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner NC, Ashworth A. Biomarkers of PARP inhibitor sensitivity. Breast Cancer Res Treat. 2011;127(1):283–286. doi: 10.1007/s10549-011-1375-8. [DOI] [PubMed] [Google Scholar]
- 21.Guha M. PARP inhibitors stumble in breast cancer. Nat Biotechnol. 2011;29(5):373–374. doi: 10.1038/nbt0511-373. [DOI] [PubMed] [Google Scholar]
- 22.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 23.Dent R, Lindeman G, Clemons M, Wildiers H, Chan A, McCarthy N, Singer C, Lowe E, Kemsley K, Carmichael J (2010) Safety and efficacy of the oral PARP inhibitor olaparib (AZD2281) in combination with paclitaxel for the 1st or 2nd line treatment of patients with metastatic triple negative breast cancer: results from the safety cohort of a Phase 1/2 multicentre trial. Proc Am Soc Clin Oncol 28(suppl):abstr 1018
- 24.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, Macpherson E, Carmichael J, Oza A. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 25.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 26.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 27.Weigelt B, Warne PH, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene. 2011;30(29):3222–3233. doi: 10.1038/onc.2011.42. [DOI] [PubMed] [Google Scholar]
- 28.Heiser LM, Sadanandam A, Kuo WL, Benz SC, Goldstein TC, Ng S, Gibb WJ, Wang NJ, Ziyad S, Tong F, Bayani N, Hu Z, Billig JI, Dueregger A, Lewis S, Jakkula L, Korkola JE, Durinck S, Pepin F, Guan Y, Purdom E, Neuvial P, Bengtsson H, Wood KW, Smith PG, Vassilev LT, Hennessy BT, Greshock J, Bachman KE, Hardwicke MA, Park JW, Marton LJ, Wolf DM, Collisson EA, Neve RM, Mills GB, Speed TP, Feiler HS, Wooster RF, Haussler D, Stuart JM, Gray JW, Spellman PT. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci USA. 2012;109(8):2724–2729. doi: 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulder S, Yan K, Huang F, Hess KR, Liedtke C, Lin F, Hatzis C, Hortobagyi GN, Symmans WF, Pusztai L. Development of candidate genomic markers to select breast cancer patients for dasatinib therapy. Mol Cancer Ther. 2010;9(5):1120–1127. doi: 10.1158/1535-7163.MCT-09-1117. [DOI] [PubMed] [Google Scholar]
- 30.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Weaver D. The ups and downs of DNA repair biomarkers for PARP inhibitor therapies. Am J Cancer Res. 2011;1(3):301–327. [PMC free article] [PubMed] [Google Scholar]
- 32.Holstege H, Horlings HM, Velds A, Langerod A, Borresen-Dale AL, van de Vijver MJ, Nederlof PM, Jonkers J. BRCA1-mutated and basal-like breast cancers have similar aCGH profiles and a high incidence of protein truncating TP53 mutations. BMC Cancer. 2010;10:654. doi: 10.1186/1471-2407-10-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, Kornblau SM. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5(10):2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 34.Goncalves A, Finetti P, Sabatier R, Gilabert M, Adelaide J, Borg JP, Chaffanet M, Viens P, Birnbaum D, Bertucci F. Poly(ADP-ribose) polymerase-1 mRNA expression in human breast cancer: a meta-analysis. Breast Cancer Res Treat. 2011;127(1):273–281. doi: 10.1007/s10549-010-1199-y. [DOI] [PubMed] [Google Scholar]
- 35.Domagala P, Huzarski T, Lubinski J, Gugala K, Domagala W. Immunophenotypic predictive profiling of BRCA1-associated breast cancer. Virchows Arch. 2011;458(1):55–64. doi: 10.1007/s00428-010-0988-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotter M, Pierce A, McGowan P, Madden S, Flanagan L, Quinn C, Evoy D, Crown J, McDermott E, Duffy M (2011) PARP1 in triple-negative breast cancer: expression and therapeutic potential. J Clin Oncol 29 (15_suppl):1061
- 37.Zaremba T, Ketzer P, Cole M, Coulthard S, Plummer ER, Curtin NJ. Poly(ADP-ribose) polymerase-1 polymorphisms, expression and activity in selected human tumour cell lines. Br J Cancer. 2009;101(2):256–262. doi: 10.1038/sj.bjc.6605166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saal LH, Gruvberger-Saal SK, Persson C, Lovgren K, Jumppanen M, Staaf J, Jonsson G, Pires MM, Maurer M, Holm K, Koujak S, Subramaniyam S, Vallon-Christersson J, Olsson H, Su T, Memeo L, Ludwig T, Ethier SP, Krogh M, Szabolcs M, Murty VV, Isola J, Hibshoosh H, Parsons R, Borg A. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40(1):102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100(2):229–235. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 40.Graeser M, McCarthy A, Lord CJ, Savage K, Hills M, Salter J, Orr N, Parton M, Smith IE, Reis-Filho JS, Dowsett M, Ashworth A, Turner NC. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010;16(24):6159–6168. doi: 10.1158/1078-0432.CCR-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M (2010) KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res 38(Database issue):D355–360. doi:10.1093/nar/gkp896 [DOI] [PMC free article] [PubMed]
- 42.Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25(43):5864–5874. doi: 10.1038/sj.onc.1209874. [DOI] [PubMed] [Google Scholar]
- 43.Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002;8(12):571–576. doi: 10.1016/S1471-4914(02)02434-6. [DOI] [PubMed] [Google Scholar]
- 44.CHEK2 Breast Cancer Case-Control Consortium CHEK2*1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. 2004;74(6):1175–1182. doi: 10.1086/421251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher O, Johnson N, Dos Santos Silva I, Kilpivaara O, Aittomaki K, Blomqvist C, Nevanlinna H, Wasielewski M, Meijers-Heijerboer H, Broeks A, Schmidt MK, Van’t Veer LJ, Bremer M, Dork T, Chekmariova EV, Sokolenko AP, Imyanitov EN, Hamann U, Rashid MU, Brauch H, Justenhoven C, Ashworth A, Peto J. Family history, genetic testing, and clinical risk prediction: pooled analysis of CHEK2 1100delC in 1,828 bilateral breast cancers and 7,030 controls. Cancer Epidemiol Biomarkers Prev. 2009;18(1):230–234. doi: 10.1158/1055-9965.EPI-08-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11(2):175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinhardt HC, Hasskamp P, Schmedding I, Morandell S, van Vugt MA, Wang X, Linding R, Ong SE, Weaver D, Carr SA, Yaffe MB. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Mol Cell. 2010;40(1):34–49. doi: 10.1016/j.molcel.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams GJ, Lees-Miller SP, Tainer JA. Mre11-Rad50-Nbs1 conformations and the control of sensing, signaling, and effector responses at DNA double-strand breaks. DNA Repair. 2010;9(12):1299–1306. doi: 10.1016/j.dnarep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Cancer Genome Atlas Data Portal. http://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp. Accessed 13 Jan 2012
- 50.Esserman LJ, Berry DA, Cheang MC, Yau C, Perou CM, Carey L, Demichele A, Gray JW, Conway-Dorsey K, Lenburg ME, Buxton MB, Davis SE, Van’t Veer LJ, Hudis C, Chin K, Wolf D, Krontiras H, Montgomery L, Tripathy D, Lehman C, Liu MC, Olopade OI, Rugo HS, Carpenter JT, Livasy C, Dressler L, Chhieng D, Singh B, Mies C, Rabban J, Chen YY, Giri D, Au A, Hylton N (2011) Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. doi:10.1007/s10549-011-1895-2. Accessed 13 Jan 2012
- 51.Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L, Lluch A, Vidaurre T, Holmes F, Souchon E, Wang H, Martin M, Cotrina J, Gomez H, Hubbard R, Chacon JI, Ferrer-Lozano J, Dyer R, Buxton M, Gong Y, Wu Y, Ibrahim N, Andreopoulou E, Ueno NT, Hunt K, Yang W, Nazario A, DeMichele A, O’Shaughnessy J, Hortobagyi GN, Symmans WF. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. J Am Med Assoc. 2011;305(18):1873–1881. doi: 10.1001/jama.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilar E, Bartnik CM, Stenzel SL, Raskin L, Ahn J, Moreno V, Mukherjee B, Iniesta MD, Morgan MA, Rennert G, Gruber SB. MRE11 deficiency increases sensitivity to poly(ADP-ribose) polymerase inhibition in microsatellite unstable colorectal cancers. Cancer Res. 2011;71(7):2632–2642. doi: 10.1158/0008-5472.CAN-10-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen Q, Scorah J, Phear G, Rodgers G, Rodgers S, Meuth M. A mutant allele of MRE11 found in mismatch repair-deficient tumor cells suppresses the cellular response to DNA replication fork stress in a dominant negative manner. Mol Biol Cell. 2008;19(4):1693–1705. doi: 10.1091/mbc.E07-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koberle B, Masters JR, Hartley JA, Wood RD. Defective repair of cisplatin-induced DNA damage caused by reduced XPA protein in testicular germ cell tumours. Curr Biol. 1999;9(5):273–276. doi: 10.1016/S0960-9822(99)80118-3. [DOI] [PubMed] [Google Scholar]
- 55.Okano S, Kanno S, Nakajima S, Yasui A. Cellular responses and repair of single-strand breaks introduced by UV damage endonuclease in mammalian cells. J Biol Chem. 2000;275(42):32635–32641. doi: 10.1074/jbc.M004085200. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez AA, Makris A, Wu MF, Rimawi M, Froehlich A, Dave B, Hilsenbeck SG, Chamness GC, Lewis MT, Dobrolecki LE, Jain D, Sahoo S, Osborne CK, Chang JC. DNA repair signature is associated with anthracycline response in triple negative breast cancer patients. Breast Cancer Res Treat. 2010;123(1):189–196. doi: 10.1007/s10549-010-0983-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File The supplementary file contains a detailed description of materials and methods, Supplementary Table 1, Supplementary Table 2 and the matlab code used for signature development and signature validation. Supplementary Table 1 shows the association of individual DNA repair biomarkers with response to olaparib in the breast cancer cell line panel with use of the non-parametric Wilcoxon rank sum test. Supplementary Table 2 lists the 118 unique DNA repair biomarkers from [Wang X, Weaver D (2011) The ups and downs of DNA repair biomarkers for PARP inhibitor therapies. Am J Cancer Res 1 (3):301–327] and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, divided according to the principal DNA repair pathways BER, NER, MMR, HR/FA, NHEJ and DDR (DOCX 159 kb)
Supplementary Data 1 Processed exon array data for the 22 breast cancer cell lines (TXT 2885 kb)
Supplementary Data 2 Log-transformed RNA-seq data for 20/22 breast cancer cell lines (TXT 4577 kb)