Abstract
Phosphatidylinositol 3-kinase (PI3K) is essential for both G protein-coupled receptor (GPCR)- and receptor tyrosine kinase (RTK)-mediated cancer cell migration. Here, we have shown that maximum migration is achieved by full activation of phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 (P-Rex1) in the presence of Gβγ and PI3K signaling pathways. Lysophosphatidic acid (LPA)-induced migration was higher than that of epidermal growth factor (EGF)-induced migration; however, LPA-induced activation of Akt was lower than that stimulated by EGF. LPA-induced migration was partially blocked by either Gβγ or RTK inhibitor and completely blocked by both inhibitors. LPA-induced migration was synergistically increased in the presence of EGF and vice versa. In correlation with these results, sphingosine-1-phosphate (S1P)-induced migration was also synergistically induced in the presence of insulin-like growth factor-1 (IGF-1). Finally, silencing of P-Rex1 abolished the synergism in migration as well as in Rac activation. Moreover, synergistic activation of MMP-2 and cancer cell invasion was attenuated by silencing of P-Rex1. Given these results, we suggest that P-Rex1 requires both Gβγ and PI3K signaling pathways for synergistic activation of Rac, thereby inducing maximum cancer cell migration and invasion.
Keywords: cell movement; GTP-binding protein β subunits; GTP-binding protein γ subunits; phosphatidylinositol 3-kinase; P-Rex1 protein; receptor protein-tyrosine kinases; receptors, G-protein-coupled
Introduction
While cell migration is essential for normal embryonic development, immune function and angiogenesis, it is also associated with inflammatory disease, vascular impairment and tumor cell invasion (Lauffenburger and Horwitz, 1996; Ridley et al., 2003). Cell migration is initiated by the activation of cell surface receptors such as receptor tyrosine kinase (RTK) and G protein-coupled receptor (GPCR), leading to the activation of phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. Chemical gradients lead to the local activation of PI3K and the establishment of a phosphatidylinositol 3,4,5-trisphosphate (PtdIns-P3) gradient that ultimately creates polarized Rac or Ras activity (Srinivasan et al., 2003; Sasaki et al., 2004).
The mammalian rho subfamily of small G proteins consists of distinct proteins including Rho, Rac and CDC42 (Hall, 1994). Activation of Rac requires GTP/GDP exchange factors (GEFs), and PtdIns-P3-dependent Rac exchanger 1 (P-Rex1), which is a Rac-GEF, selectively activates Rac through its catalytic Dbl homology (DH) domain. P-Rex1 can be directly activated by Gβγ subunits and PtdIns-P3 (Welch et al., 2002). It has been reported that P-Rex1 is activated by GPCR (Hill et al., 2005; Yoshizawa et al., 2005). In addition, P-Rex1 is stimulated by nerve growth factor, which acts through TrkA to activate PI3K and their downstream effectors in the developing nervous system. P-Rex1 is also stimulated by insulin-like growth factor-1 (IGF-1), which modulates cancer cell migration (Kim et al., 2011). Moreover, P-Rex1 associates with mammalian target of rapamycin complex 2 (mTORC2) and regulates cancer cell migration via activation of Rac (Hernandez-Negrete et al., 2007; Dada et al., 2008; Kim et al., 2011).
Lysophosphatidic acid (LPA, l-acyl-sn-glycerol 3-phosphate) is a phospholipid ligand that normally exists in serum and body fluids (Goetzl and An, 1998). LPA is also regarded as a biomarker for ovarian cancer, and a high level of LPA is detected in ascitic fluids and plasma of ovarian cancer patients (Xu et al., 1998; Jeon et al., 2010). It has been reported that LPA exerts its biological function by interacting with GPCRs, such as LPA1/Edg-2, LPA2/Edg-4 and LPA3/Edg-7 (Contos et al., 2000). Occupation of its cognate receptors by LPA triggers activation of various signaling molecules during cell migration. Among them, PI3K is a major signal transducer for LPA-induced cell migration (Kim et al., 2008b). Although LPA-induced Akt activation is relatively weaker than that of growth factors, migration is strongly induced by LPA compared to growth factors (Kim et al., 2008b; Shida et al., 2008). However, it is still unclear how LPA triggers the activation of PI3K, and LPA induces cell migration through different modulation mechanisms compared to growth factors. In the present study, we provide evidence that LPA induces synergistic activation of P-Rex1 via PI3K and Gβγ, thereby leading to maximum migratory activity of cancer cells.
Results
Akt activation and migration are significantly induced by LPA in A549 cells
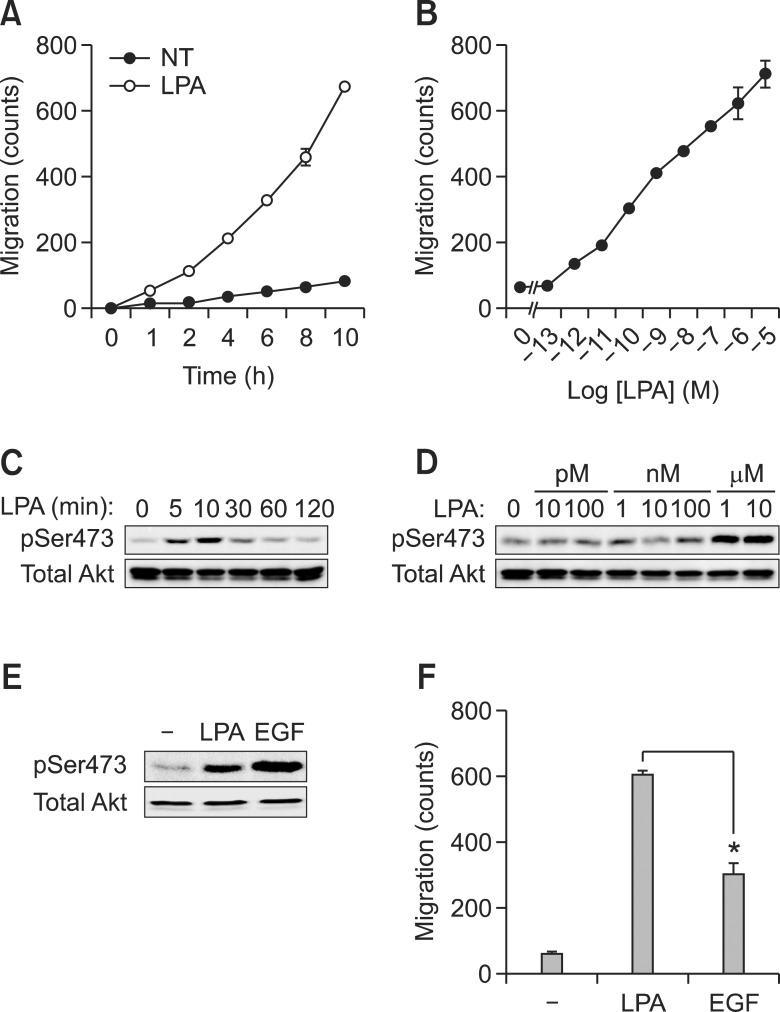
LPA is reportedly involved in a variety of diseases such as atherosclerosis and tumorigenesis (Fang et al., 2002; Xie et al., 2002). In fact, LPA is originally identified as a tumor-stimulating factor that promotes cancer cell migration (Fang et al., 2002; Kim et al., 2008b). Our results also showed that LPA strongly induced the migration of A549 lung epithelial cancer cells (Figures 1A and 1B). It has been reported that PI3K plays a major role in downstream signaling pathway for LPA-induced MEF cell migration. Indeed, Akt, which is downstream of PI3K, was also activated by LPA treatment as shown in Figures 1C and 1D. However, the activation of Akt by LPA was relatively weaker than that of EGF stimulation (Figure 1E). In contrast, LPA-induced A549 lung cancer cell migration was significantly higher than EGF-dependent migration (Figure 1F). These results indicate that LPA-induced signaling pathway includes additional signaling pathways besides PI3K and Akt signaling pathways during the regulation of cancer cell migration.
Figure 1.
LPA dramatically induces cancer cell migration compared to EGF. A549 cell migration was stimulated with LPA (10 µM) for the indicated time or at the indicated dose for 10 h (A, B). Akt phosphorylation was treated with LPA (10 µM) for the indicated time or at the indicated dose of LPA for 10 min and detected by western blotting with phospho-Akt (Ser473) and total Akt (C, D). Western blotting (E) and migration (F) were determined by individually treatment with LPA (10 µM) or EGF (50 ng/ml). *P < 0.05.
LPA-induced migration is controlled by activation of Gβγ and RTK
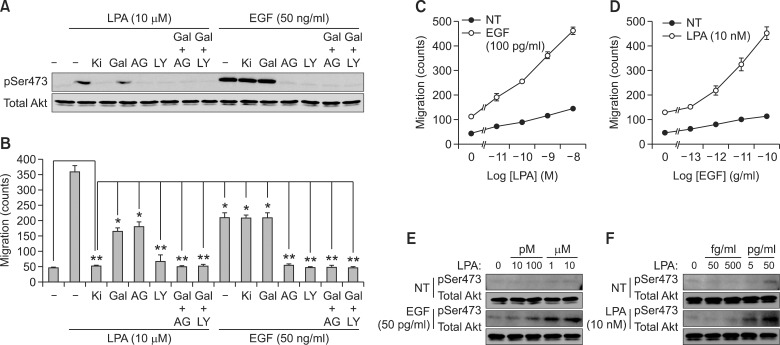
In order to investigate major signaling pathways that regulate LPA-induced Akt activation and cancer cell migration, we next assessed the effect of specific inhibitors of signaling pathways involved in Akt activation and cell migration. As shown in Figures 2A and 2B, LPA-induced Akt activation and cell migration were completely blocked by LPA 1/3 receptor inhibitor (Ki16425) and PI3K inhibitor (LY294002). However, EGF-induced Akt activation and cell migration did not affected by Ki16425. Interestingly, pretreatment of RTK inhibitor (AG1478) significantly blocked LPA-induced Akt activation, whereas LPA-induced cancer cell migration was partially blocked. Moreover, the inhibition of Gβγ by allein also partially blocked LPA-induced Akt activation and cell migration. Although LPA-induced migration was partially inhibited by either gallein or AG1478, LPA-induced cancer cell migration was completely blocked by simultaneous treatment of gallein and AG1478. On the other hand, the inhibition of RTK completely eliminated EGF-induced Akt activation and cancer cell migration, whereas inhibition of Gβγ had no effect (Figures 2A and 2B). These findings support the idea that both Gβγ and RTK signaling pathways are necessary for LPA-induced cancer cell migration, whereas EGF-induced cancer cell migration is regulated by only RTK signaling pathway. GPCRs transmit signals through heterotrimeric G proteins composed of Gα, Gβ, and Gγ subunits. As shown in Figures 2C and 2D, LPA-induced cancer cell migration was synergistically increased in the presence of low concentration of EGF. In addition, EGF-induced cancer cell migration was also synergistically increased in the presence of low concentration of LPA. Moreover, synergistic increment of Akt activation was regulated by both Gβγ and PI3K and vice versa (Figures 2E and 2F). Therefore, co-activation of Gβγ and PI3K is required for maximum cancer cell migration.
Figure 2.
The activation of Gβγ and RTK is critical for LPA-induced cancer cell migration. (A) Akt activation by LPA (10 µM) and EGF (50 ng/ml) for 10 min was detected by western blotting with phospho-Akt (Ser473) and total Akt antibodies. (B) Migration of A549 cells was determined in the absence or presence of various inhibitors such as LPA receptor inhibitor (Ki: Ki16425, 2 nM), Gβγ inhibitor (Gal: gallein, 10 µM), RTK inhibitor (AG: AG1478 100 nM), PI3K inhibitor (LY: LY294002, 10 µM) or gallein together with AG1478 or LY294002 upon LPA (10 µM) or EGF (50 ng/ml) stimulation for 10 h. The chemotactic migration of A549 cells induced by either LPA (10 nM) with the indicated dose of EGF (C) or EGF (50 pg/ml) with the indicated dose of LPA for 10 h (D), and phosphorylation at Ser473 of Akt and total Akt levels were assessed by western blotting (E, F). *P < 0.05, **P > 0.05.
Synergism of cancer cell migration by GPCR and RTK is generalized
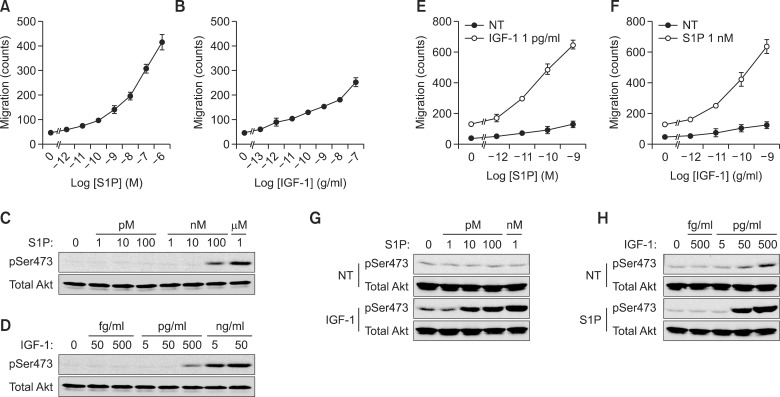
To further generalize the synergistic migration by Gβγ and PI3K, we have evaluated the synergistic activation of cancer cell migration using different set of agonists. Since A549 cells dominantly express insulin-like growth factor-1 (IGF-1) and sphingosine-1-phosphate (S1P) receptors, we verified synergistic induction of migration by IGF-1 and S1P. As shown in Figures 3A and 3B, S1P strongly induced the migration of cancer cells compared to IGF-1-dependent cancer cell migration. In contrast, the activation of Akt by S1P was relatively weaker than that of IGF-1 stimulation as well as LPA-induced Akt activation (Figures 3C and 3D). Interestingly, S1P-induced cancer cell migration was synergistically induced in the presence of low concentration of IGF-1. Moreover, IGF-1-induced cancer cell migration was also synergistically increased in the presence of low concentration of S1P (Figures 3E and 3F). In correlation with this, activation of Akt was synergistically induced by both treatment of S1P and IGF-1 (Figures 3G and 3H). These results indicate that synergistic increment of cancer cell migration by GPCR and RTK could be generalized.
Figure 3.
Synergistic acceleration of cancer cell migration is regulated by both GPCR and RTK. A549 cell migration was stimulated with S1P and IGF-1 at the indicated dose for 10 h (A, B). Akt phosphorylation was stimulated by the indicated dose of S1P (C) and IGF-1 (D) for 30 min and verified by western blotting with phospho-Akt (Ser473) and total Akt. The chemotactic migration of A549 cells induced by either IGF-1 (500 pg/ml) with the indicated dose of S1P (E) or S1P (1 nM) with the indicated dose of IGF-1 for 10 h (F). Phosphorylation at Ser473 of Akt and total Akt were stimulated by either IGF-1 (500 pg/ml) with the indicated dose of S1P (G) or S1P (1 nM) with the indicated dose of IGF-1 for 30 min (H).
P-Rex1 is the merge point in the synergism of cancer cell migration
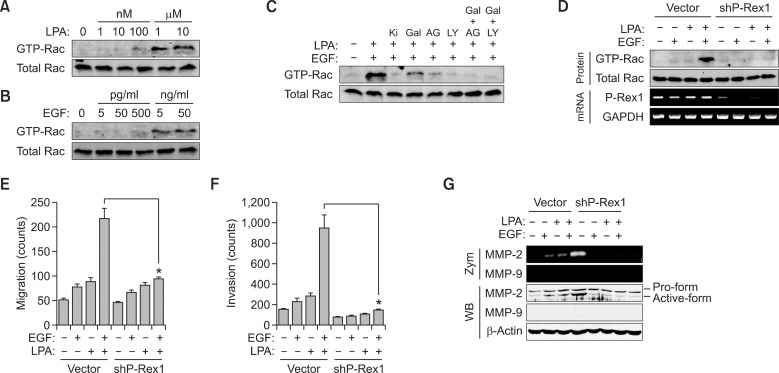
Rac small G proteins have critical roles during migration in a variety of cell types (Chung et al., 2000). Our results also showed that LPA and EGF dose-dependently induced the activation of Rac (Figures 4A and 4B). Since Rac small G protein plays critical roles in GPCR- or RTK-mediated cell migration (Chung et al., 2000; Barber and Welch, 2006; Kim et al., 2008a; Qin et al., 2009), we evaluated synergistic activation of Rac by Gβγ and RTK. As shown in Figure 4C, Rac was synergistically activated in the presence of both LPA and EGF. Synergistic activation of Rac was partially blocked by inhibition of either Gβγ or RTK. In addition, inhibition of both Gβγ and RTK completely blocked activation of Rac. These results suggest that LPA-induced Rac activation requires both Gβγ and RTK. Recently, it was reported that P-Rex1, a Rac guanine nucleotide exchange factor, stably interacts with the mTOR complex and Akt (Higuchi et al., 2001; Welch et al., 2002; Hernandez-Negrete et al., 2007; Kim et al., 2011). Therefore, we examined the effect of P-Rex1 on the synergistic activation of Rac. Silencing of P-Rex1 attenuated the synergistic activation of Rac (Figure 4D). Moreover, LPA- and EGF-induced synergism of migration and invasion was abolished by knock-down of P-Rex1 (Figures 4E and 4F). Since GRCR- and RTK-induced cancer cell invasion is mediated by matrix metalloproteinases (MMP) including MMP-2 and MMP-9, we examined the synergistic activation of MMP-2 and MMP-9 by Gβγ and RTK. As shown in figure 4G, MMP-2 was also synergistically activated by LPA and EGF, however, MMP-9 was not affected. Moreover, synergistic activation of MMP-2 was abolished by silencing of P-Rex1. These results indicate that RTK- and GPCR-mediated synergistic activation of Rac and MMP-2 are controlled by P-Rex1.
Figure 4.
P-Rex1 plays an essential role in synergism of Gβγ- and PI3K-dependent migration. LPA- and EGF-induced dose-dependent activation of Rac for 5 min was determined by measuring the GTP form of Rac as described in 'Materials and methods' (A, B). (C) A549 cells were pretreated for 20 min with various inhibitors such as Ki16425 (2 nM), gallein (10 µM), AG1478 (100 nM), LY294002 (10 µM) or gallein together with AG1478 or LY294002, followed by stimulation with LPA (10 nM) and EGF (50 pg/ml) for 5 min. (D) After knock-down of P-Rex1, activation of Rac was determined by measuring the GTP form of Rac and expression of P-Rex1 was determined by RT-PCR. (E) Motility after silencing P-Rex1 was determined by migration assay for 10 h. (F) LPA (10 nM)- and EGF (50 pg/ml)-induced cancer cell invasion for 24 h was measured as described in 'Materials and methods' section. *P < 0.05. (G) Cells were stimulated with LPA (10 nM), EGF (50 pg/ml) or together with LPA and EGF for 24 h. MMP-2 protein expression and gelatinolytic activity were analyzed by western blotting (WB) and gelatin zymography (Zym), respectively.
Discussion
In this study, we examined synergistic pathway of cancer cell migration. Plethora of reports suggests that ascites from cancer patients contain inducing factors for migration in many cell types. In fact, LPA is a major component of ascites from ovarian cancer patients (AOCP) that induces MEF cell migration (Kim et al., 2008b; Lee et al., 2008) and LPA is an important predictor of cancer diagnosis (Xu et al., 1998; Fang et al., 2002). Indeed, LPA dramatically stimulated A549 lung epithelial cancer cell migration (Figures 1A and 1B). Although PI3K is mainly activated by the RTK-mediated signaling pathway, it seems likely that PI3K signaling pathway is also important for LPA-induced cancer cell migration. For example, the inhibition of PI3K virtually blocks LPA-induced migration in a number of cancer cells (Barber and Welch, 2006). In addition, Akt, which is downstream of PI3K, was also activated by LPA treatment as shown in Figures 1C and 1D. Therefore, it seems likely that PI3K plays crucial roles in LPA-induced cancer cell migration.
Although PI3K seems to be a key regulator of GPCR-dependent cancer cell migration, the degree of cancer cell migration does not match with the degree of PI3K activity. For instance, LPA-induced cancer cell migration was about 2-fold higher than that of EGF-induced migration, whereas LPA-induced activation of Akt was 3~4-fold lower than that of EGF stimulation (Figures 1E and 1F). Similarly, it has been reported that LPA-induced gastric cancer cell migration is also higher than EGF-induced migration (Shida et al., 2008). Moreover, although S1P-induced Akt activation was relatively weaker than that of IGF-1 stimulation, S1P strongly induced cancer cell migration compared to IGF-1 (Figures 3A-3D). Therefore, it is possible that GPCR-dependent signaling pathway includes additional signaling pathways besides PI3K/Akt signaling pathways during the regulation of cancer cell migration.
LPA receptors are mainly coupled to Gαi, Gα12/13, and Gq interacting with Gβγ proteins (Oldham and Hamm, 2008). The inhibition of both Gαi and Gβγ with pertussis toxin completely blocks LPA-induced migration (Do et al., 2009). In addition, it has been reported that Gβγ rather than Gαi, Gα12/13, and Gq plays crucial roles in chemotaxis of HEK293 cells (Neptune and Bourne, 1997; Neptune et al., 1999). In line with this, our results also showed that selective inhibition of Gβγ by gallein or RTK by AG1478 suppressed but not completely LPA-induced cancer cell migration. It is also notable that inhibition of both Gβγ and RTK completely blocked LPA-induced cancer cell migration. On the other hand, inhibition of RTK completely blocked EGF-induced cancer cell migration, whereas inhibition of Gβγ did not affect (Figure 2B), indicating that EGF can evoke migration of cancer cells in the absence of Gβγ activation unlike LPA. Therefore, these results suggest that LPA renders RTK-dependent activation of PI3K as well as Gβγ activation, and the activation of both PI3K and Gβγ results in higher migration in comparison with EGF stimulation by which PI3K is activated solely.
If additional Gβγ signaling pathway in LPA stimulation is one of the reasons for higher migration in comparison with EGF stimulation, EGF-stimulated cancer cell migration should be significantly enhanced by additional Gβγ activation. Indeed, EGF-induced cancer cell migration was synergistically enhanced in the presence of low concentration of LPA (Figure 2D). In addition, IGF-1-induced cancer cell migration was also synergistically accelerated in the presence of low concentration of S1P (Figure 3F). Since Gα is not involved in cell migration (Neptune et al., 1999), the responsible factor for synergistic increment of cancer cell migration would be Gβγ. Requirement of both PI3K and Gβγ signaling pathways for maximum cancer cell migration was examined by similar experiment. For instance, LPA-induced cancer cell migration was also synergistically elevated in the presence of low concentration of EGF (Figure 2C). Likewise, S1P-induced migration of cancer cell was synergistic increased in the presence of low concentration of IGF-1 (Figure 3E), indicating that saturation of PI3K activity is critical for maximum cancer cell migration. Therefore, these results suggest that the activation of both Gβγ and PI3K is generalized to induce maximum cancer cell migration.
The activation of Rac is the most important determinant for many types of cell migration. (Chung et al., 2000; Barber and Welch, 2006; Kim et al., 2008a; Qin et al., 2009). Indeed, the activation of Rac was dramatically induced by LPA and EGF in a dose-dependent manner (Figures 4A and 4B). Since cancer cell migration was synergistically induced by simultaneous activation of Gβγ and PI3K, it is possible that maximum Rac activation will be acquired in the presence of both Gβγ and PI3K signaling pathway. As shown in Figures 4A and 4B, the activation of Rac was not observed in the presence of minimum dose of either LPA or EGF. By contrast, Rac was significantly activated in the presence of both LPA and EGF. It is also notable that synergistic activation of Rac was partially blocked by inhibition of either Gβγ or RTK, whereas the inhibition of both Gβγ and RTK completely blocked synergistic activation of Rac by LPA and EGF (Figure 4C). Therefore, these results suggest that LPA-induced maximum cancer cell migration is achieved by synergistic activation of Rac through Gβγ and PI3K signaling pathways.
It is likely that LPA can stimulate RTK signaling pathway in addition to Gβγ signaling pathway. Transactivation of RTK seems to be achieved by the generation of active growth factors. For example, our results showed that LPA-induced cancer cell migration was completely blocked by PI3K inhibitor (Figure 2B). In addition, PI3K inhibitor also impaired LPA- and EGF-induced synergistic activation of Rac (Figure 4C). On the other hand, inhibition of RTK partially blocked LPA-induced cancer cell migration indicating that LPA possesses signaling pathways leads to Rac activation in addition to transactivation of EGF receptor. Similarly, it has been reported that Gβγ is one of the important molecules of PI3K activity and regulates cancer cell migration (Hill et al., 2005). In fact, as shown in Figure 4C, synergistic activation of Rac was not completely blocked by RTK inhibitor in comparison with PI3K inhibitor. Indeed, it has been reported that stimulation of squamous cell carcinoma cell lines with GPCR agonists evokes the tyrosine phosphorylation of EGFR and activation of PI3K (Gschwind et al., 2002; Shah et al., 2006). Since the inhibition of metalloproteinase blocks GPCR agonist-induced activation of EGFR, the communication between GPCR and EGFR signaling systems involves cell surface proteolysis of EGF precursors (Gschwind et al., 2003). In addition, expression of metalloproteinase is completely blocked by the inhibition of Gβγ (von Offenberg Sweeney et al., 2004; Zou et al., 2011).
One possible mediator for synergistic activation of Rac seems to be P-Rex1, which is a Rac guanine nucleotide exchange factor. Indeed, it has been reported that P-Rex1 is dually activated by Gβγ and PtdIns-P3 which is a product of PI3K (Welch et al., 2002; Hill et al., 2005). In fact, synergistic activation of Rac was completely abolished by silencing of P-Rex1 (Figure 4D). Moreover, silencing of P-Rex1 also eliminated synergism of cancer cell migration and invasion (Figure 4E and 4F). Synergistic activation of MMP-2 was abolished by silencing of P-Rex1, however, MMP-9 was not affected (Figure 4G). In this regard, LPA can activate both Gβγ and PI3K through transactivation of RTK, thereby leads to full activation of Rac and MMP-2 as well as P-Rex1. However, it seems likely that PI3K alone partially activates P-Rex1 thereby weakly inducing activation of Rac and MMP-2, which is the reason of relatively low migration and invasion of EGF stimulation in comparison with LPA stimulation.
Concerning about synergistic activation of Akt by GPCR and RTK (Figures 2E, 2F, 3G and 3H), positive feedback mechanism would be one plausible explanation. It has been reported that P-Rex1 forms molecular complex with mTORC2, Akt, and Rac (Yoshizawa et al., 2005; Kim et al., 2011). Particularly, expression of constitutively active form of Rac enhances activation of Akt and expression of constitutively active form of Akt enhances activation of Rac (Higuchi et al., 2001). In correlation with this, synergistic activation of P-Rex1 by providing both LPA and EGF resulted in synergistic activation of Rac (Figure 4D), thereby leaded to synergistic activation of Akt (Figures 2E, 2F, 3G and 3H). Therefore, these results suggest that positive feedback activation of Rac by Akt is important for full activation of Rac in addition to activation of Rac by synergistic activation of P-Rex1 through Gβγ and PI3K.
In conclusion, co-activation of Gβγ and PI3K regulates synergistic activation of P-Rex1, thereby full activation of Rac as well as migration. This study provides mechanistic insight into the activation of P-Rex1 by LPA and the synergism between Gβγ and PI3K pathways. Moreover, our results suggest that P-Rex1 could provide advanced strategies for cancer therapy.
Methods
Reagents and antibodies
DMEM, FBS, trypsin-EDTA, and antibiotics were purchased from Hyclone Laboratories, Inc. (Logan, UT). Anti-pan-Akt and anti-phospho-Akt (Ser473) were obtained from Cell Signaling Technology (Boston, MA). Anti-Rac antibody was purchased from Millipore (Billerica, MA). Anti-MMP-2 and Anti-MMP-9 antibodies were purchased from Santa Cruz Biotechnology Inc. (Beverly, MA). DAPI was purchased from Molecular Probes, Inc. (Carlsbad, CA). IRDye700- or IRDye800-conjugated rabbit or mouse secondary antibody was obtained from Li-COR Bioscience (Lincoln, NE). Ki16425 (a LPA receptor 1/3 inhibitor), gallein (a Gβγ inhibitor), AG 1478 (a tyrosine kinase inhibitor of EGFR), LY294002 (a PI3K inhibitor), recombinant human IGF-1, recombinant human LPA, recombinant human EGF, recombinant human S1P and all other reagents were high quality and were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise indicated.
Cell culture and transfection
A549 cells were cultured in DMEM supplemented with 10% (v/v) FBS and penicillin/streptomycin, and maintained at 37℃ in 5% CO2. For transient expression, HEK293FT cells were transfected with various plasmids by the calcium phosphate method. Cell-free culture supernatant and cells were harvested for viral infection.
Western blotting and analysis of mRNA expression
Western blotting and analysis of mRNA expression were performed as described in a previous report (Kim et al., 2011).
Gelatin zymography
The extracellular medium was concentrated using an Amicon Centricon from Millipore, and then electrophoretically separated onto 8% SDS-polyacrylamide gels containing 1 mg/ml gelatin. After being washed with wash buffer (2.5% Triton X-100 in 50 mM Tris-HCl, pH 7.4), the gel was stained with 0.2% Coomassie Brilliant Blue R-250 from Sigma-Aldrich for 2 h and then distained in the same solution without dye. Zymographic results were expressed as MMP proteolytic activity.
Rac activation assay
The level of active GTP-bound Rac was determined by pulling-down GTP-bound Rac with GST-PAK-RBD coupled to glutathione agarose beads. Cells were co-stimulated with LPA and EGF for 5 min and then lysed with lysis buffer containing 50 mM Tris (pH 7.5), 1% Nonidet P-40, 150 mM NaCl, 5 mM MgCl2, 10% glycerol, 1 mg/ml leupeptin, 1 mg/ml aprotinin and 1 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged, and supernatants were incubated with beads coupled to GST-PAKRBD for 2 h at 4℃. Beads were washed with lysis buffer and bound GTP-loaded Rac was eluted with sample buffer. The amount of active Rac was determined by western blot analysis.
Migration assay and invasion assay
The migration of A549 cells was measured as described previously (Kim et al., 2011). For invasion assays, A549 cells were serum-starved for 12 h (1 × 105) and overlaid on top of a 24-well Trans-well plate (Corning Costar Corp., Cambridge, MA) containing artificial basement membrane produced by 1 mg/ml of Matrigel (BD Bioscience, San Jose, CA). Invasion was induced by placing the cells on overlaid inserts of serum-free medium either in the absence or presence of LPA and EGF for 24 h. The insert was fixed with 4% paraformaldehyde, and non-migratory cells on the top-side of the membrane were removed by gently wiping with a cotton swab. The membrane was stained with DAPI, and invasive cells were counted under the fluorescence microscope at × 10 magnification (Axiovert200, Carl Zeiss, Jena).
Lentiviral gene silencing
For generation of lentiviruses expressing shRNA, HEK293FT cells were co-transfected with pLKO.1 constructs (2 µg), pVSV-G (0.2 µg) and Δ8.9 (2 µg) by the calcium phosphate method as described previously (Kim et al., 2011). Target sequence was 5'-ggaccatgctggaggacatct-3' (sh-P-Rex1).
Statistical analysis
Results are expressed as the means ± S.D. of two independent experiments (n = 3 for each experiment). When comparing two groups, an unpaired Student's t-test was used to address differences. P-values < 0.05 were considered significant and indicated by *. P-values > 0.05 were considered insignificant and indicated by **.
Acknowledgements
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A090086).
Abbreviations
- EGF
epidermal growth factor
- GPCR
G protein-coupled receptor
- LPA
lysophosphatidic acid
- PI3K
phosphatidylinositol 3-kinase
- P-Rex1
PtdIns-P3-dependent Rac exchanger 1
- PtdIns-P3
phosphatidylinositol 3,4,5-trisphosphate
- RTK
receptor tyrosine kinase
- S1P
sphingosine-1-phosphate
References
- 1.Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93:E44–E52. [PubMed] [Google Scholar]
- 2.Barber MA, Donald S, Thelen S, Anderson KE, Thelen M, Welch HC. Membrane translocation of P-Rex1 is mediated by G protein betagamma subunits and phosphoinositide 3-kinase. J Biol Chem. 2007;282:29967–29976. doi: 10.1074/jbc.M701877200. [DOI] [PubMed] [Google Scholar]
- 3.Chung CY, Lee S, Briscoe C, Ellsworth C, Firtel RA. Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells. Proc Natl Acad Sci USA. 2000;97:5225–5230. doi: 10.1073/pnas.97.10.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 5.Dada S, Demartines N, Dormond O. mTORC2 regulates PGE2-mediated endothelial cell survival and migration. Biochem Biophys Res Commun. 2008;372:875–879. doi: 10.1016/j.bbrc.2008.05.154. [DOI] [PubMed] [Google Scholar]
- 6.Do KH, Choi YW, Kim EK, Yun SJ, Kim MS, Lee SY, Ha JM, Kim JH, Kim CD, Son BG, Kang JS, Khan IA, Bae SS. Pinoresinol-4,4'-di-O-beta-D-glucoside from Valeriana officinalis root stimulates calcium mobilization and chemotactic migration of mouse embryo fibroblasts. Phytomedicine. 2009;16:530–537. doi: 10.1016/j.phymed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, Jaffe R, Erickson J, Mills GB. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 8.Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. Faseb J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- 9.Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002;62:6329–6336. [PubMed] [Google Scholar]
- 10.Gschwind A, Hart S, Fischer OM, Ullrich A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J. 2003;22:2411–2421. doi: 10.1093/emboj/cdg231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernandez-Garcia R, Calderon-Salinas JV, Reyes-Cruz G, Gutkind JS, Vazquez-Prado J. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007;282:23708–23715. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi M, Masuyama N, Fukui Y, Suzuki A, Gotoh Y. Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and in invasive PTEN knockout cells. Curr Biol. 2001;11:1958–1962. doi: 10.1016/s0960-9822(01)00599-1. [DOI] [PubMed] [Google Scholar]
- 14.Hill K, Krugmann S, Andrews SR, Coadwell WJ, Finan P, Welch HC, Hawkins PT, Stephens LR. Regulation of P-Rex1 by phosphatidylinositol (3,4,5)-trisphosphate and Gbetagamma subunits. J Biol Chem. 2005;280:4166–4173. doi: 10.1074/jbc.M411262200. [DOI] [PubMed] [Google Scholar]
- 15.Jeon ES, Heo SC, Lee IH, Choi YJ, Park JH, Choi KU, Park DY, Suh DS, Yoon MS, Kim JH. Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1α from human mesenchymal stem cells. Exp Mol Med. 2010;42:280–293. doi: 10.3858/emm.2010.42.4.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim EK, Tucker DF, Yun SJ, Do KH, Kim MS, Kim JH, Kim CD, Birnbaum MJ, Bae SS. Linker region of Akt1/protein kinase Balpha mediates platelet-derived growth factor-induced translocation and cell migration. Cell Signal. 2008a;20:2030–2037. doi: 10.1016/j.cellsig.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Kim EK, Yun SJ, Do KH, Kim MS, Cho M, Suh DS, Kim CD, Kim JH, Birnbaum MJ, Bae SS. Lysophosphatidic acid induces cell migration through the selective activation of Akt1. Exp Mol Med. 2008b;40:445–452. doi: 10.3858/emm.2008.40.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EK, Yun SJ, Ha JM, Kim YW, Jin IH, Yun J, Shin HK, Song SH, Kim JH, Lee JS, Kim CD, Bae SS. Selective activation of Akt1 by mammalian target of rapamycin complex 2 regulates cancer cell migration, invasion, and metastasis. Oncogene. 2011;30:2954–2963. doi: 10.1038/onc.2011.22. [DOI] [PubMed] [Google Scholar]
- 19.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Jeon ES, Lee JS, Cho M, Suh DS, Chang CL, Kim JH. Lysophosphatidic acid in malignant ascites stimulates migration of human mesenchymal stem cells. J Cell Biochem. 2008;104:499–510. doi: 10.1002/jcb.21641. [DOI] [PubMed] [Google Scholar]
- 21.Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci USA. 1997;94:14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neptune ER, Iiri T, Bourne HR. Galphai is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem. 1999;274:2824–2828. doi: 10.1074/jbc.274.5.2824. [DOI] [PubMed] [Google Scholar]
- 23.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 24.Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, Scofield MA, Dowd FJ, Lin MF, Tu Y. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene. 2009;28:1853–1863. doi: 10.1038/onc.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167:505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah BH, Neithardt A, Chu DB, Shah FB, Catt KJ. Role of EGF receptor transactivation in phosphoinositide 3-kinase-dependent activation of MAP kinase by GPCRs. J Cell Physiol. 2006;206:47–57. doi: 10.1002/jcp.20423. [DOI] [PubMed] [Google Scholar]
- 28.Shida D, Fang X, Kordula T, Takabe K, Lepine S, Alvarez SE, Milstien S, Spiegel S. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008;68:6569–6577. doi: 10.1158/0008-5472.CAN-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Offenberg Sweeney N, Cummins PM, Birney YA, Cullen JP, Redmond EM, Cahill PA. Cyclic strain-mediated regulation of endothelial matrix metalloproteinase-2 expression and activity. Cardiovasc Res. 2004;63:625–634. doi: 10.1016/j.cardiores.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y, Gibbs TC, Meier KE. Lysophosphatidic acid as an autocrine and paracrine mediator. Biochim Biophys Acta. 2002;1582:270–281. doi: 10.1016/s1388-1981(02)00181-6. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, Kennedy AW, Belinson J, Markman M, Casey G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 34.Yoshizawa M, Kawauchi T, Sone M, Nishimura YV, Terao M, Chihama K, Nabeshima Y, Hoshino M. Involvement of a Rac activator,P-Rex1, in neurotrophin-derived signaling and neuronal migration. J Neurosci. 2005;25:4406–4419. doi: 10.1523/JNEUROSCI.4955-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou Y, Fu Y, Davies MG. Role for gbetagamma G-Proteins in protease regulation during remodeling of the murine femoral artery. J Surg Res. 2011 doi: 10.1016/j.jss.2011.08.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]