Abstract
Background and Purpose
Few studies have addressed outcomes among patients ≥80 years treated with acute stroke therapy. In this study, we outline in-hospital outcomes in (1) patients ≥80 years compared to their younger counterparts, and (2) those over age 80 receiving intra-arterial therapy (IAT) compared to those treated with intravenous recombinant tissue plasminogen activator (IVrtPA).
Methods
Stroke centers within the Specialized Program of Translational Research in Acute Stroke (SPOTRIAS) prospectively collected data on all patients treated with IVrtPA or IAT from 1/1/2005 to 12/31/2010. IAT was defined as receiving any endovascular therapy; IAT was further divided into bridging therapy (BT) when the patient received both IAT and IVrtPA, and endovascular therapy alone (ETA). In-hospital mortality was compared in (1) all patients age ≥80 versus younger counter-parts, and (2) IAT, BT, and ETA versus IVrtPA only among those age ≥80 using multivariable logistic regression. An age-stratified analysis was also performed.
Results
A total of 3768 patients were included in the study; 3378 were treated with IVrtPA alone, 808 with IAT (383 with ETA and 425 with BT). Patients ≥80 (n=1182) had a higher risk of in-hospital mortality compared to younger counterparts regardless of treatment modality (OR 2.13, 95%CI 1.60–2.84). When limited to those age ≥80, IAT (OR 0.95, 95%CI 0.60–1.49), BT (OR 0.82, 95%CI 0.47–1.45), or ETA (OR 1.15, 95%CI 0.64–2.08) versus IVrtPA were not associated with increased in-hospital mortality
Conclusions
IAT does not appear to increase the risk of in-hospital mortality among those over age 80 compared to intravenous thrombolysis alone.
Introduction
The incidence of ischemic stroke increases with age and is particularly high in people over the age of 801. Compared to younger patients, ischemic stroke is more likely to be associated with severe neurological impairment, larger infarct volume, and higher morbidity and mortality rates in older patients1. In-hospital complications including stroke expansion, hemorrhagic transformation, pneumonia, urinary tract infections, cardiac complications and mortality, are more likely in patients age ≥80 compared to younger patients2,3. Perhaps because of the greater impairment and disability in this age group, treatment with intravenous recombinant tissue plasminogen activator (IVrtPA) remains controversial for some practitioners2, and has not been approved by the European Medicines Evaluations Agency3. Clinical trials in acute stroke have previously excluded octogenarians4, 5. Controversy over how to treat older patients mainly stems from concern over excess risk of hemorrhage, lower likelihood of clinical benefit6–12 and higher inhospital and 3-month mortality13–15. Nonetheless, despite increased complications this patient population still appears benefit from thrombolysis16–20, though in the NINDS tPA trial only a small proportion of participants were over the age of 8021, 22,.
With the emergence of endovascular therapy, there has been an increased interest in determining whether this treatment modality is safe in older patients. Endovascular treatment has been associated with higher mortality rates and lower likelihood of clinical benefit among patients over the age of 8023–25, though some older patients may still benefit from endovascular therapy26. In the present study, we aimed to evaluate mortality and hospital disposition outcomes in patients ≥80 years treated with endovascular therapy. We hypothesized that because of higher complication rates overall in this population, (1) treatment with endovascular therapy would be associated with a greater risk of in-hospital mortality in patients ≥80 years compared to younger counterparts, but (2) in-hospital mortality would be similar among those ≥80 years receiving endovascular therapy vs. IVrtPA.
Methods
Patient selection and data collection
This is a retrospective analysis of prospectively collected acute stroke patients admitted to SPOTRIAS centers between January 1st 2005 and December 31st 2010. The study was approved by the institutional review board at each center.
SPOTRIAS is an NIH-funded program consisting of 8 academic stroke programs with the central aim of testing novel stroke treatments in the phase I and II stages (see acknowledgements). Each SPOTRIAS center maintains a prospective acute stroke patient database that collects admission and in-hospital characteristics, as well as clinical outcomes in all patients who either received acute stroke treatments or are enrolled in one of the SPOTRIAS clinical trials. We examined data from all patients from the SPOTRIAS database who received acute stroke therapy. Demographic and clinical data elements collected for the SPOTRIAS consortium database included age, race-ethnicity, sex, pre-treatment NIHSS, acute stroke treatment modality, discharge destination and inhospital mortality. Pre-stroke modified Rankin scale (mRS) was collected at only 6 sites (n = 2074). Ninety-day clinical outcomes, information regarding symptomatic intracranial hemorrhage (sICH), modified Rankin Scale, and causes of death were available in only a limited number of patients; time to treatment with IVrtPA and intra-arterial therapy was not captured.
Data and Statistical analysis
For our first hypothesis the principal explanatory variable was being age 80 and above. For our second hypothesis the principal explanatory variable was intra-arterial therapy (IAT), defined as receiving any intra-arterial pharmacological or mechanical endovascular treatment, regardless of preceding IVrtPA. IAT was further divided into bridging therapy (BT) when the patient received both IAT and IVrtPA, and endovascular therapy alone (ETA) when patients did not receive IVrtPA before endovascular treatment. Outcome measures were in-hospital mortality and discharge to a facility other than home.
Continuous variables were first dichotomized to relevant clinical cut-points. Patients were divided into two age categories (<80 and ≥80 years) based on common exclusion criteria of several recent clinical trials, such as PROACT-II, IMS-III and ECASS-III, and the ongoing clinical concern about treating octogenarians with IAT24. To evaluate the influence of age on mortality for each treatment group an age-stratified analysis was performed. Age was stratified in deciles, age <50 selected as the reference category. Severe stroke was defined as NIHSS ≥1227. Initial proportions for each treatment arm were calculated for descriptive statistics. Categorical variables were assessed in a univariate analysis using chi-squared analysis. Multivariable logistic regression was used to assess for independent associations between age and IAT with in-hospital mortality and discharge disposition. We first performed univariate analyses (model 1), followed by a model adjusted for baseline demographics: sex, race-ethnicity, and SPOTRIAS center (model 2). Our final model (model 3) was further adjusted for our hypothesized principal confounders: NIHSS and serum glucose level. All analyses were performed using SAS version 9.2 (SAS Institute Cary, N.C.); p ≤0.05 was set as statistically significant.
Results
Baseline characteristics
A total of 3768 patients were treated with acute stroke therapy across the SPOTRIAS consortium over 6 years; 3378 were treated with IVrtPA alone, 808 with IAT (383 with ETA and 425 with BT). Baseline demographics were similar between the different treatment groups as outlined in table 1. Patients were predominantly white non-Hispanic with approximately 50 % males. The proportion of all patients treated with IVrtPA who were ≥80 years was 34.2% and varied significantly between the centers (19.4%–50.4, p<0.0001). In comparison 23% of patients in the IAT group were over 80 (21.9% in ETA group and 24% in BT group, table 1). Octogenerians were more likely to have severe strokes (NIHSS ≥12) (64.9% vs. 48.4%, p<0.0001) and were less likely to receive BT (9.5% vs 14.5%, p<0.0001), when compared to younger patients. When limited to those patients with an NIHSS 1≥2, patients ≥80 years were less likely to receive IAT (12.8% vs 24.6%, p < 0.0001). Overall, a total of 431 (12.1%) deaths were reported and 2412 (64.0%) patients were not discharged home.
Table 1.
Baseline Demographics of Table 1. Patients treated across the SPOTRIAS consortium between January 1st 2005 and December 31st 2010
| All Treated Patients*
|
Intravenous recombinant tissue plasminogen activator#
|
Any Intra- arterial Therapy
|
Bridging Therapy&
|
Endovascular Therapy Alone^
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = | % | n = | % | n = | % | n = | % | n = | % | |
| Proportion over age 80 | 1182 | 31.4 | 1095 | 32.4 | 186 | 23 | 102 | 24 | 84 | 21.9 |
| Sex (% female) | 1859 | 49.4 | 1699 | 50.4 | 419 | 52.1 | 219 | 51.8 | 200 | 52.4 |
| Race/ethnicity | ||||||||||
| Hispanic | 379 | 10.1 | 344 | 10.2 | 64 | 7.9 | 29 | 6.8 | 35 | 9.1 |
| Non-Hispanic | 688 | 18.3 | 612 | 18.1 | 144 | 17.8 | 71 | 16.7 | 73 | 19.1 |
| Non-Hispanic white | 2530 | 67.1 | 2269 | 67.2 | 559 | 69.2 | 301 | 70.8 | 258 | 67.4 |
| Other | 171 | 4.5 | 153 | 4.5 | 41 | 5.1 | 24 | 5.6 | 17 | 4.4 |
| Deaths | 431 | 12.1 | 359 | 12.2 | 145 | 18.5 | 76 | 18.3 | 69 | 18.8 |
| Disposition (Not discharged home) | 2412 | 64 | 2119 | 62.7 | 628 | 77.7 | 340 | 80 | 288 | 75.2 |
NIHSS, median (IQR): 12 (6–18)
NIHSS, median (IQR): 16 (11–20)
NIHSS, median (IQR): 17 (13–20.5)
NIHSS, median (IQR): 15 (8–20)
In hospital outcomes in patients over age 80 compared to younger patients
Patients ≥80 years treated with IVrtPA alone had a higher risk of in-hospital mortality (model 3: adjusted OR 2.13, 95% CI 1.60–2.84) and of having a disposition other than home (model 3: adjusted OR 2.51, 95% CI 2.03–3.11) compared to younger patients. Octogenarians who were treated with IAT also demonstrated increased mortality compared to younger counterparts (model 3: adjusted OR 1.98, 95% CI 1.29–3.04). Similar results were noted in patients ≥80 years versus younger counter-parts for ETA (model 3: adjusted OR 2.44, 95% CI 1.30–4.59), but not BT (model 3: adjusted OR 1.65, 95% CI 0.91–2.98). A higher risk of not being discharged home was noted for all treatment modalities except for ETA (model 3: adjusted OR 1.55, 95% CI 0.68–3.55). In addition, the association of disposition other than home with BT was disproportionally higher (model 3: OR 9.41, 95% CI 2.64–33.6) when compared with the other treatment modalities. (electronic table 1)
Age influence on In-hospital outcomes
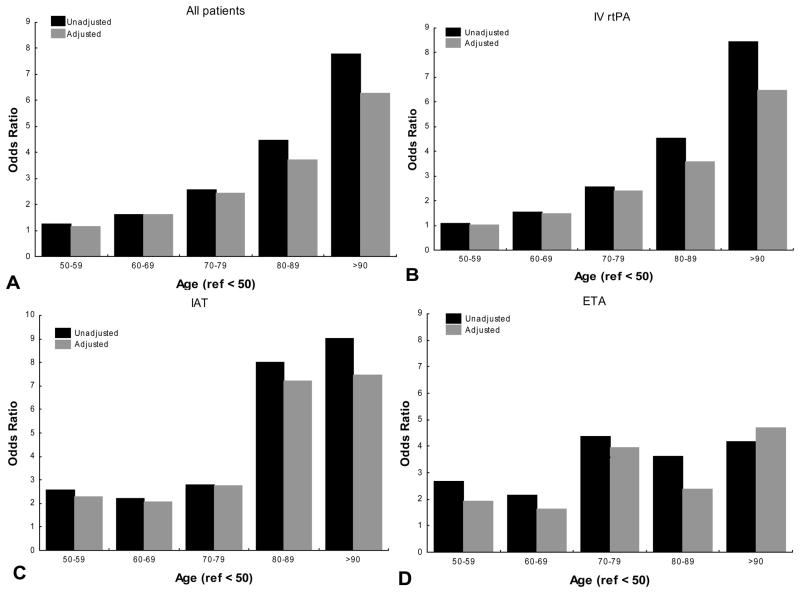
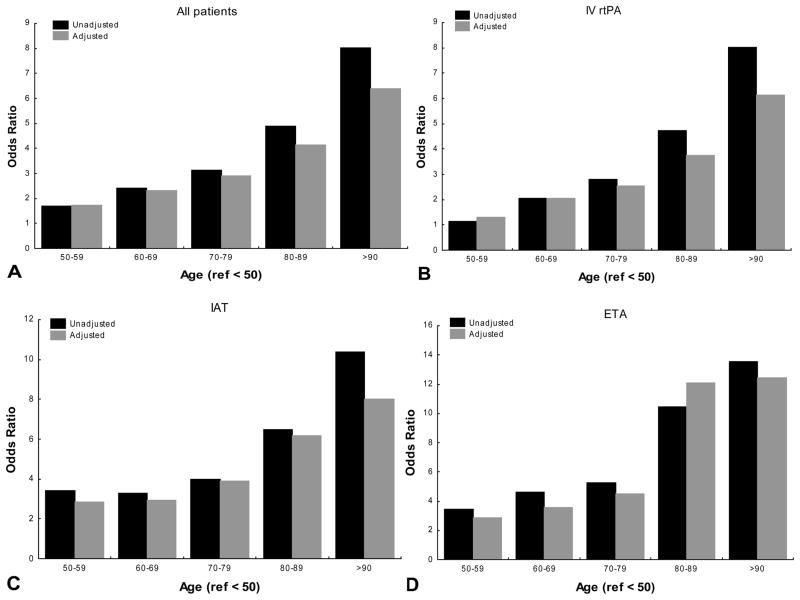
Univariate and multivariable analysis categorizing age as deciles revealed that the likelihood of mortality increased with age regardless of the treatment. Additionally the rate of rise in ORs was more notable at the >80 strata in unadjusted and adjusted models (figure 1). When IVrtPA was used, the odds of inhospital mortality in the 80–89 age strata increased 1.48 times when compared to the 70–79 category after adjusting for sex, race-ethnicity, SPOTRIAS center NIHSS and glucose serum levels (from OR: 2.53 95% CI 1.36–4.72 to OR: 3.75, 95% CI 2.03–6.94). Similarly the adjusted odds of mortality increased 1.54 times when 80–89 group was compared with 80–89 strata (OR: 3.88, 95% CI 1.68–8.98 to OR: 6.18, 95% CI 2.57–14.83). A similar pattern of increase was encountered when discharge disposition was used as outcome (figure 2).
Figure 1. Age effect on In-hospital mortality in different acute stroke therapies.
IV rtPA: intravenous recombinant tissue plasminogen activator alone
IAT: any intra-arterial therapy
ETA: endovascular therapy alone
Ref: reference category
Unadjusted: univariate analysis
Adjusted: Multivariable adjusted for sex, race-ethnicity, and SPOTRIAS center, national institutes of health stroke scale and serum glucose level.
Figure 2. Age effect on discharge disposition other than home in different acute stroke therapies.
IV rtPA: intravenous recombinant tissue plasminogen activator alone
IAT: any intra-arterial therapy
ETA: endovascular therapy alone
Ref: reference category
Unadjusted: univariate analysis
Adjusted: Multivariable analysis adjusted for sex, race-ethnicity, and SPOTRIAS center, national institutes of health stroke scale and serum glucose level.
In-hospital outcomes among octogenarians comparing endovascular therapy to IVrtPA
The univariate analyses showed that all endovascular therapies were associated with an increased risk of in-hospital mortality when compared to IVrtPA (Table 2). However in adjusted models all of the associations were no longer significant (model 3: IAT vs. IV rtPA adjusted OR 0.95, 95% CI 0.60–1.49) (model 3: BT vs. IVrtPA adjusted OR 0.82, 95%CI 0.47–1.45)(model 3: ETA vs IVrtPA adjusted OR 1.15, 95%CI 0.64–2.08). Given the importance of NIHSS on the decision to proceed with IAT, we carried out further analyses only among those age ≥80 with an NIHSS ≥12 (n = 751) and found no evidence for increased mortality. In our final models (model 3) IAT (adjusted OR 0.79, 95% CI 0.49–1.29), BT (adjusted OR 0.79, 95% CI 0.44–1.42) and ETA (adjusted OR 0.92, 95% CI 0.48–1.77) versus IVrtPA were not associated with increased mortality.
Table 2.
In-hospital mortality among acute ischemic stroke patients over age 80 based on treatment modality
| Model 1
|
Model 2
|
Model 3
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| All acute ischemic stroke patients | ||||||
| IAT vs. IV rtPA | 1.93 | (1.34–2.79) | 1.46 | (0.97–2.20) | 0.95 | (0.60–1.49) |
| BT vs. IV rtPA | 1.72 | (1.07–2.77) | 1.34 | (0.80–2.24) | 0.82 | (0.47–1.45) |
| ETA vs. IV rtPA | 2.08 | (1.27–3.43) | 1.53 | (0.89–2.63) | 1.15 | (0.64–2.08) |
| All patients where NIHSS > 12 | ||||||
| IAT vs. IV rtPA | 1.32 | (0.88–1.98) | 0.96 | (0.61–1.52) | 0.79 | (0.49–1.29) |
| BT vs. IV rtPA | 1.21 | (0.73–2.02) | 0.92 | (0.53–1.59) | 0.79 | (0.44–1.42) |
| ETA vs. IV rtPA | 1.45 | (0.82–2.57) | 1.09 | (0.58–2.04) | 0.92 | (0.48–1.77) |
Legend:
IV rtPA: intravenous recombinant tissue plasminogen activator alone
IAT: any intra-arterial therapy
BT: bridging therapy
ETA: endovascular therapy alone
Model 1: univariate analysis
Model 2: model 1 further adjusted for sex, race-ethnicity, and SPOTRIAS center
Model 3: model 2 further adjusted for national institutes of health stroke scale and serum glucose level
Since only 68 patients with age ≥80 and an NIHSS ≥12 (9.1%) were discharged home, no analysis comparing the different treatment modalities was performed on this subgroup alone.
Outcomes among those restricted to arrival within 3 hours of stroke onset
An additional analysis was performed restricted to those patients who arrived under 3 hours and received IVrtPA alone versus endovascular therapy alone, regardless of patient age. A total of 94 patients who arrived within 3 hours received ETA. Reported exclusion reasons for IVrtPA included: age ≥80 (18), international normalized ratio > 1.7 (8), abnormal platelet count (8), could not be treated within 3 hours (8), ICH history (2), elevated NIHSS (1), no other reason listed (49). Univariate analysis revealed that ETA was associated with a greater risk of in-hospital mortality when compared to IVrtPA (OR 3.80, 95% CI 2.20–6.54). These results persisted after adjusting for sex, race-ethnicity, center, and NIHSS, suggesting that ETA was associated with a greater risk of in-hospital mortality (OR 3.97, 95% CI 2.00–7.87). Interestingly, results were similar when restricted to patients who were over the age of 80 (n= 18) (adjusted OR 5.52, 95% CI 1.24–25.0).
Discussion
This is the largest study of endovascular therapy in patients ≥80 of age. The results of our study suggest that: 1) in-hospital outcome measured by mortality and disposition were worse in those age ≥80, compared to their younger counterparts; and 2) acute endovascular treatment of stroke using IAT, ETA, or BT was not associated with an increase mortality in those age ≥80 when compared to IVrtPA, including among those with severe strokes. In secondary analyses we also found that 1) aging is associated with mortality and being discharged other than home regardless of the treatment used; and 2) the use of endovascular therapy under 3 hrs without IVrtPA was associated with an increased mortality compared IVrtPA alone.
Data from the SITS-MOST registry evaluated over 1000 patients age ≥80 who received IVrtPA and compared outcomes to younger patients. In keeping with our results, the authors reported a higher mortality and a worse 3-month functional outcomes in older versus younger patients. These findings are consistent with the overall worse prognosis in this age group regardless of treatment offered28. Part of this effect may be due to the presence of a higher pre-stroke functional disability, more medical comorbidities29, 30, or a baseline risk of neurological complications such as infarct expansion. Nonetheless an independent effect of age on outcomes is noted in these studies and could reflect further unmeasured confounders, a particular susceptibility to ischemic brain injury, or poor development of collaterals31. The risks for hemorrhagic conversion and symptomatic intracranial hemorrhage, on the other hand, do not appear to be higher among octogenarians32, 33. Several case series have documented a lower probability of a good neurological outcome at discharge or at 90 days using endovascular therapy among those over age 80 compared to younger counterparts16, 24, 25. In our study however we showed that in-hospital mortality associated with endovascular therapies was not different in patients over age 80 using controls of the same age group and after adjustment for pretreatment NIHSS.
Our results are also unique in comparing IVrtPA treatment to a small sample of patients who were treated with ETA despite arriving within 3 hours of stroke onset. We noted in this group that ETA led to poorer outcomes despite having adjusted for stroke severity. The principal reasons for exclusion from IVrtPA were age and coagulopathy. Interestingly, results remained the same when the comparison was performed among patients over age 80, stressing the point IVrtPA remains gold standard for acute stroke treatment. Although there may be unmeasured confounders that could contribute to the difference in outcome, our results caution against proceeding with ETA without first administering IVrtPA to eligible patients.
Our study has several weaknesses that should be considered. First, no information regarding time from stroke onset to treatment, multi-modal imaging, or recanalization was collected: Faster treatment may have resulted in better recanalization, and those with proximal occlusions and larger penumbra based on multimodal imaging, may have been more likely to be treated with endovascular therapy, leading to a bias towards better outcomes in this group. Secondly, we did not systematically collect data on symptomatic ICH, procedural complications, or detailed pre-morbid functional status. Symptomatic ICH is associated with significant morbidity and mortality that could have skewed our results against endovascular therapy; on the other hand we did not find an increased risk of death. In addition, data on pre-morbid functional status was only collected at 6 sites and overall, in less than half of all patients. We did not have additional information on medical comorbidities, pre-morbid frailty or dementia, which are likely to contribute to post-stroke outcomes and treatment selection bias. Lastly, we did not collect 90-day outcomes such as the mRS, which is considered a standard outcome for stroke studies. However, given that our focus was on identifying negative outcomes related to each treatment modality, these are unlikely to change dramatically after hospital discharge since improvement is expected after stroke.
Our findings suggest that endovascular therapy among patients over 80 does not increase in-hospital mortality when compared to patients of the same age receiving only IVrtPA. Advance age increases the likelihood of poor outcome regardless of the treatment, however, particularly in. the transition from the 7th to the 8th decade. In addition we found increased mortality among patients who received endovascular treatment under 3 hrs when IVrtPA was contraindicated, suggesting that endovascular treatment might not benefit everyone. Whether older patients should ultimately be treated with endovascular therapy with or without IVrtPA can only be answered through a clinical trial. These trials should recruit participants over the age of 80, and include clinical variables that may influence outcomes in this age group including frailty and cognition measures, and a complete assessment of comorbidities. In the interim our data would suggest that these patients can be safely enrolled. The routine clinical use of IAT, especially in this age group however, remains as of yet experimental.
Acknowledgments
Funding
SPOTRIAS is funded by the National Institutes of Neurological Diseases and Stroke (NINDS P50 NS049060). JZW was funded by NINDS 1K23 NS 073104-01A1 and the NIH Loan Repayment Program (AG 31009). This work was funded in part by the Intramural Division of the National Institute of Neurological Disorders and Stroke/NIH.
SPOTRIAS sites:
Columbia University, New York, (PI: Randolph Marshall)
University of Cincinnati, (PI: Joseph Broderick)
Division of Intramural Research, NINDS, Bethesda, MD (PI: Steven Warach)
Washington University, St. Louis, (PI: Colin Derdeyn)
Massachusetts General Hospital, Boston, (PI: Karen Furie)
University of Texas-Houston, (PI: James Grotta) University of California, Los Angeles, (PI: Jeffrey Saver)
University of California, San Diego (PI: Brett C. Meyer)
Footnotes
Disclosures
The authors report no conflict of interest and have no relevant disclosures.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Lindley RI, Wardlaw JM, Sandercock PA. Thrombolysis in elderly people. Observational data insufficient to change treatment. BMJ. 2011;342:d306. doi: 10.1136/bmj.d306. author reply d312. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: Patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 5.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The proact ii study: A randomized controlled trial. Prolyse in acute cerebral thromboembolism. Jama. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 6.van Oostenbrugge RJ, Hupperts RM, Lodder J. Thrombolysis for acute stroke with special emphasis on the very old: Experience from a single dutch centre. Journal of neurology, neurosurgery, and psychiatry. 2006;77:375–377. doi: 10.1136/jnnp.2005.070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelter ST, Bonati LH, Lyrer PA. Intravenous thrombolysis in stroke patients of > or = 80 versus < 80 years of age--a systematic review across cohort studies. Age and ageing. 2006;35:572–580. doi: 10.1093/ageing/afl104. [DOI] [PubMed] [Google Scholar]
- 8.Simon JE, Sandler DL, Pexman JH, Hill MD, Buchan AM. Is intravenous recombinant tissue plasminogen activator (rt-pa) safe for use in patients over 80 years old with acute ischaemic stroke? - the calgary experience. Age and ageing. 2004;33:143–149. doi: 10.1093/ageing/afh031. [DOI] [PubMed] [Google Scholar]
- 9.Berrouschot J, Rother J, Glahn J, Kucinski T, Fiehler J, Thomalla G. Outcome and severe hemorrhagic complications of intravenous thrombolysis with tissue plasminogen activator in very old (> or =80 years) stroke patients. Stroke; a journal of cerebral circulation. 2005;36:2421–2425. doi: 10.1161/01.STR.0000185696.73938.e0. [DOI] [PubMed] [Google Scholar]
- 10.Mouradian MS, Senthilselvan A, Jickling G, McCombe JA, Emery DJ, Dean N, et al. Intravenous rt-pa for acute stroke: Comparing its effectiveness in younger and older patients. Journal of neurology, neurosurgery, and psychiatry. 2005;76:1234–1237. doi: 10.1136/jnnp.2004.047803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meretoja A, Putaala J, Tatlisumak T, Atula S, Artto V, Curtze S, et al. Off-label thrombolysis is not associated with poor outcome in patients with stroke. Stroke; a journal of cerebral circulation. 2010;41:1450–1458. doi: 10.1161/STROKEAHA.109.576140. [DOI] [PubMed] [Google Scholar]
- 12.Bhatnagar P, Sinha D, Parker RA, Guyler P, O’Brien A. Intravenous thrombolysis in acute ischaemic stroke: A systematic review and meta-analysis to aid decision making in patients over 80 years of age. Journal of neurology, neurosurgery, and psychiatry. 2011;82:712–717. doi: 10.1136/jnnp.2010.223149. [DOI] [PubMed] [Google Scholar]
- 13.Chen CI, Iguchi Y, Grotta JC, Garami Z, Uchino K, Shaltoni H, et al. Intravenous tpa for very old stroke patients. European neurology. 2005;54:140–144. doi: 10.1159/000089086. [DOI] [PubMed] [Google Scholar]
- 14.Engelter ST, Reichhart M, Sekoranja L, Georgiadis D, Baumann A, Weder B, et al. Thrombolysis in stroke patients aged 80 years and older: Swiss survey of iv thrombolysis. Neurology. 2005;65:1795–1798. doi: 10.1212/01.wnl.0000183702.04080.27. [DOI] [PubMed] [Google Scholar]
- 15.Toni D, Lorenzano S, Agnelli G, Guidetti D, Orlandi G, Semplicini A, et al. Intravenous thrombolysis with rt-pa in acute ischemic stroke patients aged older than 80 years in italy. Cerebrovascular diseases (Basel, Switzerland) 2008;25:129–135. doi: 10.1159/000112323. [DOI] [PubMed] [Google Scholar]
- 16.Zeevi N, Chhabra J, Silverman IE, Lee NS, McCullough LD. Acute stroke management in the elderly. Cerebrovascular diseases (Basel, Switzerland) 2007;23:304–308. doi: 10.1159/000098332. [DOI] [PubMed] [Google Scholar]
- 17.Sylaja PN, Dong W, Grotta JC, Miller MK, Tomita K, Hamilton S, et al. Safety outcomes of alteplase among acute ischemic stroke patients with special characteristics. Neurocrit Care. 2007;6:181–185. doi: 10.1007/s12028-007-0018-8. [DOI] [PubMed] [Google Scholar]
- 18.Ringleb PA, Schwark C, Kohrmann M, Kulkens S, Juttler E, Hacke W, et al. Thrombolytic therapy for acute ischaemic stroke in octogenarians: Selection by magnetic resonance imaging improves safety but does not improve outcome. Journal of neurology, neurosurgery, and psychiatry. 2007;78:690–693. doi: 10.1136/jnnp.2006.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Keyser J, Gdovinova Z, Uyttenboogaart M, Vroomen PC, Luijckx GJ. Intravenous alteplase for stroke: Beyond the guidelines and in particular clinical situations. Stroke; a journal of cerebral circulation. 2007;38:2612–2618. doi: 10.1161/STROKEAHA.106.480566. [DOI] [PubMed] [Google Scholar]
- 20.Mishra NK, Ahmed N, Andersen G, Egido JA, Lindsberg PJ, Ringleb PA, et al. Thrombolysis in very elderly people: Controlled comparison of sits international stroke thrombolysis registry and virtual international stroke trials archive. BMJ. 2010;341:c6046. doi: 10.1136/bmj.c6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. The New England journal of medicine. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 22.Generalized efficacy of t-pa for acute stroke. Subgroup analysis of the ninds t-pa stroke trial. Stroke; a journal of cerebral circulation. 1997;28:2119–2125. doi: 10.1161/01.str.28.11.2119. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: Pooled analysis of the mechanical embolus removal in cerebral ischemia (merci) and multi merci trials. Stroke; a journal of cerebral circulation. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi AI, Suri MF, Georgiadis AL, Vazquez G, Janjua NA. Intra-arterial recanalization techniques for patients 80 years or older with acute ischemic stroke: Pooled analysis from 4 prospective studies. AJNR Am J Neuroradiol. 2009;30:1184–1189. doi: 10.3174/ajnr.A1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazighi M, Labreuche J, Meseguer E, Serfaty JM, Laissy JP, Lavallee PC, et al. Impact of a combined intravenous/intra-arterial approach in octogenarians. Cerebrovascular diseases (Basel, Switzerland) 2011;31:559–565. doi: 10.1159/000324626. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Ford GA, Kidwell CS, Starkman S, Vinuela F, Duckwiler GR, et al. Intra- arterial thrombolysis for acute stroke in patients 80 and older: A comparison of results in patients younger than 80 years. AJNR Am J Neuroradiol. 2007;28:159–163. [PMC free article] [PubMed] [Google Scholar]
- 27.Sacco RL, DeRosa JT, Haley EC, Jr, Levin B, Ordronneau P, Phillips SJ, et al. Glycine antagonist in neuroprotection for patients with acute stroke: Gain americas: A randomized controlled trial. Jama. 2001;285:1719–1728. doi: 10.1001/jama.285.13.1719. [DOI] [PubMed] [Google Scholar]
- 28.Di Carlo A, Lamassa M, Pracucci G, Basile AM, Trefoloni G, Vanni P, et al. Stroke in the very old: Clinical presentation and determinants of 3-month functional outcome: A european perspective. European biomed study of stroke care group. Stroke; a journal of cerebral circulation. 1999;30:2313–2319. doi: 10.1161/01.str.30.11.2313. [DOI] [PubMed] [Google Scholar]
- 29.Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, et al. Functional recovery after ischemic stroke--a matter of age: Data from the austrian stroke unit registry. Neurology. 2012;78:279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- 30.Sharma JC, Fletcher S, Vassallo M. Strokes in the elderly - higher acute and 3-month mortality - an explanation. Cerebrovascular diseases (Basel, Switzerland) 1999;9:2–9. doi: 10.1159/000015889. [DOI] [PubMed] [Google Scholar]
- 31.Liebeskind DS. Collateral circulation. Stroke; a journal of cerebral circulation. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 32.Ford GA, Ahmed N, Azevedo E, Grond M, Larrue V, Lindsberg PJ, et al. Intravenous alteplase for stroke in those older than 80 years old. Stroke; a journal of cerebral circulation. 2010;41:2568–2574. doi: 10.1161/STROKEAHA.110.581884. [DOI] [PubMed] [Google Scholar]
- 33.Mono ML, Romagna L, Jung S, Arnold M, Galimanis A, Fischer U, et al. Intra-arterial thrombolysis for acute ischemic stroke in octogenarians. Cerebrovascular diseases (Basel, Switzerland) 2012;33:116–122. doi: 10.1159/000333429. [DOI] [PubMed] [Google Scholar]