Abstract
Despite the increasing sophistication and affordability of touch-sensitive technology, its use in the behavioral sciences has been limited. The present paper describes the design and empirical validation of a novel touch-sensitive operant conditioning chamber for use with unrestrained squirrel monkeys. In addition, results from a variant of a commonly employed animal model of learning, the repeated acquisition task, demonstrated the effectiveness of this chamber in programming an assay of complex behavior. Finally, results from a study with Δ9-tetrahyrdrocannabinol, the active ingredient in marijuana, showed that its effects in this novel touchscreen chamber were consistent with its dose-related effects on learning using more conventional approaches. Overall, these studies indicate the touchscreen apparatus provides effective means for programming complex behavioral tasks to assess the effects of pharmacological agents on cognitive function.
Keywords: Complex behavior, Cognitive function, Δ9-tetrahyrdrocannabinol, Measurement validity, Touchscreen, Squirrel monkey
1. Introduction
In recent years touch-sensitive technology, which permits the use of computer-generated stimuli, has become both more sophisticated and affordable. Despite this, its use in the behavioral sciences employing operant methodology has developed slowly. As interest increases in studies of complex behavior, including higher-order cognitive function in laboratory animals, especially in nonhuman primates (NHP), researchers may benefit from the relatively unlimited variety of complex stimuli as well as the variability in stimulus position afforded by touchscreen technology that are not possible with standard stimulus lights and levers.
Several recent studies have used touch-sensitive technology with NHP but, typically, with a portable apparatus that is attached to their homecage (e.g., Judge et al., 2011; Rodriguez et al., 2011; Takemoto et al., 2011). These studies report orderly data but also illustrate some limitations. For example, conducting studies in the homecage environment may be problematic depending on species. When describing why experimental sessions were shorter for squirrel monkeys than rhesus monkeys, Judge et al. (2011) stated, “some squirrel monkey subjects would not maintain attention to the task for longer testing sessions” (p. 59). This limitation may stem from the less-controlled and often noisy environment of NHP colonies. Indeed, advantages of isolated sound- and light-attenuating chambers were noted early on in the behavioral literature (e.g., Skinner, 1956) and remain an important feature of operant conditioning methodology. With this in mind, the purpose of the present studies was to, first, construct and empirical validate an isolated touch-sensitive chamber for squirrel monkeys. Squirrel monkeys (Saimiri sciureus) have served as subjects in experimental psychology and behavioral pharmacology for over five decades and continue to provide extremely useful information regarding the behavioral effects of psychoactive drugs (e.g., Desai et al., 2007; Justinova, et al., 2005; Kelleher et al., 1963; Morse and Kelleher, 1968; Spealman, 1979). Their small size (usually 750–1100 g), lifespan (approximately 20 years under laboratory conditions), and new world origins (free of hepatitis A and B, herpes B), make them a relatively convenient and safe nonhuman primate experimental subject (see Rosenblum and Cooper, 1968). Next, the utility of the chamber was assessed with a variant of a common cognitive task. Finally, pharmacological sensitivity under these novel testing conditions was assessed with Δ9-tetrahydrocannabinol (Δ9-THC), a drug that has been shown to have dose-related effects on hippocampal-based learning and memory in NHP (e.g., Hampson and Deadwyler, 1999; Hampson et al., 2011).
2. Experiment 1
The purpose of Experiment 1 was to empirically validate the novel apparatus by first using a stimulus fading procedure to train touch responses and then evaluating the fidelity of the screen as a response transducer by assessing relative response rates under fixed-ratio (FR) schedules of reinforcement in different areas of the screen.
2.1. Method
2.1.1. Subjects
Four adult male squirrel monkeys (Saimiri sciureus) were individually housed in a temperature- and humidity-controlled vivarium with a 12-h light/dark cycle (7am–7pm). Subjects had unlimited access to water in the home cage and were maintained at approximate free-feeding weights by post-session feedings of a nutritionally balanced diet of high protein banana-flavored biscuits (Purina Monkey Chow, St. Louis, MO). In addition, fresh fruit and environmental enrichment were provided daily. Experimental sessions were conducted 5 days a week (Monday–Friday). The experimental protocol for the present studies was approved by the Institutional Animal Care and Use Committee at McLean Hospital. Subjects were maintained in a facility licensed by the U.S. Department of Agriculture and in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
2.1.2. Apparatus
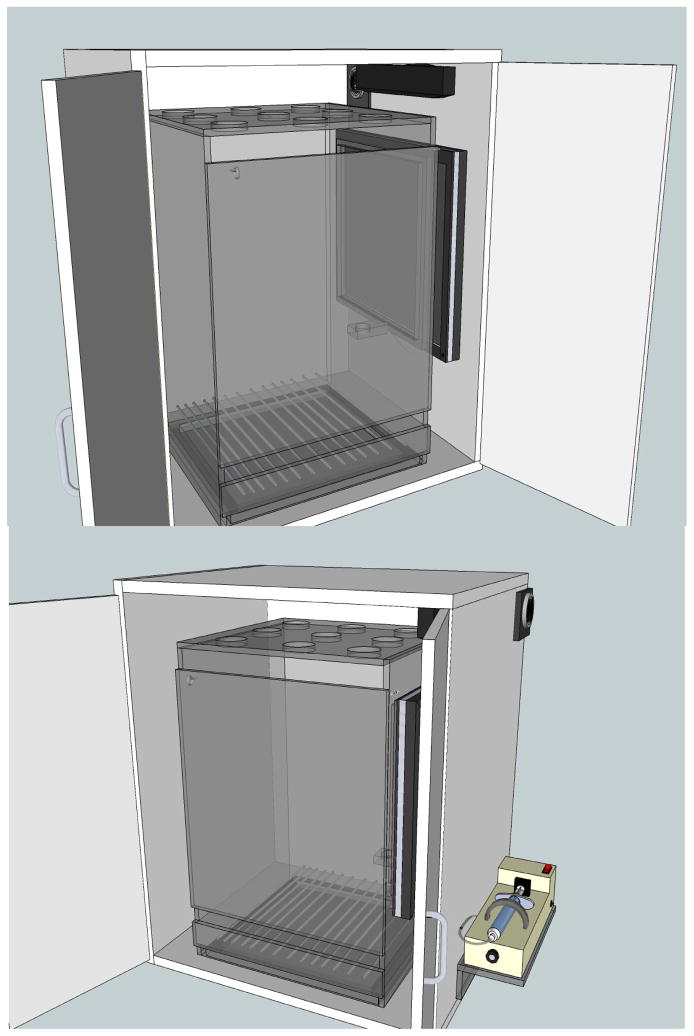
Figure 1 shows schematics and a photograph of the touch-sensitive chamber. Specifically, a custom-built Plexiglas™ chamber measuring 38 cm × 40 cm × 60 cm was housed in a custom-built 50 cm × 60 cm × 70 cm sound- and light-attenuating enclosure. The right wall of the chamber had a 34 cm × 27 cm cut-out to fit the 17″ touch-sensitive screen (1739L, ELO TouchSystems, Menlo Park, CA) mounted to the inside surface of the wall of the enclosure. Stainless steel floor bars (5.25 mm diameter) were mounted 5 cm above a removable excreta tray. An infusion pump (PHM-100-10, Med Associates, St. Albans, VT) mounted to the outside of the right wall of the enclosure was used to deliver precise quantities of a milk solution (30% sweetened condensed milk, 70% water) via Tygon® Microbore tubing (0.40 ID, 0.70 OD, Saint-Gobain Performance Plastics, Paris, France) into a custom-designed 5 cm × 3.5 cm × 1.27 cm milk well with a circle well diameter of 2.5 cm. Previous studies in our laboratory have found that this concentration serves as a powerful reinforcer for squirrel monkeys that is very resistant to satiation even under free-feeding conditions. A speaker bar (NQ576AT, Hewlett-Packard, Palo Alto, CA) mounted to the top of the inside right enclosure wall was used to emit an audible feedback click each time the subject touched a stimulus presented on the screen. This was designed to mark the subject’s response by mimicking the sound of a relay click in conventional operant chambers (see Ferster and Skinner, 1957). All experimental events and data collection were programmed in E-Prime Professional 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA).
Figure 1.

Schematic and photograph of the experimental apparatus. See apparatus section 2.1.2 for measurement details.
2.1.3. Procedure
Subjects were transported from the vivarium to the laboratory in stainless steel transfer cages using standard pole and leash techniques (see Wood, 1969). During experimental sessions, subjects were placed within the experimental chamber and were not restrained or restricted in their movement. Each subject was first trained to drink milk from the well and then trained to touch the screen with its hand using response shaping (see Catania, 1998). The initial training stimulus was a blue screen. As topographical requirements were made increasingly stringent, a stimulus fading procedure (Terrace, 1963) was programmed such that the blue training stimulus was made smaller (background always white) after reliable responding within the blue training stimulus was observed. The progression of stimulus fading was the entire screen, 18 cm × 18 cm, 12 cm × 12 cm, 7 cm × 7 cm, and finally 5 cm × 5 cm.
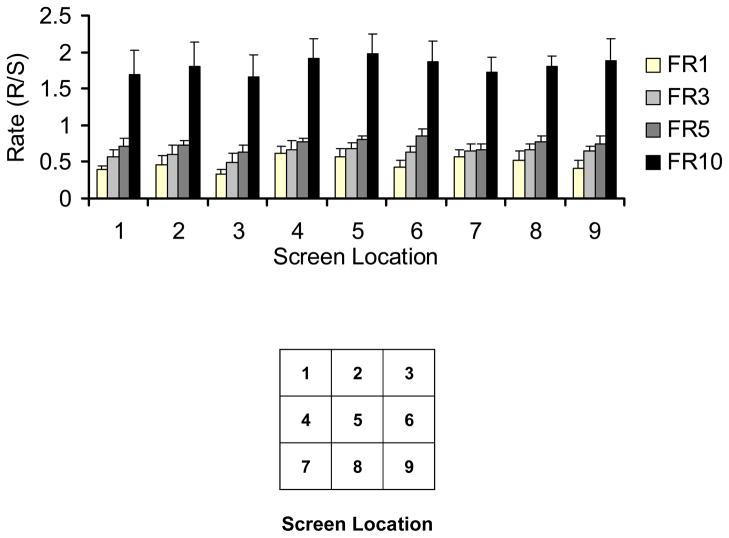
After discrete, hand-only, responses on the final 5 cm × 5 cm training stimulus were reliably observed, an automated program was introduced such that each response within the blue square was reinforced with 0.15 ml of milk, paired with an 880 ms yellow screen flash. Milk delivery was followed by a 5 s intertrial interval (ITI) in which the screen was blackened. These conditions were programmed for five 100-trial sessions before subjects were exposed to the terminal training procedure in which the 5 cm × 5 cm blue stimulus was presented in a quasi-random location on the screen each trial. Specifically, there were 9 possible locations (in an equally-spaced 3 × 3 position matrix) for the stimulus to appear on a given trial (see Fig. 2 legend), and although location varied across trials, each of the 9 stimulus positions was used exactly 10 times comprising each 90-trial session.
Figure 2.
Mean response rates (±SEM) across 9 areas of the screen under FR1, 3, 5, and 10.
In order to assess the fidelity of the touchscreen, we measured relative response rates in different areas of the screen. In addition, because both simple operant tasks as well as more complex cognitive and memorial procedures often involve schedules of reinforcement (e.g., multiple response requirements), response rates under several FR schedules of reinforcement were investigated. Each subject was exposed to an ascending FR schedule of FR1, 3, and 5 for three sessions each, followed by an FR10 schedule which was maintained over sessions until stable response rates were observed. Our criterion for stability was the occurrence of 5 consecutive sessions, after a minimum of 15 sessions under the FR10 schedule, in which the overall response rate during each session was within ±10% of the overall mean rate across the 5 sessions.
2.2. Results
The stimulus fading procedure proved to be an effective tactic to shape touch responses. Reliable responses to the final 5 cm × 5 cm stimulus were observed after approximately 2 to 4 hours of shaping. The shaded bars in Figure 2 present group-mean response rate data for responding in the 9 sections of the screen during the 3 training sessions of FR1, 3, and 5. The black bar represents the group-mean response rate under FR10 during the final 5 sessions of steady-state performance. As the figure indicates, response rates increased as the FR requirement was increased, and during steady-state performance under FR10, rates approached or exceeded 2 responses per sec, approximating those obtained under an FR10 schedule with squirrel monkeys using a standard response lever (cf. Delatte and Paronis, 2008). In addition, no systematic differences were noted in the relative response rates across areas of the screen suggesting that the entire screen was a reliable response transducer notwithstanding slight differences in topographies that might be related to screen position. These results, albeit straightforward, support the utility of the novel apparatus by demonstrating the availability of the entire screen for presentation of complex stimuli. Finally, these data also emphasize the benefit of permitting easy movement within the chamber: such a wide array of stimulus arrangement would not be possible with seated subjects.
3. Experiment 2
The results from Experiment 1 validated the first of two primary characteristics of touchscreen technology in operant conditioning chambers, namely that all areas of the screen can be used by the subject with comparable efficiency, thus providing a large variety of possible stimulus position arrangements. The second characteristic of this technology involves the near-limitless variety of visual stimuli that can be presented to the subject. The purpose of Experiment 2 was to investigate performance under a customized variant of a classic model of animal learning, the repeated acquisition task. This variant used different complex pictorial stimuli (i.e., digital photographs) during each session in order to 1) assess whether squirrel monkeys can effectively discriminate complex visual stimuli, and 2) evaluate the effectiveness of the novel chamber for evaluating complex behavior.
The repeated acquisition task is a commonly employed assay of learning. Subjects view two concurrently-presented stimuli – one arbitrarily designated as S+ (responses result in reinforcement) and the other as S− (responses result in timeout). Through trial-and-error, subjects acquire the discrimination, as evidenced by exclusive S+ responding, after which another pair of stimuli is presented (i.e., repeated acquisition). In early work with NHP, Harlow (1949) discovered a reliable effect – the rate at which a subject acquires such discriminations increase as a function of repeated exposure to the task until very few trials are required to learn the discrimination. He called this behavioral phenomenon a learning set. Although this effect has been replicated with humans, non-primates, and NHP species, including squirrel monkeys (see Warren, 1965, for an early review), its initial discovery was with 3 dimensional (i.e., physical) objects manually presented to the subjects by an experimenter, and it was not clear how these features (dimensionality) might constrain the general effect. In the present procedural variant, novel digital photographs presented on the touchscreen served as stimuli.
3.1. Method
3.1.1. Subjects and Apparatus
Same as Experiment 1.
3.1.2. Procedure
Each session began with concurrent presentation of two 7 cm × 7 cm digital photographs, each in a different randomly-selected quadrant of the screen. A response to one initiated a 0.15 ml milk delivery (S+) paired with an 880 ms yellow screen flash, followed by a 10 s ITI blackout, whereas a touch to the other immediately initiated the 10 s ITI (S−). The same two stimuli were presented during each of 200 trials comprising each session. S+ and S− assignments were determined by coin flip. Subjects were presented with a new S+/S− pair each session, with photographs randomly selected from our laboratory bank of >10,000 images. That is, the subject learned a new S+/S− discrimination each session based on distinguishing features of the two visual stimuli. The primary dependent variable was the number of trials to acquire the discrimination; our criterion for mastery was accuracy (accurate responses defined as responses to S+) in 9 of 10 consecutive trials. A 2 hr session time limit was programmed but, in the course of the present experiment, never enabled.
3.2. Results
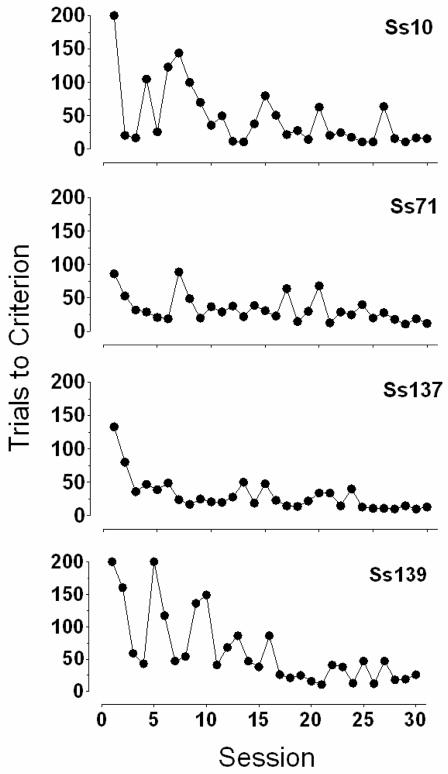
Figure 3 presents the number of trials required to acquire the discrimination across each of 30 successive sessions. Consistent with previous research on learning sets, the rate of acquisition increased across successive sessions until the subject acquired the discrimination quickly each session. This was evident in the number of trials to reach 90% accuracy which, as shown in Figure 3, was usually fewer than 20 trials by the end of the 30-session block. These data confirm that squirrel monkeys can effectively learn new discriminations with complex visual stimuli each session, and that the manner in which the rate of acquisition changes across experimental sessions is similar to that observed in conventional approaches (cf. Harlow, 1949; Warren, 1965). Moreover, the visual dimension on which the discrimination is based (e.g., color, edge), was not constant but varied from session to session. Interestingly, in preliminary studies using fixed-position stimuli (i.e., S+ and S− were presented left and right of center), discriminations were not rapidly learned as control by position dominated (data not shown). That is, side-biases were observed, a common outcome in early discrimination training with fixed manipulanda (see Kangas & Branch, 2008). This suggests that the variability in stimulus position of S+ and S− across trials improved the efficiency of discrimination acquisition and reiterates the value of unrestrained subjects having access to a large screen with multiple stimulus position arrangements. Finally, although the number of sessions required to achieve criterion varied among subjects, these data show that a stable baseline amenable to the assessment of drug effects on discrimination learning was readily obtained.
Figure 3.
The number of trials into the repeated acquisition session where the subject responded to the S+ stimulus on the previous 9 of 10 trials (i.e., our discrimination mastery criterion) across 30 successive sessions.
4. Experiment 3
Experiment 3 employed Δ9-THC, the primary psychoactive ingredient in marijuana, and most commonly abused illegal drug in the United States (SAMHSA, 2011), to determine whether the novel conditions of the experimental chamber and learning task were suitable for pharmacological studies. Δ9-THC, a CB1 partial agonist, binds to CNS CB1 receptors in brain regions that are implicated in learning processes, including basal ganglia, cerebellum, and hippocampus (Glass et al., 1997; Hampson and Deadwyler, 1999; Herkenham et al., 1990). Consistent with this binding profile, Δ9-THC can produce deficits in learning and memory, which have been well-documented in laboratory animals and humans (e.g., Abel, 1971; Darley et al., 1973a; Darley et al., 1973b). More recently, Winsauer and colleagues have examined the effects of selected CB1 agonists on a variant of the repeated acquisition task in which subjects (rodents or NHP) acquire different response sequences across three levers in each session. In those studies, acutely-administered CB1 agonists produced orderly dose-related decreases in the rate of acquisition at doses that also affected overall response rate (e.g., Nakamura-Palacios et al., 2000; Winsauer et al., 1999). Therefore, these results provide useful comparisons for the evaluation of Δ9-THC’s effects under the present conditions.
4.1. Method
4.1.1. Subjects and Apparatus
Same as Experiment 1.
4.1.2. Procedure
This experiment was conducted after steady-state baselines of acquisition rate were observed. Specifically, 10 consecutive sessions requiring fewer than 20 trials to reach our mastery criterion described above were necessary prior to initiating experiments with Δ9-THC. All procedural details were the same as Experiment 2, except that Δ9-THC was administered 1 hr prior to selected test sessions. This pretreatment time was selected to ensure the study of peak effects of Δ9-THC during the early portion of the session when, as discovered in Experiment 2, the discrimination develops. Drug test sessions occurred following control sessions in which baseline rates of acquisition were observed and were spaced at least 4 days apart. The effects of doses of Δ9-THC ranging from those without effect to those that abolished responding beyond a programmed 2 hr session time limit were studied in each subject.
Δ9-THC was provided by the NIDA Drug Supply Program (Rockville, MD) and prepared for administration in a 20:20:60 mixture of 95% ethanol, Emulphor (Alkamuls EL-620; Rhone-Poulenc, Cranbury, NJ), and saline. Solutions were refrigerated and protected from light. Injections of drug or vehicle were prepared in volumes of 0.3 ml/kg body weight or less and, were administered i.m. in the calf or thigh muscle.
4.2. Results
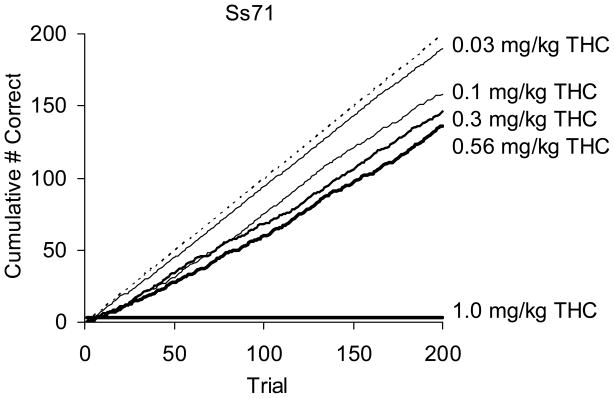
Figure 4 presents cumulative trial-by-trial acquisition data following several doses of Δ9-THC for one subject (Ss71). The figure plots the cumulative number of correct trials as a function of cumulative total trials during each drug administration session. As the figure indicates, orderly dose-related decrements in acquisition were observed. The dose of 0.03 mg/kg Δ9-THC had no effect on acquisition, producing data very similar to baseline control values (control and saline curves largely overlapped and therefore are not shown). A direct relation between the number of incorrect trials throughout the session and administration of larger doses of Δ9-THC prior to the session resulted in a dose-related decrement in slope. Following the highest dose (1.0 mg/kg), performance on the touchscreen was abolished (represented by the horizontal line above the abscissa).
Figure 4.
Effects of Δ9-THC on trial-by-trial acquisition performance. Cumulative correct trials are plotted as a function of cumulative total trials following administration of Δ9-THC for an individual squirrel monkey. The thickness of the data series is related to dose with thicker lines representing performance under larger doses of Δ9-THC. Dashed reference line represents 100% accuracy.
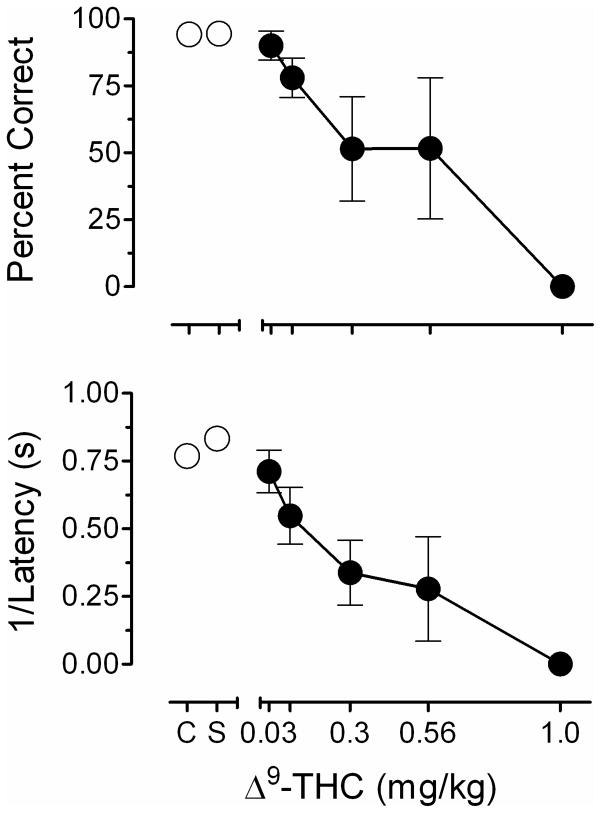
The top panel of Figure 5 presents the mean effects of Δ9-THC and saline (S) on overall session accuracy (i.e., percent correct) for the group of 4 subjects. Control (C) values represent the average accuracy of all sessions immediately prior to drug administration sessions. Accuracy values were set to 0% correct to represent sessions in which discrimination performance was abolished throughout the 2-hr session. As the figure shows, dose-related decrements in performance accuracy were observed. Graded dose-related effects were obtained in 3 of 4 subjects; the fourth subject, Ss137, showed negligible effects of small and moderate doses of Δ9-THC, but performance was abolished following administration of 1.0 mg/kg. The lower panel of Figure 5 shows effects of Δ9-THC and saline (S) on the latency to respond with data transformed by dividing 1 by the latency value. This was done for ease of comparison with accuracy (top panel). As the figure indicates, a similar dose-related increase on latency to respond that mirrored effects on accuracy was observed.
Figure 5.
Upper panel: Effects of Δ9-THC and saline (S) on overall session accuracy (±SEM). Control (C) values represent the average accuracy of all sessions immediately prior to drug administration sessions. Accuracy values were set to 0% correct to represent sessions in which discrimination performance was abolished throughout the 2-hr session.
Lower panel: Effects of Δ9-THC and saline (S) on latency to respond (±SEM). Control (C) values represent the average accuracy of all sessions immediately prior to drug administration sessions. Latency values were set to 7,200 s to represent sessions in which discrimination performance was abolished throughout the 2-hr session. Data transformed by dividing 1 by the latency for ease of comparison with the top panel.
Taken together, these data indicate that the novel apparatus and task used in the present studies are suitable for examining the effects of behaviorally-active drugs on complex performance, as evidenced by orderly dose-related effects on this repeated acquisition task. Importantly, the dose-related effects on accuracy were observed under an identical dose range as in a different type of repeated acquisition with squirrel monkeys described above (Nakamura-Palacios et al., 2000), indicating that the present findings comport well with previous studies of Δ9-THC-related decrements on learning.
5. Discussion
The present results validate our novel isolated touch-sensitive chamber for squirrel monkeys as an experimentally robust means for studying complex behavior. The touchscreen appears to be an effective response transducer, and is capable of exposing subjects to a near-limitless quantity of complex stimuli in a variety of stimulus-position arrangements. The chamber/task conditions were also suitable for determining the dose-related effects of Δ9-THC, a compound well-known to produce effects on learning under more conventional approaches.
Importantly, the isolated conditions allowed for long sessions (e.g., 200 trials/session of repeated acquisition exposure), with no indication of the attention deficits (e.g., latency changes) noted in previous research with squirrel monkeys tested in their home colonies (e.g., Judge et al., 2011). Overall, the present results strongly support the utility of the novel touchscreen chamber for studying the effects of psychoactive drugs on complex behavior and higher-order cognitive performance.
Finally, it should be noted that CANTAB (Cambridge Neuropsychological Test Automated Batteries, CeNes, Cambridge, UK) has also proven effective in obtaining orderly data of a similar sort, including a different, but related variant of a repeated acquisition task (e.g., Spinelli et al., 2004; Weed et al., 1999; Zürcher et al., 2010). One of the most attractive features of the CANTAB software is its adaptation of several human cognitive tasks (e.g., the Wisconsin card sort) for nonhuman primate subjects. Unlike CANTAB, the chamber described here is not intended to evaluate performance under a fixed battery of cognitive tests. Rather it was designed to serve as a generic and experimentally flexible interface that exploits technological advances for behavioral research. Like standard operant conditioning equipment, it can be programmed for a wide variety of simple and complex behavioral tasks as dictated by research objectives.
Highlights.
Touch-sensitive technology use in the behavioral sciences has been limited
Describes the design and empirical validation of a novel touch-sensitive operant chamber for use with squirrel monkeys
Results demonstrated the effectiveness of this chamber in programming an assay of complex behavior
Results with Δ9-tetrahyrdrocannabinol showed consistent dose-related effects on learning with more conventional approaches
Touchscreen provided effective means for programming complex behavioral tasks to assess the effects of drugs on cognition
Acknowledgments
The authors thank Michael Z. Leonard for assistance conducting this study.
This research was supported by grants DA023142 and DA007252 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Marihuana and memory: Acquisition or Retrieval? Science. 1971;173:1038–1040. doi: 10.1126/science.173.4001.1038. [DOI] [PubMed] [Google Scholar]
- Catania AC. Learning. 4. New Jersey: Prentice Hall; 1998. [Google Scholar]
- Darley CF, Tinklenberg WT, Roth JR, Hollister LE, Atkinson RC. Influence of marihuana on storage and retrieval processes in memory. Mem Cognition. 1973a;1:196–200. doi: 10.3758/BF03198094. [DOI] [PubMed] [Google Scholar]
- Darley CF, Tinklenberg WT, Roth JR, Hollister LE, Atkinson RC. Marihuana and retrieval from short-term memory. Psychopharmacologia (Berl) 1973b;29:231–38. doi: 10.1007/BF00414037. [DOI] [PubMed] [Google Scholar]
- Delatte MS, Paronis CA. Evaluation of cannabinoid agonists using punished responding and midazolam discrimination procedures. Psychopharmacology. 2008;198:521–28. doi: 10.1007/s00213-007-0918-5. [DOI] [PubMed] [Google Scholar]
- Desai RI, Neumeyer JL, Paronis CA, Nguyen P, Bergman J. Behavioral effects of the R(+)- and S-(−)-enantiomers of the dopamine D(1)-like partial receptor agonist SKF 83959 in monkeys. Eur J Pharmacol. 2007;558:98–106. doi: 10.1016/j.ejphar.2006.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of Reinforcement. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65:715–23. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Sweatt AJ, Goonawardena AV, Song D, Chan RH, Marmarelis VZ, Berger TW, Deadwyler SA. Memory encoding in hippocampal ensembles is negatively influenced by cannabinoid CB1 receptors. Behav Pharmacol. 2011;22:335–46. doi: 10.1097/FBP.0b013e3283473bfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF. The formation of learning sets. Psychol Rev. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proceed Natl Acad Sci USA. 1990;87:1932–36. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005;25:5645–50. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge PG, Evans DW, Schroepfer KK, Gross AC. Preservation on a reversal-learning task correlates with rates of self-directed behavior in nonhuman primates. Behav Brain Res. 2011;222:57–65. doi: 10.1016/j.bbr.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Branch MN. Empirical validation of a procedure to correct position and stimulus biases in matching-to-sample. J Exp Anal Behav. 2008;90:103–112. doi: 10.1901/jeab.2008.90-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of the behavioral effects of drugs. Erg Physiol Biol Ch. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Gill CA, Riddle WC, Cook L. On the use of the squirrel monkey in behavioral and pharmacological experiments. J Exp Anal Behav. 1963;6:249–52. doi: 10.1901/jeab.1963.6-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Winsauer PJ, Moerschbaucher JM. Effects of cannabinoid ligand SR141716A alone or in combination with Δ9-tetrahrdrocannabinol or scopolamine on learning in squirrel monkeys. Behav Pharmacol. 2000;11:377–86. doi: 10.1097/00008877-200008000-00003. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Rodriguez JS, Zvrcher NR, Bartlett TQ, Nathanielsz PW, Nijland MJ. CANTAB delayed matching to sample task performance in juvenile baboons. J Neurosci Meth. 2011;196:258–63. doi: 10.1016/j.jneumeth.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum LA, Cooper RW. The Squirrel Monkey. New York: Academic Press; 1968. [Google Scholar]
- Skinner BF. A case history in scientific method. Am Psychol. 1956;11:221–233. [Google Scholar]
- Spealman RD. Behavior maintained by termination of a schedule of self-administered cocaine. Science. 1979;204:1231–3. doi: 10.1126/science.109920. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR. Performance of the marmoset monkey on computerized tasks of attention and working memory. Cognitive Brain Res. 2004;19:123–137. doi: 10.1016/j.cogbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. NSDUH Series H-41, HHS Publication No. (SMA):11–4658. [Google Scholar]
- Takemoto A, Izumi A, Miwa M, Nakamura K. Development of a compact and general-purpose experimental apparatus with a touch-sensitive screen for use in evaluating cognitive functions in common marmosets. J Neurosci Meth. 2011;199:82–6. doi: 10.1016/j.jneumeth.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Terrace HS. Discrimination learning with and without “errors”. J Exp Anal Behav. 1963;6:1–27. doi: 10.1901/jeab.1963.6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM. Primate learning in comparative perspective. In: Schrier AM, Harlow HF, Stollnitz F, editors. Behavior of Nonhuman Primates. Vol. 1. New York: Academic; 1965. pp. 249–81. [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Cognitive Brain Res. 8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Wood RW. A pole and leash handling system for primates. J Exp Anal Behav. 1969;12:758. doi: 10.1901/jeab.1969.12-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, McDonald TJ, Nathanielsz PW, Nijland MJ. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Meth. 2010;188:219–225. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]