Abstract
Background
Cognitive models of bipolar I disorder (BD) may aid in identification of children who are especially vulnerable to chronic mood dysregulation. Information-processing biases related to memory and attention likely play a role in the development and persistence of BD among adolescents; however, these biases have not been extensively studied in youth with BD.
Methods
We administered the self-referent encoding task and the dot-probe task to adolescents with bipolar I disorder (BD, n = 35) and a demographically similar healthy comparison group (HC, n = 25) at baseline, and at a 1-year follow-up in a subset of this cohort (n = 22 per group).
Results
At both baseline and 1-year follow-up, there were significant interactions of group (BD, HC) and valence of stimulus (positive, negative adjective) on endorsement and recall of self-referent adjectives. HC adolescents endorsed and recalled more positive self-referent adjectives at baseline and follow-up while adolescents with BD endorsed and recalled more negative self-referent adjectives at baseline but not follow-up. Over time, depression symptomatology was associated with impaired memory for positive self-referent adjectives. There were no group differences in attentional bias at either time points.
Conclusions
Adolescents with BD exhibit bias away from endorsement and recall of positive adjectives, which remained stable over time and independent of mood state.
Keywords: Adolescence, bipolar disorder, information-processing, memory bias, longitudinal
Introduction
Bipolar disorder (BD) in children may present with impairments in cognitive function that might serve as early indicators of brain dysfunction and vulnerability to illness progression. Psychological factors, especially as they relate to cognitive function, may help to explain why some children and adolescents are more vulnerable to chronic mood dysregulation than are others (Rosen & Rich, 2010). Cognitive models of unipolar depression in youth have been investigated in some depth, including examination of children at elevated risk for depression (Joormann, Gilbert, & Gotlib, 2010; Joormann, Talbot, & Gotlib, 2007; Kujawa et al., 2011). However, few studies have examined cognitive models of BD, with only a single cross-sectional study examining how information is processed in healthy offspring of parents with BD (Gotlib, Traill, Montoya, Joormann, & Chang, 2005). In this study, Gotlib et al. (2005) compared healthy offspring of parents with BD to a healthy comparison group, and found that asymptomatic but high-familial-risk children of parents with BD recalled more self-referent negative adjectives than did healthy control children on a self-referent encoding task (SRET). Selective memory for negative stimuli has been documented in adults with depression (Matt, Vazquez, & Campbell, 1992), and is enhanced for negative adjectives that have been endorsed as self-referent (Baños, Medina, & Pascual, 2001).
Although information-processing biases such as selective memory have not yet been extensively studied in pediatric BD, cognitive deficits in a variety of domains have been documented in this population that may contribute to the development and persistence of symptoms of BD. For example, examination of specific cognitive processes in individuals with (Joseph, Frazier, Youngstrom, & Soares, 2008; Pavuluri, West, Hill, Jindal, & Sweeney, 2009; Pavuluri et al., 2006), and at risk for (Singh, DelBello, Fleck, Shear, & Strakowski, 2009) for BD, has identified deficits in working memory, visual memory, and attention despite pharmacologic treatment for their underlying mood disorder (Pavuluri et al., 2009). Passarotti, Sweeney, and Pavuluri (2010) showed that on functional magnetic resonance imaging, children with BD deployed emotion-processing circuitry to a greater extent and working memory circuitry to a lesser extent than did children with attention-deficit/hyperactivity disorder (ADHD). Taken together, the burden of these deficits in memory and attention may lead children and adolescents with BD to experience difficulties in their reception and processing of information.
The aim of the present study was to compare information-processing biases in adolescents who met full DSM-IV diagnostic criteria (American Psychiatric Association, 2000) for bipolar I disorder and in a healthy comparison (HC) group. Adolescents with BD experienced their first manic episode less than a year before enrollment (M 4.70 months from onset of mania to initial enrollment), increasing the likelihood that they were symptomatic at the time of enrollment and minimizing their lifetime exposure to psychotropic medications and to previous mood episodes. We sought to expand the Gotlib et al. (2005) study by examining adolescents with fully syndromal BD, and by assessing attention and memory biases at baseline and after 1 year. We also examined whether adolescents with syndromal BD are characterized by an attentional bias for mood-congruent face expression stimuli, as previously demonstrated in samples of depressed adults (Gotlib, Krasnoperova, Yue, & Joormann, 2004; Gotlib et al., 2004). We hypothesized that in processing adjectives on the SRET, adolescents with BD would endorse more negative than positive adjectives as self-referent while HC adolescents would endorse more positive than negative adjectives as self-referent. We further predicted that adolescents with BD would exhibit enhanced recall for negative adjectives, including those they had endorsed as self-referent, whereas HC adolescents would exhibit enhanced recall of positive adjectives, including those they had endorsed as self-referent. Finally, we hypothesized that, because a majority of adolescents with BD would be in a depressed mood state at the time of evaluation (Bopp et al., 2010; Perlis et al., 2005), they would selectively attend to negative facial expressions on a dot-probe task, whereas HC adolescents would selectively attend to positive facial expressions. We expected that these memory and attention biases would worsen frominitial assessment to follow-up 1 year later, and would correlate with symptom progression.
Methods
Participants and procedure
The university panel of medical research in human subjects approved this research protocol. Adolescents (aged 13–18 years at baseline) with bipolar I disorder, who were recruited by referral to a pediatric BDs program and from the surrounding community, completed study assessments at the initial time of enrollment (T1, n = 35) and a subset completed the same assessments 1 year later (T2, n = 22). Adolescents were eligible for inclusion in the study if they had experienced a single, first manic episode within 12 months of enrollment. Adolescents who had experienced more than one manic episode during their lifetime or who had experienced their first manic episode more than a year prior to T1 were not eligible for the study to avoid potential exposure to multiple mood episodes and medications. Healthy adolescents (n = 25 at T1 and n = 22 at T2) were recruited through internet and print advertisements in the local community. Adolescents who participated as healthy comparisons (HC) had no first-degree relatives with psychopathology, no family history of BD, and had never taken psychotropic medication during their lifetime. A screening telephone call with a parent or guardian established that all participants were fluent in English, had no history of head injury with loss of consciousness lasting more than 5 min, no seizures, and no developmental or substance dependence disorders. History of substance use was an exclusion criterion for HC but not BD participants, who could not have had their manic episode in the context of substance use, and were required to be substance-free for at least 1 month prior to study participation at both time points. To avoid the risk of mood destabilization, participants with BD were allowed to continue to take psychotropic medications. After describing the study to the participants, written informed consent and assent were obtained, and participants were invited with their parents or guardians for interviews and testing.
Measures
Clinical assessments
All participants were evaluated for current and lifetime psychiatric disorders using the Washington University in St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia (WASH-U KSADS, Geller, Zimerman, Williams, Bolhofner, and Craney, et al. 2001) for depressive and BDs, and the KSADS-PL (Kaufman et al., 1997) for remaining psychiatric diagnoses at both T1 and T2. These interviews were administered separately to adolescents about themselves and to parents or guardians about their children by a child psychiatrist or masters-level interviewer with established inter-rater reliability (kappa >.90). Formal DSM-IV diagnoses (American Psychiatric Association, 2000) were subsequently determined at a consensus conference attended by board-certified child and adolescent psychiatrists (M.K.S. and K.D.C.). Trained psychometricians with high inter-rater reliability (kappa > .90) obtained an estimate of IQ by administering the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) to all adolescents at T1 only. Inclusion criteria required IQ > 80. To estimate developmental level, adolescents completed the Peterson Pubertal Development Scale (PDS, Petersen, Crockett, Richards, & Boxer, 1988). Symptoms of anxiety were assessed using the Multidimensional Anxiety Scale for Children (MASC, March, Sullivan, Stallings, & Conners, 1997). The presence and severity of ADHD symptoms was determined by parent or guardian report on the ADHD Rating Scale (Conners, Sitarenios, Parker, & Epstein, 1998). Current manic mood state was confirmed with a score greater than or equal to 20 on the Young Mania Rating Scale (YMRS, Young, Biggs, Ziegler, & Meyer, 1978) while current depressed mood state was confirmed with a score greater than or equal to 40 on the Children’s Depression Rating Scale-Revised (CDRS-R, Poznanski & Mokros, 1995). The Adolescent Longitudinal Interview Follow-up Evaluation (A-LIFE, Keller et al., 1987) is a measurement of functional status in several different domains that was used to provide clinical context at T1 and T2, and the change in this clinician-rated measure was compared to change in major outcome measures from baseline to follow-up.
Information-processing assessments
Adolescents in the BD and HC groups completed two information-processing tasks at T1 and T2: the self-referent encoding task (SRET) and the emotion face dot-probe task. All participants were administered the dot-probe task followed by the SRET. The SRET assesses endorsement of positive and negative adjectives and selective memory for valenced stimuli. For administration of the SRET task, participants sat before a computer screen on which the words ‘Describes me?’ were displayed, followed by one positive or negative stimulus word.
Participants indicated ‘yes’ or ‘no’ for each word by using assigned keys on the computer keyboard, and the process was repeated for 40 stimulus words. The stimulus words were composed of 20 positive and 20 negative adjectives that had been reviewed by research assistants and rated on a seven-point Likert scale. Adjectives that were included as stimulus words had received a mean rating above 4.0 on one dimension (e.g., positive) and below 2.0 on the other dimension (e.g., negative). Examples of positive adjectives included ‘friendly, helpful, lucky, nice, winner,’ and examples of negative adjectives included ‘angry, lazy, lonely, strange, bad.’ Participants then completed the Digit Span task from the WAIS-III (Wechsler, 1997) to distract their attention from the adjectives they had just seen. Finally, participants completed an incidental recall task, in which they were given 3 min to recall as many of the SRET adjectives as they could, regardless of whether they had endorsed the words as self-referent (i.e., ‘Describes me?’ had been answered in the affirmative). For each item, responses and response times were recorded.
The dot-probe task assessed selective attention toward neutral compared to emotionally valenced stimuli. Pairs of photographs of faces, one photo in the pair displaying a neutral and one photo displaying an emotional expression of the same actor, were presented side-by-side on a computer screen. After the offset of the faces, a dot appeared in the place where either the neutral face or the emotionally expressive face had been, and participants’ response time to indicate the dot’s location was recorded. These response times assessed attentional orientation toward the emotional material. If participants oriented toward the emotional face, response times should have been faster when the dot was presented in the location of the emotional face. The photographs, all of adult faces, had been selected from the MacArthur Network Face Stimuli Set (Research Network on Early Experience and Brain Development, http://www.macbrain.org/resources.htm) according to the protocol described by Joormann and Gotlib (2007). Stimuli were displayed for 500 or 1500 milliseconds (ms) rather than for 1000 ms because previous studies have shown that different stimulus presentations capture different components of attention (Joormann & Gotlib, 2007). Selective attention to sad faces among individuals with depression has been demonstrated when stimuli are presented for a longer duration and are therefore subject to conscious processing (Matthews & MacLeod, 2005), whereas for individuals with anxiety, attentional bias occurs in the context of very brief stimulus exposure and is subject to automated processing (Mogg & Bradley, 2005).
Response times were used to calculate an attentional bias score according to the formula below in which R = right position, L = left position, p = probe, and e = emotional face (Mogg, Bradley, & Williams, 1995). For example, RpLe corresponds to the mean reaction time for response when the right position contains the probe and the left position contains the emotional face.
When this equation yields a positive value, it indicates that participants were faster to respond to a dot that appeared behind an emotional (i.e., happy or sad) face compared to a neutral face and, thus, oriented their attention toward happy or sad faces. A negative bias score indicates attention shifted away from the happy or sad face and toward the neutral face. We calculated the attentional bias score using the formula once for comparing response times to neutral and happy faces, and again for comparing response times to neutral and sad faces.
Following Joormann et al. (2007), we implemented two measures of quality control to address variance in attentional bias scores during this task. First, only response times associated with correct responses were analyzed. Second, data that reflected anticipation errors or lapses in attention were excluded: anticipation errors were trials with a response time less than or equal to 100 ms, and a lapse in attention was defined as a trial with a response time greater than 1000 ms.
Data analysis
Chi-squared analyses and t-tests were used to examine group differences in demographic variables. Two-way analyses of variance (ANOVAs) were conducted on the SRET data to examine the effects of group (BD, HC) and valence of adjective (positive, negative) on the proportion of words endorsed, words recalled, and self-referent words recalled. A three-way repeated measures ANOVA was conducted on the dot-probe attentional bias scores for correct responses to examine the effects of group (BD, HC), face emotion (happy, sad), and length of stimulus exposure (500, 1500 ms). Post hoc one-sample t-tests comparing attentional bias scores to zero within each group were used to examine the degree and directionality of significant group differences. A Levene’s test of homogeneity was used to examine the variance of response times across groups.
The effects of co-occurring ADHD, oppositional defiant disorder (ODD), or conduct disorder (CD), and medication exposure on T1 and T2 outcomes were examined by use of ANCOVA. The effect of these factors was examined by first comparing BD group participants without medication exposure or without comorbid Axis I disorders to participants with medication exposure or comorbid conditions, and then by serially excluding BD group participants with exposure to specific medication classes or with specific comorbidities and then reanalyzing the results. To examine the potential effect of mood state on task responses, the BD group was subdivided into manic, depressed, and mixed mood states, and each of these subgroups was compared to the HC group. Pearson correlations were used to examine the association between proportion of positive or negative words endorsed on the SRET and degree of depressive or manic symptomatology in adolescents with BD who were in a depressed, manic mood, or mixed state at the time of assessment. Pearson correlations were also used to examine the change in depressive or manic symptomatology from T1 to T2 to the change in performance on the SRET and dot-probe tasks from T1 to T2.
Results
Baseline demographic and clinical characteristics of the two participant groups are presented in Table 1. Compared to HC adolescents, adolescents with BD were older (BD: M = 15.7, SD = 1.5, HC: M = 14.9, SD = 1.4, p = .03) but were not different in terms of pubertal development at the time of enrollment (T1) or follow-up (T2), BD at T1: M = 3.3, SD = .4, HC at T1: M = 3.2, SD = .6, t(60) = −.4, p > .05; BD at T2: M = 3.4, SD = .5, HC at T2: M = 3.4, SD = .5, t(20) = .1, p > .05). Adolescents with BD had a lower M IQ (M = 106.6, SD = 9.8) than did HC adolescents (M = 112.3, SD = 10.7, t(60) = 2.1, p = .03). At T1, 8 (23%) adolescents with BD were in a manic mood state, 12 (34%) were depressed, 12 (34%) were in a mixed state of mania and depression, and 3 (9%) were euthymic. At T2, no adolescent was in a manic mood state (0%), 5 (24%) of adolescents with BD were depressed, 7 (33%) were in a mixed mood state, and 9 (43%) were euthymic. From T1 to T2, mood state remained the same for 6 of 21 adolescents with BD (29%) whereas it changed for 15 (72%), including 3 from depressed to manic and 2 from manic to depressed. Among participants in the BD group, the number of mood episodes between T1 and T2 was as follows: manic, M = 2.9, SD = 4.2, median = 1, range = 0–14; depressed, M = 4.1, SD = 5.3, median = 3 range = 1–19; mixed, M = 14.9, SD = 10.7, median = 14, range = 0–37. Twenty-seven of 35 adolescents with BD (77%) were taking psychotropic medications at T1, and 19 of 22 adolescents with BD (89%) were treated with medication at T2. There were three participants who were started on psychotropic medications after T1 and none who discontinued them between T1 and T2.
Table 1.
Baseline demographic and clinical characteristics
| Variable | Healthy adolescents (N = 25) | Adolescents with bipolar I disorder (N = 35) |
|---|---|---|
| Age*, M (SD) | 14.88 (1.42) | 15.74 (1.50) |
| Tanner stage, M (SD) | 3.19 (0.56) | 3.25 (0.43) |
| Full-scale IQ*, M (SD) | 112.32 (10.65) | 106.55 (9.81) |
| Sex, (%) Male | 40% | 46% |
| Race, N (%) | ||
| Asian | 4, 16% | 0, 0% |
| Black (non-Hispanic) | 1, 4% | 1, 3% |
| Hispanic | 3, 12% | 2, 6% |
| Pacific islander | 0, 0% | 1, 3% |
| White (non-Hispanic) | 12, 48% | 21, 60% |
| Mixed race | 3, 12% | 7, 20% |
| Lifetime medication exposure (N, M longest exposure in weeks) | ||
| Any* | 0, 0 | 27, 12.37 |
| Atypical antipsychotics* | 0, 0 | 23, 3.64 |
| Lithium* | 0, 0 | 18, 2.28 |
| Anti-depressants* | 0, 0 | 13, 3.63 |
| ADHD medications | 0, 0 | 10, 4.82 |
| Anxiolytics | 0, 0 | 4, 0.11 |
| YMRS*, M (SD) | 0.17 (0.48) | 18.81 (7.06) |
| CDRS-R*, M (SD) | 17.75 (1.26) | 45.88 (13.61) |
| MASC, M (SD) | ||
| Physical* | 3.42 (3.42) | 11.07 (8.48) |
| Harm avoidance* | 14.54 (4.03) | 11.07 (5.70) |
| Social anxiety | 6.54 (4.47) | 9.38 (7.49) |
| Separation anxiety | 3.50 (2.48) | 4.36 (4.46) |
| Mood state*, (N, %) | ||
| Euthymic | 25, 100% | 3, 9% |
| Manic | 0, 0% | 8, 23% |
| Depressed | 0, 0% | 12, 34% |
| Mixed mood state | 0, 0% | 12, 34% |
| Comorbid diagnoses, (N, %) | ||
| ADHD* | 0, 0% | 15, 43% |
| Any anxiety disorder* | 0, 0% | 5, 14% |
| ODD | 0, 0% | 2, 6% |
| Conduct disorder | 0, 0% | 1, 3% |
ADHD, attention-deficit/hyperactivity disorder; BD, adolescents with bipolar I disorder; CDRS-R, Children’s Depression Rating Scale-Revised; HC, healthy comparison group; IQ, intelligence quotient; MASC, Multidimensional Anxiety Scale for Children; ODD, oppositional defiant disorder; SD, standard deviation; SES, socioeconomic status; YMRS, Young Mania Rating Scale.
Significant p < .05.
SRET
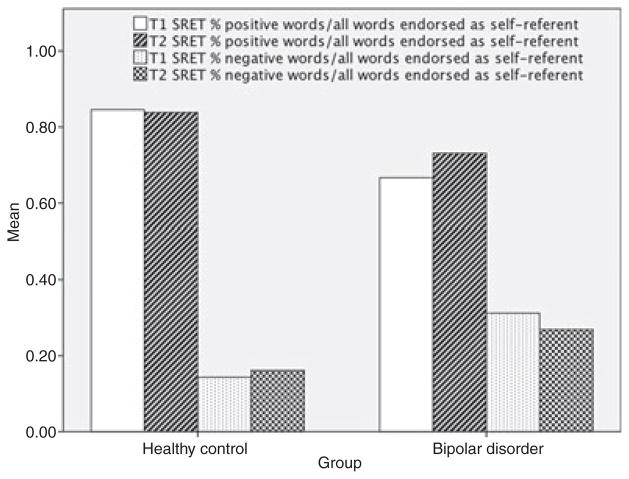
For adjective endorsement, a two-way repeated measures ANOVA examining the effects of group (BD, HC) and valence of adjective (positive, negative) on adjective endorsement yielded no main effect of group, F(1,58) = .01, p > .05, but a significant effect of valence, F(1,58) = 155.8, p < .001. There was a significant interaction between group and valence, F(1,58) = 16.3, p < .001. Follow-up tests indicated that at T1, compared with HC adolescents, adolescents with BD endorsed a significantly greater proportion of negative adjectives (BD: M = .30, SD = .22, HC: M = .13, SD = .16, t(58) = −3.38, p = .03) and a significantly smaller proportion of positive adjectives (BD: M = .68, SD = .21, HC: M = .85, SD = .12, t(58) = 3.71, p = .02). At T2, repeated measures ANOVA examining the effects of group and valence as well as time of assessment (T1, T2), demonstrated again a main effect of valence, F(1,22) = 163.5, p < .001, and a significant interaction of group and valence, F(1,22) = 10.7, p = .002. There was no significant main effect of group, time, or an interaction between group and time or valence and time, or between group, valence, and time (all p > .05). There was no significant difference at T2 between the proportion of negative (BD: M = .2, SD = .2, HC: M = .3, SD = .2, t(20) = −1.8, p > .05) or positive adjectives (BD: M = .7, SD = .2, HC: M = .8, SD = .2, t(20) = 1.8, p > .05) endorsed by each group.
For recall of all adjectives at T1, a two-way ANOVA yielded a significant main effect of valence, F(1,51) = 12.9, p = .001, but no main effect of group, F(1,58) = 1.47, p > .05, and no significant interaction of group and valence, F(1,51) = .20, p > .05. At T1, participants in both groups recalled more positive than negative adjectives. At T2, repeated measures ANOVA examining adjectives recalled based on group, valence, and time of assessment yielded a significant main effect of group, F(1,22) = 8.9, p = 0.005, and valence, F(1,22) = 15.7, p < .001, but no significant main effect of time. There were no significant interactions between valence and group, group and time, valence and time, or between valence, group, and time (all p > .05). Post hoc t-tests showed that HC recalled more positive adjectives than did BD at T2 (BD: M = 6.7, SD = 2.7, HC: M = 4.8, SD = 2.4, t(41) = 2.5, p = .02), and there was no group differences in the recall of negative adjectives (p > .05).
Figure 1 shows the valence of adjectives endorsed as self-referent by participants in each group at T1 and T2. For recall of self-referent adjectives, a two-way ANOVA conducted on recall of the adjectives that participants rated as self-referent yielded a significant main effect of valence, F(1,58) = 83.31, p < .001, and a significant interaction of group and valence, F(1,58) = 14.59, p < .001). The effect of group was not significant, F(1,58) = .73, p > .05. At T2, repeated measures ANOVA examining recall of self-referent adjectives yielded a significant main effect of group, F(1,22) = 11.7, p = .001, valence, F(1,22) = 115.0, p < .0001, and time, F(1,22) = 4.6, p = .04. There was also a significant interaction between group and valence, F(1,22) = 18.7, p < .001, and a trend-level effect of the interaction between time and valence, F(1,22) = 3.5, p = .07, but no significant interactions between group and time or between group, time, and valence (all p > .05). As predicted at T1, HC adolescents recalled significantly more self-referent positive adjectives than did adolescents with BD (BD: M = .2, SD = .1, HC: M = .3, SD = .1, t(58) = 2.7, p = .01), whereas adolescents with BD recalled significantly more self-referent negative adjectives than did HC adolescents (BD: M = .1, SD = .1, HC: M = .02, SD = .04, t(58) = −2.6, p = .001). At T2, HC again recalled significantly more self-referent positive adjectives than BD (BD: M = .2, SD = .1, HC: M = .3, SD = .1, t(20) = 3.3, p = .002) with no significant difference in recall of self-referent negative adjectives (BD: M = .05, SD = .1, HC: M = .05, SD = .1, t(22) = .02, p > .05).
Figure 1.
Proportion of valenced adjectives endorsed as self-referent by group and time
Repeated measures ANOVA including date from T2 showed a main effect of valence on reaction time to endorse adjectives as self-referent, F(1,22) = 35.0, p = .02, but no main effect of group or time and no significant interactions. At both time points, there was no association between the proportion of positive adjectives endorsed and the number of positive adjectives recalled (T1: r = .01, n = 60, T2: r = .1, all p > .05) or between the proportion of negative adjectives endorsed and the number of negative adjectives recalled (T1: r = .06, n = 60, T2: r = .1, all p > .05). Therefore, endorsing adjectives as self-referent did not seem to enhance recall at either time point.
The effect of group on words endorsed and/or recalled at T1 was unchanged when covarying for baseline age, IQ, YMRS, CDRS-R, or MASC scores (all p > .05). Excluding participants with co-occurring ADHD (n = 15), ODD (n = 2), or CD (n = 1), or those who were exposed to any psychotropic medications including atypical antipsychotics (n = 23), mood stabilizers (n = 18), antidepressants (n = 13), or ADHD medications, including psychostimulants and atomoxetine (n = 10), did not change the effect of group on words endorsed or recalled. There were no significant associations within the BD group between words endorsed and/or recalled and medication exposure (all p > .05).
At T2, the effects and interactions of group on words endorsed (no significant effect) and on words recalled and self-referent words recalled (significant effect) were unchanged when covarying for age, IQ, YMRS, and CDRS-R scores at T2 (all p > .05) and when serially excluding participants with Axis I comorbidities (ADHD, n = 8; ODD, n = 2; CD, n = 1) or exposure to medication classes (atypical antipsychotics, n = 10; mood stabilizers, n = 10; antidepressants, n = 11; ADHD medications, n = 8, all p > .05).
Exploratory analyses were conducted to examine the impact of mood state on endorsement of self-referent adjectives at T1 and T2. Participants in the BD group showed a significant main effect of depressed mood state on the proportion positive words that were endorsed as self-referent at T1, F(1,31) = 6.4, p = .02; this was not the case at T2 (p > .05). Participants with BD in a depressed mood state at T1 showed a significant inverse association between CDRS-R and the proportion of positive adjectives endorsed at baseline, r = −.7, n = 32, p < .001. Participants with BD in a depressed mood state endorsed a lower proportion of positive words as self-referent (M = .6, SD = .2) than did participants with BD who were not depressed at the time of assessment (M = .8, SD = .1; t(30) = 2.5, p = .02). There was a significant negative correlation within the BD group between CDRS-R and number of positive words recalled (r = −.4, n = 32, p = .03), and between CDRS-R and positive self-referent words recalled (r = −.5, n = 31, p = .01). At T2, adolescents with BD in a depressed mood state did not show an association between CDRS-R score and the proportion of positive words endorsed as self-referent, p > .05. There was no difference in the proportion of positive words endorsed as self-referent based upon depressed/not depressed mood state within the BD group (depressed: M = .7, SD = .3; not depressed: M = .7, SD = .2, t(19) = .04, p > .05). There was no correlation between CDRS-R and the proportion of positive or negative words endorsed by BD participants, p > .05. However, there was a significant negative correlation between CDRS-R and recall of positive self-referent words (r = −.4, n = 21, p = .05).
To examine the longitudinal effect of mood symptoms from T1 to T2, we compared the change in CDRS-R scores to the change in endorsement and recall of self-referent adjectives. Change in CDRS-R score was negatively associated with change in recall of positive self-referent adjectives, r = −.6, n = 20, p = .005, indicating that worsening depression was associated with reduced recall of positive self-referent adjectives. At T1, there was no significant association between YMRS score and the proportion of positive adjectives endorsed as self-referent (r = .06, n = 35, p = .8). There were no significant correlations between YMRS and number of total, positive words recalled, negative words recalled, or positive or negative self-referent words recalled (all p > .05). The change in YMRS score from T1 to T2 was not associated with the change in endorsement or recall of self-referent adjectives between time points (all p > .05).
To examine change in clinical status and progression in a different way, we examined associations between the change in A-LIFE scores from T1 to T2 and the change in SRET performance from T1 to T2; there was no significant association between change in A-LIFE and change in SRET performance from baseline to follow-up (all p > .05).
Dot-probe
An average of 2.2% of participants’ T1 data were discarded due to anticipation errors or lapses in attention. Two anticipation errors occurred across all participants and trials, both of which were associated with responses from the HC group. There were significantly more instances of attention lapse in the BD group (M = 3.7, SD = 5.8) than in the HC group (M = 1.0, SD = 2.4, t(58) = −2.2, p = .04). The two groups did not differ with respect to percentage of correct responses (97.2% BD vs. 97.9% HC, t(58) = 1.6, p > .05) or overall reaction time (BD: M = 500.8, SD = 93.6 ms; HC: M = 467.1, SD = 60.2 ms, t(51) = −1.5, p > .05). At T2, one participant in the BD group had more than 100 anticipation errors so was excluded from the analysis. 4.2% of the remaining data were discarded due to anticipation errors or lapses in attention. There was no difference between groups in the rate of anticipation errors, attention lapses, or correct answers (all p > .05).
A three-way ANOVA (Group: BD, HC; Valence of stimuli: happy, sad faces; Duration of stimulus exposure: 500, 1500 ms) conducted on attentional bias scores at T1 yielded a significant main effect only of duration of stimulus exposure, F(1,58) = 8.5, p = .01; neither the main effect of group, F(1,58) = .5, p > .05, nor the main effect of valence, F(1,58) = .1, p > .05, was significant. None of the interactions of group, valence, or duration of exposure were significant (all p > .05). Follow-up t-tests yielded no significant differences from zero in the bias scores for the BD or HC participants (all p > .05). At T2, four-way repeated measures ANOVA examining attentional bias scores by group, valence of stimuli, duration of stimulus exposure, and time point of assessment (T1, T2) replicated the main effect of duration of stimulus exposure, F(1,22) = 11.7, p = .02, but showed no other significant main effects or interactions (all p > .05).
An ANCOVA revealed that the effect of group on bias scores at T1 continued to be nonsignificant after co-varying separately for age, IQ, YMRS, CDRS-R, and MASC scores (all p > .05). Excluding participants who had co-occurring ADHD (n = 15), ODD (n = 2), or CD (n = 1), or who were exposed to any psychotropic medications, including atypical antipsychotics (n = 23), mood stabilizers (n = 18), antidepressants (n = 13), and ADHD medications (n = 10), did not change the nonsignificant effect of group on attentional bias scores. There were no significant associations within the BD group between attentional bias scores and maximum length of exposure to any medication (all p > .05). With regard to mood state, there was no main effect of group on positive or negative attentional bias scores when comparing adolescents with BD in a manic, depressed, or mixed mood states to HC adolescents (all p > .05). Results did not change at T2 after co-varying for age, IQ, CDRS-R, and YMRS, or serially excluding participants with Axis I comorbidities (ADHD, n = 8; ODD, n = 2; CD, n = 1) or previous exposure to different classes of psychotropic medications (atypical antipsychotics, n = 10; mood stabilizers, n = 10; antidepressants, n = 11; ADHD medications, n = 8). There was also no change in the effect of group on bias scores when comparing adolescents with BD by mood state to HC, all p > .05. There were no significant associations between change in A-LIFE scores from T1 to T2 or the change in attentional bias scores over this time period (all p > .05).
Discussion
In this study, we predicted that adolescents who had experienced the onset of BD within 1 year of enrollment would be characterized by biases in memory and attention at initial assessment and after 1-year follow-up. At baseline, adolescents with BD endorsed and recalled more negative than positive self-referent adjectives, whereas HC adolescents endorsed and recalled significantly more positive than negative self-referent adjectives. Within the BD group, recall of positive self-referent adjectives was inversely related to depressed mood. At 1-year follow-up, a negative memory bias persisted in adolescents with BD, and those who became less depressed had improved recall for positive self-referent adjectives. Neither adolescents in the BD nor the HC group exhibited attentional biases for happy or sad faces at either time point. Instead they consistently allocated their attention about equally toward neutral and emotional faces. These findings suggest that adolescents with BD use negatively charged words to describe themselves and have memory biases that persist with illness, but do not demonstrate any biases in attention toward emotionally valenced facial expressions.
Consistent with previous literature (Geller et al., 2001), two-thirds of adolescents in the BD group had significant depressive symptoms at both baseline and 1-year follow-up, suggesting that symptom burden on information-processing may have been influenced more by depression than by mania. In adolescents with BD, depressive symptomatology was related to endorsement and recall of positive adjectives. Moreover, longitudinal analysis demonstrated that improvement in depressive symptomatology over time was related to improvement in recall of positive self-referent words, suggesting that symptoms of depression contributed to how adolescents with BD recalled having described themselves. In their study using the SRET with adolescents at high risk for BD, Gotlib et al. (2005) found no difference in the proportion of valenced (positive vs. negative) adjectives recalled by adolescents at risk for BD compared to healthy adolescents; however, adolescents at risk for BD demonstrated enhanced recall for negatively valenced adjectives. Our SRET results suggest that depressive symptoms are associated with a progression to endorsement of negative words and a negative memory bias in the participants with BD. Thus, memory bias among adolescents with BD appears to be a function of the disorder rather than the mood state. Enhanced recall of negative self-referent words has also been previously reported in adults with depression (Derry & Kuiper, 1981; Heim-Dreger, Kohlmann, Eschenbeck, & Burkhardt, 2006). If BD that begins in childhood is continuous with adult BD (Chang, 2007) with a course of illness dominated by depression (Perlis et al., 2005), we speculate that adolescents in this study with bipolar I disorder have a negative memory bias that may persist into adulthood coincident with symptom persistence. The fact that negative memory bias was found to be disorder-dependent and stable over a 1-year follow-up period informs clinical management and increases our understanding of the contribution of depression to cognitive processing differences in youth with BD. It is possible, therefore, that cognitive interventions indicated for depression may be able to attenuate or even reverse the negative self-concept and negative memory bias that seems to progress with illness in bipolar youth.
A longitudinal study conducted with a sample of adolescents with BD that was exposed to multiple medications documented impairment in neurocognitive processes that continued over 3 years despite treatment of the underlying disorder (Pavuluri et al., 2009). In the present study we found improvement in memory for positively valenced self-descriptors with improvement in depressive symptomatology in a cohort of adolescents assessed soon after their first manic episode and then again a year later, with some degree of treatment between these time points. This suggests that interventions aimed at reducing depressive symptomatology may in fact relieve the burden of long-term negative cognitive biases. Further study is needed to determine the mechanism by which interventions aide in reducing symptoms and cognitive biases.
Few studies have examined attention biases in adults or children diagnosed with BD, and the results of these investigations have been mixed. Jongen, Smulders, Ranson, Arts, and Krabbendam (2007) reported that adults with BD directed their attention away from positive words, and whether they directed their attention away from depression-related words was mood state-dependent. Previous studies using the dot-probe task that have found differences in attentional bias in adolescent girls at risk for depression (Joormann et al., 2007), and that have found memory bias in adolescents who are at risk for BD (Gotlib et al., 2005) administered a sad mood induction to participants before the dot-probe task, which was not done in the present study. Adolescents with BD may have been affected with symptoms at the time of assessment that were not sufficiently severe to lead to biases in attention in the absence of a mood induction. Divergent findings may also be accounted for by differences in types of visual stimuli presented (happy and sad vs. angry or threat-relevant faces), or type of mood disorder (e.g., Joormann & Gotlib, 2007).
Brotman et al. (2007) found that only children diagnosed with BD comorbid with an anxiety disorder oriented toward threat-relevant stimuli, and no bias was found for children who had BD without anxiety. This study suggests that in the context of BD, the presence of information- processing biases may be determined in part by and the presence of co-occurring anxiety symptoms. The significance of anxiety in attentional biases among children has also been described (Heim-Dreger et al., 2006; Waters, Henry, Mogg, Bradley, & Pine, 2009); however, in the present sample, MASC subscale scores were not related to our group findings on either of the information-processing tasks. Previous studies have also documented an association between ADHD and attentional bias toward emotionally valenced over neutral cues; in this sample, however, presence of ADHD or current medication treatment for ADHD had no impact on major outcome measures for the dot-probe task or the SRET.
Limitations that may have affected the results of this study include a small sample size, and sample heterogeneity, which may have attenuated potential group differences, particularly in terms of attentional bias. Although beyond the scope of the current study, which was aimed at comparisons to typically developing youth, the addition of a morbid control group would add information concerning specificity to information-processing biases in youth with BD, compared to youth in other psychopathological states. Despite these potential limitations, this study is the first to describe the biases in memory of adolescents with BD at a pivotal point early in the course of illness. Adolescents with BD, who demonstrated enhanced recall for negative adjectives, may have organized a cognitive framework early in the course of their illness that reflects low self-esteem and increases the likelihood of future mood episodes.
Conclusion
The present results increase our understanding of potential early biases in memory but not attention in a group of adolescents with bipolar I disorder. Future studies are needed to examine whether the pattern of negative self-reference and negative memory bias described here persists, is enhanced, or remits over the course of bipolar illness.
Key points.
Adolescents with bipolar I disorder diagnosed shortly after their first manic episode had significant depressive symptoms at the time of assessment and at 1-year follow-up.
Adolescents with bipolar I disorder demonstrated a memory bias at baseline and follow-up.
Memory biases in adolescents with bipolar I disorder appear to be a trait feature of the disorder and not mood state dependent.
Depressive symptom severity may contribute to perpetuating negative memory biases in adolescents with bipolar I disorder.
Adolescents with bipolar I disorder do not exhibit attentional biases during a dot-probe task at baseline or at 1-year follow-up.
Acknowledgments
This research was supported by funding from the Klingenstein Third Generation Foundation Depression Fellowship and Grant MH085919 (PI: M.K.S.). The authors thank Melissa Pease and Erica Weitz for their assistance with recruiting and training participants, Mark Lum, Simone Vernez, and Yuliana Noniyeva for help with data processing, and Allison Libby for help with data entry.
Footnotes
Conflicts of interest statement: Drs. Singh, Joormann, and Gotlib, Mr. Kelley, Ms. Adams, Ms. Acquaye, Ms. Howe, and Ms. Whitney report no competing interests. Dr. Chang is a consultant for GlaxoSmithKline, Eli Lilly and Company, Merck, and Bristol-Myers Squibb, and he receives research support from GlaxoSmithKline, Merck, National Institute of Mental Health, and National Alliance for Research in Schizophrenia and Depression.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Association; 2000. Text revision. [Google Scholar]
- Baños RM, Medina PM, Pascual J. Explicit and implicit memory biases in depression and panic disorder. Behaviour Research and Therapy. 2001;39:61–74. doi: 10.1016/s0005-7967(99)00158-8. [DOI] [PubMed] [Google Scholar]
- Bopp JM, Miklowitz DJ, Goodwin GM, Stevens W, Rendell JM, Geddes JR. The longitudinal course of bipolar disorders as revealed throughweekly textmessaging:A feasibility study. Bipolar Disorders. 2010;12:327–334. doi: 10.1111/j.1399-5618.2010.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Schmajuk M, Reising M, Monk CS, Dickstein DP, Mogg K, Bradley BP, Pine DS, Libenluft E. Attention bias to threat faces in children with bipolar disorder and comorbid lifetime anxiety disorders. Biological Psychiatry. 2007;61:819–821. doi: 10.1016/j.biopsych.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Chang K. Adult bipolar disorder is continuous with pediatric bipolar disorder. The Canadian Journal of Psychiatry. 2007;52:407–408. doi: 10.1177/070674370705200703. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Derry PA, Kuiper NA. Schematic processing and self-reference in clinical depression. Journal of Abnormal Psychology. 1981;90:286–297. doi: 10.1037//0021-843x.90.4.286. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. American Journal of Psychiatry. 2001;158:125–127. doi: 10.1176/appi.ajp.158.1.125. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:450– 455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information- processing biases in depression and social phobia. Journal of Abnormal Psychology. 2004;113:386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113:121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Traill SK, Montoya RL, Joormann J, Chang K. Attention and memory biases in the offspring of parents with bipolar disorder: Indications from a pilot study. The Journal of Child Psychology and Psychiatry. 2005;46:84–93. doi: 10.1111/j.1469-7610.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- Heim-Dreger U, Kohlmann CW, Eschenbeck H, Burkhardt U. Attentional biases for threatening faces in children: Vigilant and avoidant processes. Emotion. 2006;6:320– 325. doi: 10.1037/1528-3542.6.2.320. [DOI] [PubMed] [Google Scholar]
- Jongen EM, Smulders FT, Ranson SM, Arts BM, Krabbendam L. Attentional bias and general orienting processes in bipolar disorder. Journal of Behavior Therapy and Experimental Psychology. 2007;38:168–183. doi: 10.1016/j.jbtep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. Journal of Child Psychology and Psychiatry. 2010;51:575–582. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Joseph MF, Frazier TW, Youngstrom EA, Soares JC. A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2008;18:595–605. doi: 10.1089/cap.2008.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children- Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The longitudinal interval follow-up evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, Klein DN. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology. 2011;39:125– 135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Matt GE, Vazquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clinical Psychology Review. 1992;12:227–255. [Google Scholar]
- Matthews A, MacLeod CC. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy and Research. 2005;29:29–45. [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression – The role of awareness. British Journal of Clinical Psychology. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, West A, Hill SK, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: 3-year follow-up shows cognitive development lagging behind healthy youths. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Delbello MP, Miyahara S, Wisniewski SR, Sachs GS, Nierenberg AA. Revisiting depressive- prone bipolar disorder: Polarity of initial mood episode and disease course among bipolar I systematic treatment enhancement program for bipolar disorder participants. Biological Psychiatry. 2005;58:549–553. doi: 10.1016/j.biopsych.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R) manual. Los Angeles, CA: Western Psychological Services; 1995. [Google Scholar]
- Research Network on Early Experience and Brain Development. [last accessed 15 December 2011];MacArthur network face stimuli set. Available from: http://www.macbrain.org/resources.htm.
- Rosen HR, Rich BA. Neurocognitive correlates of emotional stimulus processing in pediatric bipolar disorder: A review. Postgraduate Medicine. 2010;122:94–104. doi: 10.3810/pgm.2010.07.2177. [DOI] [PubMed] [Google Scholar]
- Singh MK, DelBello MP, Fleck DE, Shear PK, Strakowski SM. Inhibition and attention in adolescents with nonmanic mood disorders and a high risk for developing mania. Journal of Clinical and Experimental Neuropsychology. 2009;31:1–7. doi: 10.1080/13803390801945038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Henry J, Mogg K, Bradley BP, Pine DS. Attentional bias towards angry faces in childhood anxiety disorders. Journal of Behavior Therapy and Experimental Psychiatry. 2009;41:158–164. doi: 10.1016/j.jbtep.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation, Harcourt Brace and Company; 1999. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. The British Journal of Psychiatry: The Journal of Mental Science. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]