Abstract
Background:
A cluster of risk factors for cardiovascular diseases and type 2 diabetes mellitus, which occur together more often than by chance alone, have been known as the metabolic syndrome. Various definitions have been proposed by different organizations over the past decade. This study was designed to evaluate a new definition of the metabolic syndrome for the prediction of diabetes mellitus among the Iranian population.
Methods:
This study was carried out in an urban population, aged 20 to 74 years, from Yazd, a city in the center of Iran. The study is a part of the phase I of Yazd Healthy Heart Program, that is, a community-based intervention study for the prevention of cardiovascular disease. The significance level has been defined as P<0.05.
Results:
Prevalence of the metabolic syndrome by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria was 21.3 ± .017%, and by International Diabetes Federation (IDF) criteria it was 30.16 ± .02%. The multivariate analysis showed that the most important relevant factors of diabetes mellitus were: Increased age and metabolic syndrome by both definitions of NCEP and IDF criteria, and also, the most important relevant factors of stable angina were: Increased age, male sex, and metabolic syndrome by only IDF definitions, but the NCEP definition of the metabolic syndrome cannot predict diabetes mellitus independent of age and sex.
Conclusion:
This study showed that increased age and metabolic syndrome are the most important relevant factors for diabetes mellitus, especially by using the IDF criteria for definition of the metabolic syndrome.
Keywords: Diabetes mellitus, International diabetes federation, metabolic syndrome
INTRODUCTION
A cluster of risk factors for cardiovascular diseases and type 2 diabetes mellitus, which occur together, more often than by chance alone, have been known as the metabolic syndrome (MS).[1,2] New definitions have been proposed over the past decade.[3–7] Most recently, these have come from the International Diabetes Federation (IDF) and the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI). The IDF definition introduced lower measures for waist circumference in different ethnicities. Hence, regional cut off points for waist circumference can be used.[8] The IDF recommended that the threshold for waist circumference to define abdominal obesity in people of European origin should be ≥94 cm for men and ≥80 cm for women; the AHA/NHLBI, in contrast, recommended cut off points of ≥102 cm and ≥88 cm, for the two sexes, respectively. The later values are consistent with the definitions of abdominal obesity by then National Institute of Health's obesity guidelines,[9] which equates to a body mass index of approximately 30 kg/m2 in males. The IDF values are closer to a body mass index of 25 kg/m2 in males. The IDF guidelines also confirmed the need to adopt different values for waist measurement in different ethnic groups, based on the relationship of waist measurement, either to the other metabolic syndrome components or to longer-term outcome studies such as those with a risk of type 2 diabetes mellitus and cardiovascular disease (CVD).[8] Prevalence of metabolic syndrome increased with age from 6.7% in the age group of 20 to 29 years to 43.5% in the age group of 60 to 69 years. The prevalence of the metabolic syndrome in Iran is approximately 30% by NCEP criteria in adults above 20 years.[9] Patients with metabolic syndrome are at twice the risk of developing CVD over the next five to ten years, compared to those without the syndrome. The risk over a lifetime is undoubtedly even higher. Furthermore, metabolic syndrome predicts a five-fold increase in the risk for type-2 diabetes mellitus.[8] This article concerns these two different definitions of metabolic syndrome; NCEP and IDF criteria. And the aim of this study is to evaluate how the metabolic syndrome, based on the two definitions, is associated with diabetes mellitus and coronary artery disease (CAD) among the Iranian population.
METHODS
Sampling procedure
This study was carried out in an urban population, of age 20 to 74 years, from Yazd, a central city in Iran during the period 2004 – 2005. It is a cross-sectional evaluation of the current status (phase I) of the Yazd healthy heart program that is a community intervention study for the prevention of cardiovascular disease. Individuals were recruited by cluster sampling. One hundred clusters were randomly assigned. From each cluster, 20 families and one person from each family were selected. Family addresses were found based on healthcare center records. The participants were classified in five age groups: 20 – 34, 35 – 44, 45 – 54, 55 – 64, and 65 – 74-year-olds. One man and one woman from each group were interviewed and examined. Cluster sample size with cluster coefficient of 1.4 was estimated according to the previous CVD risk factor prevalence studies.
Data collection
The participants were invited via mail, were informed about the study and the content of the interview. Informed consent forms were completed by the participants. Interview and completion of questionnaires were performed by 20 trained health professionals at the houses of the participants. The questionnaire included questions about the demographic characteristics, socioeconomic status, knowledge and perception of subjects on cardiovascular disease, risk factors, and methods of control and their prevention. The participants were invited to health care center for laboratory examinations. Their blood pressure (BP) was measured four times on two different visits, with five-minute intervals, at each visit. With the help of a mercury sphygmomanometer, KorotKoff first and fifth phase sounds were recorded as systolic and diastolic blood pressure, and following that, the participants were referred to the health care centre to perform biochemical tests and anthropometric measurements. The average of these four readings was used for analyses. Biochemical-tests were taken after at least 12 hours of fasting and comprised blood glucose, total cholesterol (TC), triglycerides (TG), LDL-cholesterol, and HDL-cholesterol.
Definition of conventional risk factors[3,4]
Hypertension was defined as: Systolic BP equal and over 140 mmHg or diastolic BP equal and over 90 mmHg on two different occasions or taking antihypertensive medication.
Hyperlipidemia was defined as: A history of taking anti-hyperlipidemic drugs or lipoprotein disorders according to NCEP ATPIII (TC>5.17 mmol/l or TG>1.7 mmol/l or HDL-c< 1.03 mmol/l in men, and HDL-c<1.29 mmol/l in women or LDL-c>4.1 mmol/l).
Diabetes: A history of using hypoglycemic agents or a fasting blood sugar (FBS) of at least 7 mmol/l that was confirmed by a blood sugar of at least 11.1 mmol/l after taking 75 g of oral glucose (glucose tolerance test, GTT). The glucose tolerance test was not performed for known diabetic patients.
Obesity: A body mass index (BMI) of ≥30 is defined as obesity and 25<BMI<30 is considered as overweight.
Abdominal obesity was defined as: Waist >90 cm in men and >80 cm in women. Waist circumference measured at 2 – 3 cm over the umbilicus (or waist circumference at the middle of nipple and top of thigh) and hip circumference defined as the greatest diameter between waist and knee.[10]
NCEP criteria of the metabolic syndrome were defined as:
At least three of the following criteria: Waist circumference >102 cm in men or >88 cm in women, plasma triglycerides ≥150 mg/dl (1.7 mmol/L), HDL cholesterol <40 mg/dl (1.03 mmol/l) in men or <50 mg/dL (1.29 mmol/l) in women, blood pressure ≥130/85 mm Hg, and fasting plasma glucose ≥110 mg/dl (6.1 mmol/l).
Angina pectoris: Angina pectoris was assessed via the Rose questionnaire.[11] A score of ≥3 was considered typical angina pectoris and a score of <3 and >0 was considered as atypical angina pectoris.
The IDF criteria of metabolic syndrome were defined as:
Central obesity was defined by waist circumference >94 cm in white Caucasian or Afro-Caribbean men, >90 cm in Indian Asian men, or >80 cm in Indian Asian women,[12] plus two of the following four factors:
Serum triglycerides ≥ 1.7 mmol/l or specific treatment for this lipid abnormality.
HDL cholesterol < 1.03 mmol/l in males and < 1.29 mmol/l in females or specific treatment for this lipid abnormality.
Systolic blood pressure ≥ 130 or diastolic BP ≥ 85 mm Hg or treatment of previously diagnosed hypertension.
Fasting plasma glucose (FPG) ≥ 5.6 mmol/l, or previously diagnosed type 2 diabetes.
If plasma glucose is higher than 5.6 mmol/l, OGTT is strongly recommended, but it is not necessary to define the presence of the syndrome.
Statistical analysis
Logistic regression analysis was used to assess the association of the metabolic syndrome and conventional risk factors, such as, hypertension, hyperlipidemia, obesity, smoking, with diabetes mellitus, and coronary artery disease. The significance level was defined as P<0.05.
We conducted the Hosmer and Lemeshow goodness-of-fit test to assess whether the models significantly predicted diabetes mellitus and coronary artery disease.
The ability of the metabolic syndrome to predict diabetes and CVD was examined by the receiver operating characteristic (ROC) curves. The ROC curves were constructed by plotting the sensitivity against the corresponding false-positive rate (FPR), which equaled 1-specificity. Areas under the ROC curves were compared.
All statistical analyses were performed using SPSS version 16.
RESULTS
This study comprised of two thousand participants from the Yazd city, in the center of Iran. The mean age was 48.75 years with 15 years standard deviation, which ranged from 20 to 74 years. The mean age and its standard deviation was 48.8 ± 15 in males and 48.6 ± 15 in females. Among the participants 454 women and 376 men had a metabolic syndrome based on NCEP ATP-III criteria and 508 women and 396 men had the IDF definition metabolic syndrome. After age-adjustment using the community age distribution, prevalence of the metabolic syndrome by the NCEP criteria was 21.3 ± .017%, (27.3 ± .019% in women and 12.5 ± .015%, in men). The prevalence of metabolic syndrome by the IDF criteria was 30.16 ± .02%, (30.2 ± .02 in women and 31.6 ± .02, in men [Table 1]).
Table 1.
The prevalence of metabolic syndrome defined by two NCEP and IDF criteria in study participants
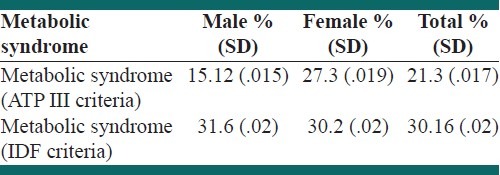
Agreement between ATP III and IDF criteria in the definition of the metabolic syndrome was high (Kappa=0.849; P<0.001).
The prevalence of diabetes mellitus was 9.3 ± 1.2% (8.7 ± 11% in men and 9.7 ± 1.2% in women).
Typical chest pain was seen in 222/1000 women and 122/1000 men.
After the age was adjusted based on population age distribution, the prevalence of typical chest pain (angina pectoris) that was assessed via the Rose questionnaire was 14.6% + 0.015 (13.2 + 0.014 in men and 16.2 + 0.016 in women).
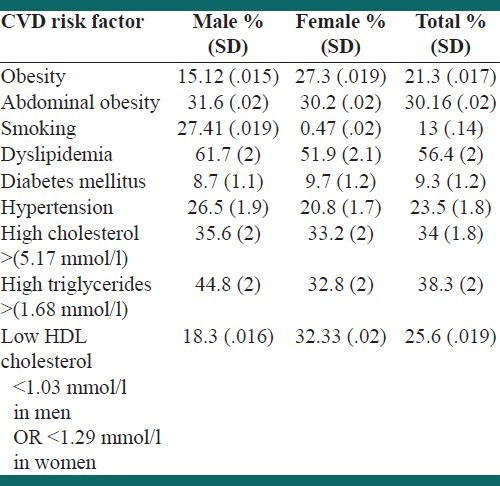
The prevalence of CVD risk factors in men and women in the 20 – 74-year-old age group, after age and sex adjustment, is shown in Table 2.
Table 2.
The prevalence of various CVD risk factors in study participants after adjustment for age and sex
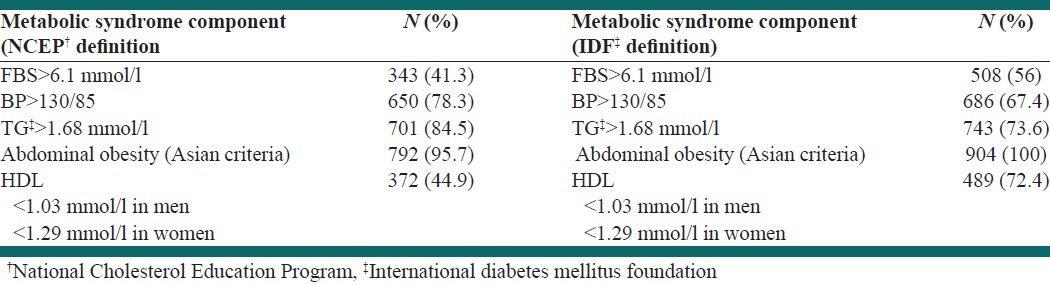
The prevalence of various components of metabolic syndrome by NCEP ATP-III and IDF criteria is shown in Table 3. Abdominal obesity and high triglycerides were the most prevalent risk factors by both definitions.
Table 3.
Prevalence of various components of metabolic syndrome by NCEP and IDF criteria
The Receiving Operating Characteristic (ROC) curve for predicting diabetes by two definitions of the metabolic syndrome is shown in Figure 1. The area under the curve in the IDF definition was more than that in the ATP definition of the metabolic syndrome, (Area=0.827; 95%CI=0.807 – 0.848 in IDF; and Area=0.776; 95%CI=0.750 – 0.808 in NCEP ATP-III definition) and both were significantly different from 0.5 of the reference line, P<0.0001.
Figure 1.
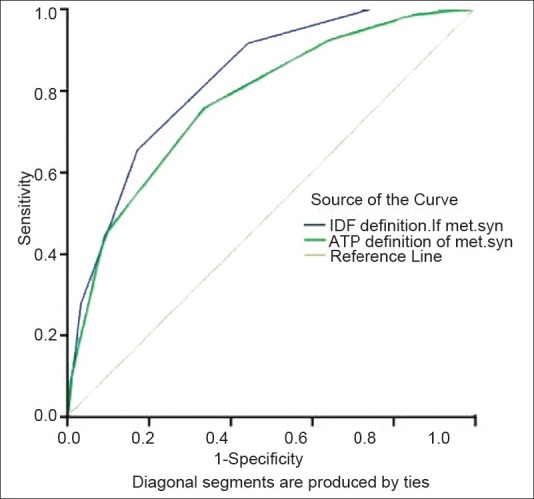
Area under the ROC curve for the IDF and NCEP ATP III definition of the metabolic syndrome in the prediction of diabetes mellitus
Multivariate analysis was performed with logistic regression analysis using the backward conditional model.
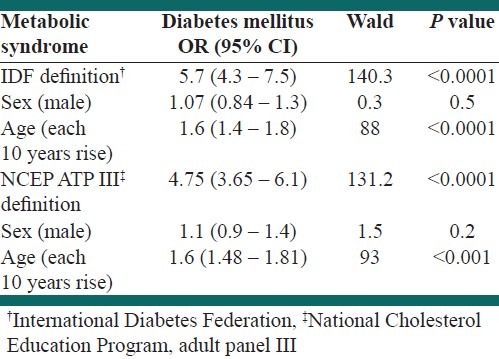
Table 4 shows that MS by IDF definition (OR=5.7, 95%CI: 4.3 – 7.5, P<0.0001) and NCEP definition (OR=4.75, 95%CI: 3.65 – 6.01, P<0.0001) was strongly associated with diabetes mellitus (DM). Age is also associated with DM in both definitions.
Table 4.
The logistic regression results of the prediction value of two definitions of the metabolic syndrome for diabetes mellitus
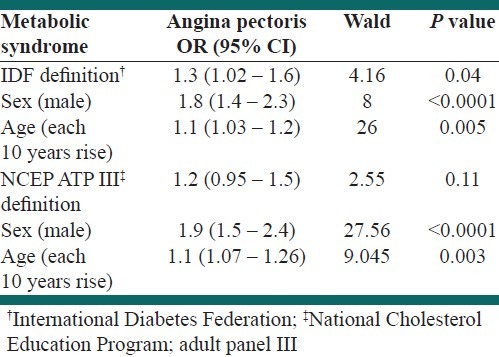
However, the MS only based on the IDF definition, was associated with angina pectoris (OR=1.3, 95%CI: 1.02 – 1.6, P=0.04). The MS based on the NCEP definition was not associated with angina pectoris. Both age and sex, by either definition, were associated with angina pectoris [Table 5].
Table 5.
The logistic regression results of the prediction value of two definitions of the metabolic syndrome for angina pectoris
DISCUSSION
This study showed that the prevalence of the metabolic syndrome with the International Diabetes Federation (IDF) definition was more than its prevalence by the NCEP ATP III definition; 30.16 ± .02% versus 21.3 ± .017%. This is consistent with the result of another study, which reported that the overall prevalence of MS was 31.6% (95% CI, 28.5 – 34.9) and 36.8% (95% CI 33.5 – 40.3), according to an update of the ATPIII-NCEP and IDF criteria, respectively.[13] It was also reported in Brazil to be 50.3 versus 56.9%.[9] However, a few studies reported a lower prevalence of MS based on IDF definition. In Portugal it was 66.4 versus 70.3%,[14] in China 37.8 versus 44.5%,[15] and in Iran it was 41.9 versus 50.8%.[16]
Therefore, the prevalence of MS has shown a wide variation depending on the target population and the diagnostic criteria used.[9] Prevalence of MS tends to be higher with the diagnostic criteria of the IDF.
Agreement between the two criteria of NCEP ATP III and IDF was 92% and Kappa index=0.849; P<0.0001. Ma XJ showed that the agreement in these criteria was 92.9% and the Kappa index was 0.85; P<0.01.[15] Also 90% concordance was reported by Valenzuela.[14]
The results of our study showed that both the definitions of IDF and NCEP of the metabolic syndrome were strongly associated with diabetes mellitus, but the IDF definition had a stronger related factor. Peter W performed a cohort study for six years and found that the age-adjusted relative risk (RR) of diabetes mellitus was RR=6.92 (95% CI 4.47 – 10.81) in the NCEP ATP III definition of the metabolic syndrome in men.[17] Other studies showed that the metabolic syndrome conferred an increased risk for the development of T2DM, with a variety of RR measures, including an RR of 3.5 in WOSCOPS, equal to 2 in the Strong Heart Study, 5.9 with 3 metabolic syndrome traits, and 17.9 with ≥4 metabolic syndrome traits in the Beaver Dam Study, ≥1.5 in the Pima Indian Study, and 6.3 in the San Antonio Heart Study.[18] One study in Texas showed that all three definitions of IDF, WHO, and ATP III had a similar diabetes risk, but the IDF definition had both a higher sensitivity and FPR when compared with the others. The NCEP ATPIII (OR 6.90 [95% CI 4.97 – 9.58]), IDF (5.76 [4.11 – 9.07]), and WHO (6.67 [4.75 – 9.35]) definitions predicted incident diabetes independently of age, sex, ethnic origin, and family history of diabetes.[19] Naveed Sattar observed that the metabolic syndrome increased the risk for diabetes; HR=3.50 (95% CI 2.51 to 4.90).[18]
The area under the ROC curves for IDF definition and NCEP ATP III was compared. We saw that the areas under the ROC curves in the IDF definition were greater than those under the NCEP ATP III, for definition of the metabolic syndrome and for prediction of diabetes mellitus [Figure 1]. Carlos Lorenzo showed that the area under the curve of a model containing age, sex, ethnic origin, family history of diabetes, and two-hour fasting glucose values increased by adding either modified ATPIII (0.842 vs. 0.857, P=0.013) or the IDF metabolic syndrome (0.858, P=0.004).[19]
The MS only based on the IDF definition, independent from age and sex, was associated with angina pectoris.
Zhou H in a 6.5-year cohort study on the Chinese population observed that the ATP-III criterion has the shortest distance in ROC curve and the lowest false positive rate and false negative rate for identifying CVD and type-2 diabetes mellitus.[20] Guzer RN in the UK, via a cross-sectional study, showed that the metabolic syndrome by the NCEP ATP-III criterion increased the risk of CVD to 2.05, independent of sex, age, total cholesterol, and lipid lowering therapy.[21] Bonora E reported the association and prediction of the metabolic syndrome for CVD independent of sex, age, smoking, and HbA1C in Type 2 diabetes mellitus.[22] Naveed Sattar observed that the metabolic syndrome increased the risk for a CHD event, HR=1.76 (95% CI, 1.44 – 2.15)] and for diabetes; HR=3.50 (95% CI 2.51 – 4.90). The metabolic syndrome continued to predict CHD events (HR=1.30, 95% CI, 1.00 – 1.67, P=0.045) in a multivariate model incorporating conventional risk factors.[18]
The association between angina pectoris and MS was only based on IDF definition and also the association between MS and DM was stronger based on the IDF definition. As the necessary factor in IDF definition is abdominal obesity, the differences in associations could be a result of abdominal obesity and its related insulin resistance. Although a strong association between DM and also angina pectoris and MS was detected in this study, this relation should be evaluated in future prospective studies.
CONCLUSION
This study showed that increased age and metabolic syndrome were the most significant and relevant factors of diabetes mellitus, especially when using the IDF criteria for definition of the metabolic syndrome. Also male sex, increased age, and only the IDF definition of the metabolic syndrome were the most significant and relevant factors of stable angina in 20 – 74-year-olds in the Yazd urban population.
ACKNOWLEDGMENT
This study was supported by the Yazd Medical University and Heart Research Center. We appreciate the cooperation of the Yazd Heart Research Center personnel and our participants in this study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hanley AJ, Karter AJ, Festa A, D’Agostino R, Jr, Wagenknecht LE, Savage P, et al. Factor analysis of metabolic syndrome using directly measured insulin sensitivity: The Insulin Resistance Atherosclerosis Study. Diabetes. 2002;51:2642–7. doi: 10.2337/diabetes.51.8.2642. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Alwan A, King H, editors. Department of Noncommunicable Disease Surveillance. Geneva: World Health Organization; 1999. World Health Organization: Definition, Diagnosis, and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus: Report of a WHO consultation; pp. 1–59. [Google Scholar]
- 4.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, et al. Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–76. [PubMed] [Google Scholar]
- 6.Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–52. [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Saely CH, Koch L, Schmid F, Marte T, Aczel S, Langer P, et al. Adult treatment panel III 2001 but not international diabetes federation 2005 criteria of the metabolic syndrome predict clinical cardiovascular events in subjects who underwent coronary angiography. Diabetes Care. 2006;29:901–7. doi: 10.2337/diacare.29.04.06.dc05-2011. [DOI] [PubMed] [Google Scholar]
- 9.Rigo JC, Vieira JL, Dalacorte RR, Reichert CL. Prevalence of Metabolic Syndrome in an Elderly Community: Comparison between Three Diagnostic Methods. Arq Bras Cardiol. 2009;93:80–6. doi: 10.1590/s0066-782x2009000800004. [DOI] [PubMed] [Google Scholar]
- 10.Sarraf-Zadegan N, Boshtam M, Rafiei M. Risk factors for coronary artery disease in Isfahan, Iran. Euro J Public Health. 1999;9:20–6. [Google Scholar]
- 11.Fischbacher C, Bhopal R, Unwin N, White M, Alberti K. The performance of the rose angina questionnaire in south Asian and European origin population: A comparative study in Newcastle, UK. Int J Epidemiol. 2001;30:1009–16. doi: 10.1093/ije/30.5.1009. [DOI] [PubMed] [Google Scholar]
- 12.Zhou H, Hu XS, Guo JT, Zhou ZY, Yao CL. A comparison of applicability in three diagnostic criteria of metabolic syndrome in Jiangsu population. Zhoghua Ya Fang Yi Xue Za Zhi. 2009;43:117–21. [PubMed] [Google Scholar]
- 13.Valenzuela AA, Maíz A, Margozzini P, Ferreccio C, Rigotti A, Olea R, et al. Prevalence of metabolic syndrome among Chilean adults. Rev Med Chil. 2010;138:707–14. doi: 10.4067/s0034-98872010000600007. [DOI] [PubMed] [Google Scholar]
- 14.Correia F, Poínhos R, Freitas P, Pinhão S, Maia A, Carvalho D, et al. Prevalence of the metabolic syndromecomparison between ATP III and IDF criteria in a feminine population with Severe Obesity. Acta Med Port. 2006;19:286–94. [PubMed] [Google Scholar]
- 15.Ma XJ, Jia WP, Hu C, Zhou J, Lu HJ, Zhang R, et al. Comparison of applicability in three diagnostic criteria of metabolic syndrome in familial type 2 diabetes pedigrees. Zhoghua Ya Fang Yi Xue Za Zhi. 2009;43:489–94. [PubMed] [Google Scholar]
- 16.Hadaegh F, Zabetian A, Tohidi M, Ghasemi A, Sheikholeslami F, Azizi F. Prevalence of metabolic syndrome by the adult treatment Panel III, International Diabetes Federation, and World Health Organization Definitions and their association with coronary heart disease in an elderly Iranian Population. Ann Acad Med Singapore. 2009;38:142–9. [PubMed] [Google Scholar]
- 17.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 18.Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–9. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The national cholesterol education program–adult treatment panel III, international diabetes federation, and world health organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes metabolic syndrome as a disease marker. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Guo ZR, Yu LG, Hu XS, Xu BH, Liu HB, et al. Evidence on the applicability of the ATPIII and CDS metabolic syndrome diagnostic criteria to identify CVD and T2DM in the Chinese population from a 6.3-year cohort study in mid-eastern China. Diabetes Res Clin Pract. 2010;90:319–25. doi: 10.1016/j.diabres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Guzder RN, Gatling W, Mullee MA, Byrne CD. Impact of metabolic syndrome criteria on cardiovascular disease risk in people with newly diagnosed type 2 diabetes. Diabetologia. 2006;49:49–55. doi: 10.1007/s00125-005-0063-9. [DOI] [PubMed] [Google Scholar]
- 22.Bonora E, Targher G, Formentini G, Calcaterra F, Lombardi S, Marini F, et al. The Metabolic Syndrome is an independent predictor of cardiovascular disease in Type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabetes Med. 2003;21:52–8. doi: 10.1046/j.1464-5491.2003.01068.x. [DOI] [PubMed] [Google Scholar]


