Abstract
Although the prognosis of patients with differentiated thyroid carcinoma (DTC) is generally encouraging, a diagnostic dilemma is posed when an increasing level of serum thyroglobulin (Tg) is noted, without detection of a recurrent tumor using conventional imaging tools such as the iodine-131 whole-body scanning (the [131I] scan) or neck ultrasonography (US). The objective of the present study was to evaluate the diagnostic value of [124I]-PET/CT and [18F]-FDG-PET/CT in terms of accurate detection of both iodine- and non-iodine-avid recurrence, compared with that of conventional imaging such as the [131I] scan or neck ultrasonography (US). Between July 2009 and June 2010, we prospectively studied 19 DTC patients with elevated thyroglobulin levels but who do not show pathological lesions when conventional imaging modalities are used. All involved patients had undergone total thyroidectomy and radioiodine (RI) treatment, and who had been followed-up for a mean of 13 months (range, 6-21 months) after the last RI session. Combined [18F]-FDG-PET/CT and [124I]-PET/CT data were evaluated for detecting recurrent DTC lesions in study patients and compared with those of other radiological and/or cytological investigations. Nine of 19 patients (47.4%) showed pathological [18F]-FDG (5/19, 26.3%) or [124I]-PET (4/19, 21.1%) uptake, and were classed as true-positives. Among such patients, disease management was modified in six (66.7%) and disease was restaged in seven (77.8%). In particular, the use of the described imaging combination optimized planning of surgical resection to deal with locoregional recurrence in 21.1% (4/19) of patients, who were shown to be disease-free during follow-up after surgery. Our results indicate that combination of [18F]-FDG-PET/CT and [124I]-PET/CT affords a valuable diagnostic method that can be used to make therapeutic decisions in patients with DTC who are tumor-free on conventional imaging studies but who have high Tg levels.
Keywords: [124I]-PET, [18F]-FDG-PET, PET/CT, Elevated Thyroglobulin Levels, Negative [131I] Whole-Body Scan, Differentiated Thyroid Carcinoma, Recurrence
INTRODUCTION
Although the prognosis of patients with differentiated thyroid carcinoma (DTC) is favorable, recurrence is noted in up to 30% of such patients (1-5). Until recent years, serum thyroglobulin (Tg) measurement and the [131I] scan were the mainstays of DTC patient evaluation after treatment and during follow-up. The [131I] scan has high specificity, yet some of papillary and follicular thyroid carcinoma recurrences are [131I]-negative (6-9). Because the most common site of recurrence is the cervical lymph nodes, neck ultrasonography (US) may also be helpful in early detection of small cervical metastases (10-12). However, a diagnostic dilemma is posed by patients who exhibit increased levels of Tg, in the absence of detection of recurrent cancer using conventional imaging tools such as the iodine-131 whole-body scanning (the [131I] scan) or neck US. Therefore, advanced diagnostic imaging permitting anatomical tumor localization has been used to accurately detect both iodine- and non-iodineavid recurrence; the relevant modalities include positron emission tomography (PET) using either iodine-124 [124I] or [18F]-fluoro-2-deoxy-D-glucose ([18F]-FDG). Moreover, if [18F]-FDG-PET and [124I]-PET are both performed before considering whether repeated high-dose radioiodine (RI) treatment is appropriate, unnecessary RI may be excluded, and further optimal management such as surgery or irradiation can be usefully indicated.
The benefits of [18F]-FDG-PET to diagnose recurrent and metastatic disease, particularly when the [131I] scan is negative, have been assessed previously (13-18). The loss of a capacity to concentrate iodine when Tg levels are elevated demand the use of imaging tools other than the [131I] scan. Cervical US is another valuable tool for treatment of recurrent DTC. However, it is also necessary to perform whole-body assessment of disease extent, using a different diagnostic technique. Although recurrent or metastatic DTC tumors grow rather slowly, such tumors consume more glucose than does normal tissue. Consequently, use of [18F]-FDG-PET has been suggested to be valuable in patients who are negative (in terms of tumor recurrence) on conventional imaging but who show elevated Tg levels. The technique has been used to detect both local DTC recurrence and distant metastasis (9, 14-18).
Recently, [124I]-PET has emerged as a valuable diagnostic tool for the detection of recurrent or residual DTC disease, and the data afforded are helpful in the planning of therapy during follow-up of DTC patients (13, 19-23). [124I]-PET imaging may offer a higher sensitivity than the conventional [131I] scan because the spatial resolution of the former modality is greater. Moreover, the recent introduction of combined PET/CT (computed tomography) scanners allows thyroid cancers to be imaged using a high-resolution PET technique. This may increase the clinical application of such imaging in thyroid cancer patients because detailed anatomical information is obtained and iodine-positive tissue can be located (22).
The aim of the present study was to prospectively evaluate the utility of [18F]-FDG-PET/CT and [124I]-PET/CT in detection of recurrent DTC in patients with increasing serum Tg levels but who showed no pathological finding upon conventional imaging such as cervical US and the [131I] scan.
MATERIALS AND METHODS
Inclusion criteria
Between July 2009 and June 2010, 19 patients with histologically proven DTC were studied. All patients had previously undergone total thyroidectomy and more than one session of postoperative RI therapy; the cumulative administered mean dose was 10,905 MBq (range, 5,500-18,500 MBq). At a mean of 16 months (range, 9-41 months) of follow-up after the last RI therapy session, all patients showed increasing pathological Tg levels (Tg > 9-10 ng/mL) after TSH stimulation (TSH > 30 mU/L). However, neither tumor recurrence nor metastasis could be detected in any patient by post-therapeutic [131I] scanning, neck US, or chest radiography. Patients with obvious cervical pathology in US or positive fine-needle aspiration cytology (FNAC) were excluded from the study.
Study protocol
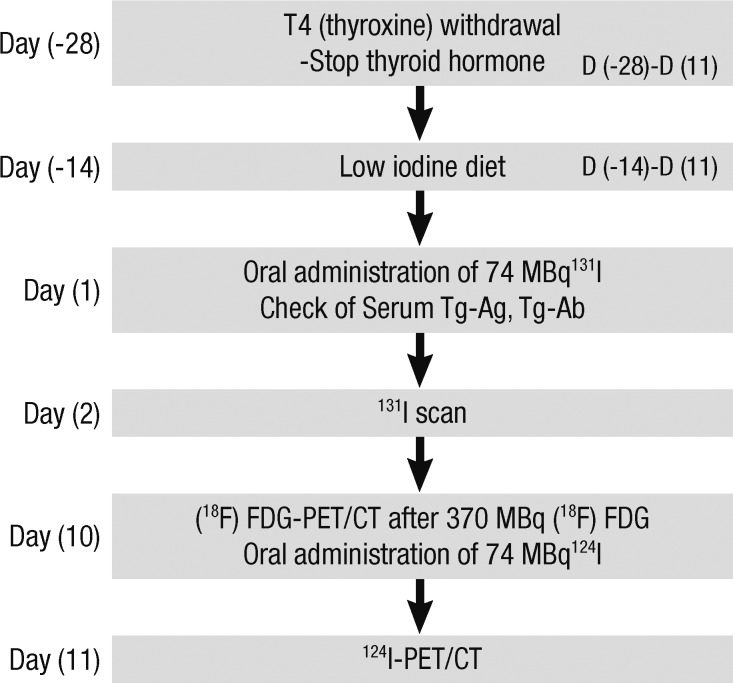
On the first day (D1) of the study, blood and urine were collected for routine examination; to measure blood TSH, Tg, and anti-Tg antibody levels; and to assess urine iodine excretion after 4 weeks of levothyroxine withdrawal (Fig. 1). All patients had consumed a low-iodine diet for the prior 2 weeks, following written instructions and assisted by a dietician. On the second day (D2) of the study, an [131I] scan was obtained 48 hr after administration of 74 MBq of [131I]. On day 10 (D10) of the study, patients fasted for at least 4 hr before examination, and were (intravenously) given 370 MBq [18F]-FDG. All patients were instructed to rest comfortably for 60 min and to empty the bladder before scanning. Whole-body PET/CT images were obtained using a Discovery ST scanner (GE Healthcare, Milwaukee, WI). Seven or eight frames (3 min/frame) of emission PET data were acquired in the two-dimensional mode after noncontrast CT scans had been performed from the base of the skull to the upper thigh (tube rotation time of 1 sec per revolution; 120 kV; 60 mA; 7.5 mm per rotation; and an acquisition time of 60.9 sec for a scan length of 867 mm). Emission PET images were reconstructed via non-contrast CT using an iterative method (ordered-subsets expectation maximization with two iterations and 30 subsets; field of view 600 mm; slice thickness 3.27 mm). Attenuation-corrected PET/CT images were reviewed on an Xeleris workstation (GE Healthcare). All images were independently interpreted by two experienced nuclear medicine physicians and screened for "hot spots" indicative of hypermetabolic abnormalities.
Fig. 1.
Diagnostic and therapeutic flow-chart of treatment performed at least 6 months after high-dose ablation.
On day 11 (D11) of the study, 24 hr after administration of an [124I] trace dose (74 MBq), whole-body PET/CT scans were obtained using the Discovery ST scanner. The [124I] radioisotope was supplied by the Department of Molecular Imaging, Korea Institute of Radiological and Medical Sciences (KIRAMS). First, a non-enhanced CT scan was performed, from the base of the skull to the upper thigh (tube rotation time of 1 sec per revolution; 140 kV; 80 mA; 3.75 mm per rotation; acquisition time of 23.9 sec for a scan length of 804 mm). Subsequently, seven or eight frames (5 min per frame) of emission PET data were acquired in the two-dimensional mode and reconstructed via CT using an iterative method (software from General Electric Medical Systems; ordered-subsets expectation maximization with two iterations and 21 subsets; field of view 600 mm; slice thickness 3.27 mm).
Data analysis
To ensure that the interpretation of [18F]-FDG-PET/CT and [124I]-PET/CT data was performed under similar conditions, all physicians who initially read images of one type were deliberately blinded to the results of the other type of imaging, and to patient clinical data. This was achieved by ensuring that the interpreting physicians were not involved in patient clinical care. Findings on [18F]-FDG-PET and [124I]-PET scanning were compared with data from diagnostic and post-therapeutic [131I] scans. Moreover, the data were compared with those of radiological imaging (US, CT, and MRI information), and/or those of cytological investigation (FNAC), to confirm (or otherwise) the findings of the [131I] scans, and those of both forms of PET/CT (using [18F]-FDG and [124I]). For each patient, the presence or absence, and number and localization of any recurrent lesions (if present) were determined. The data of both types of PET/CT scans were classified as follows: True-positive, if pathologic [18F]-FDG or [124I] uptake was proven by histology, cytology, or other imaging techniques, and, therefore, lead to a restaging of cancer or a change of therapy; False-positive, if pathologic [18F]-FDG or [124I] uptake could not verified (such observations were of no clinical consequence); True-negative, if no [18F]-FDG or [124I] uptake was found and the patient had neither an elevated Tg level (Tg ≤ 1 ng/mL after TSH elevation) nor any evidence of recurrence upon subsequent follow-up; and, False-negative if no [18F]-FDG or [124I] uptake was noted despite elevated Tg levels, even if positive findings were obtained when other imaging methods were employed.
Ethics statement
The work was reviewed and approved by the institutional review board of Ajou University Hospital (#AJIRB-CRO-09-068). Written informed consent was obtained from each patient.
RESULTS
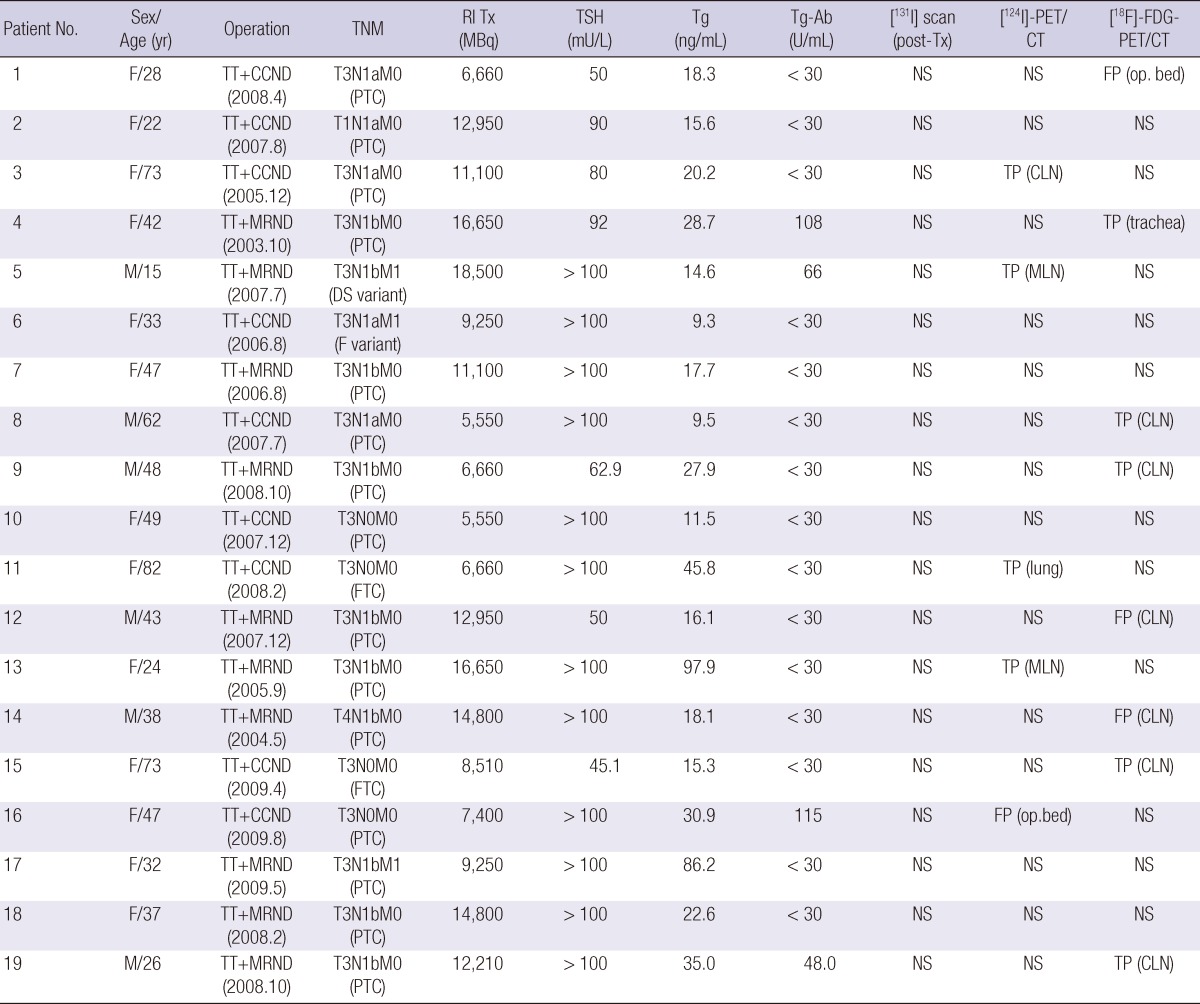
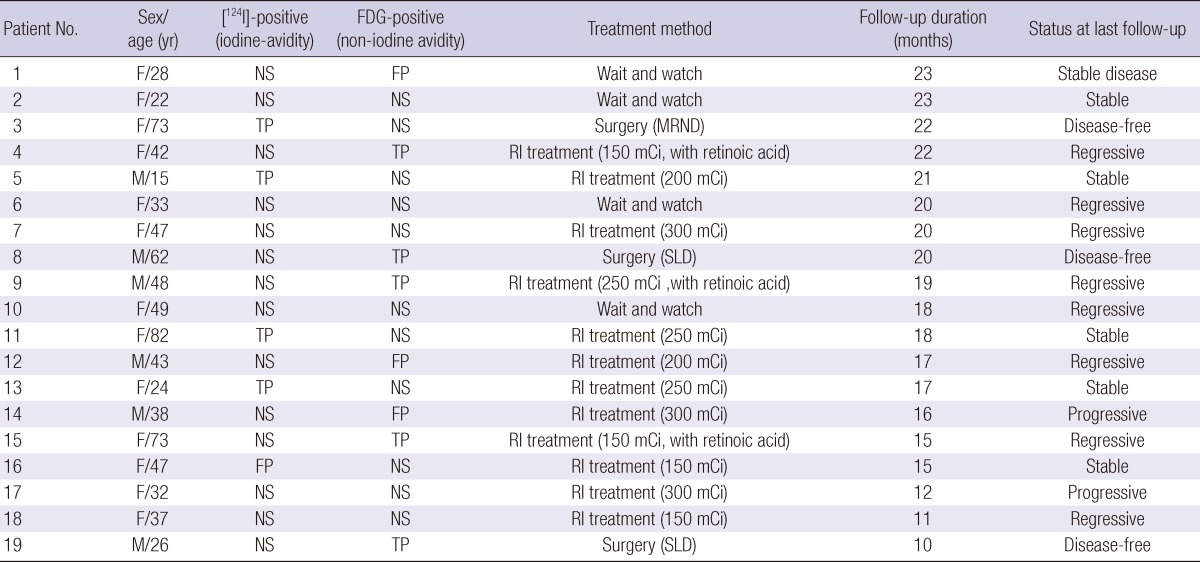
All participants underwent [18F]-FDG-PET/CT and [124I]-PET/CT between July 2009 and July 2010. No pathological lesion was observed in any patient on either the diagnostic or post-therapeutic [131I] scans. The study group consisted of 6 males and 13 females, of a median age of 43 yr (range, 15-82 yr). The characteristics of the 19 participants are listed in Table 1. All patients underwent regular follow-up examinations for at least 10 months after treatment.
Table 1.
Patient characteristics and radiological findings
TT, total thyroidectomy; CCND, central compartment node dissection; NS, no specific finding; FP, false-positive result; PTC, papillary thyroid carcinoma; TP, true-positive result; CLN, cervical lymph node; MRND, modified radical neck dissection; DS variant, diffuse sclerosing variant of PTC; MLN, mediastinal lymph node; F variant, follicular variant of PTC; FTC, widely invasive follicular carcinoma.
Nine of our 19 patients (47.4%) showed pathological [18F]-FDG or [124I]-PET uptake, and were classified as true positives. The treatment strategies for six of the nine patients (66.7%) were changed immediately. In 10 patients (52.6%), no uptake was observed on either scan. Validation of the [18F]-FDG-PET/CT and [124I]-PET/CT findings is summarized in Table 1.
[18F]-FDG-PET/CT vs [124I]-PET/CT
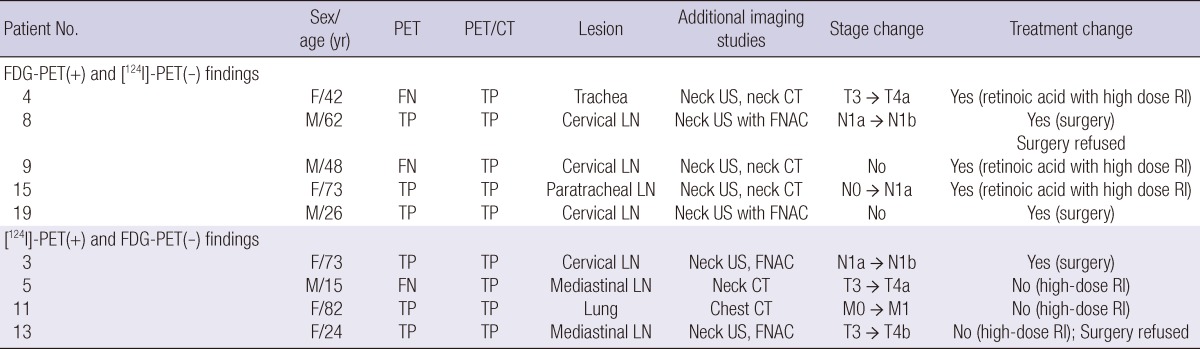
Eight patients showed uptake by [18F]-FDG-PET; however, only five patients (62.5%) were true positives. Two patients underwent further surgery, whereas three received high-dose RI therapy after preparation with retinoic acid. Also, [18F]-FDG-PET correctly restaged three of five patients (60.0%). In contrast, the remaining three patients (37.5%) were false-positives upon FDG-PET imaging. This was confirmed by histological findings or via use of other radiological information, and subsequent measurement of Tg levels during follow-up. The details of the additional radiological imaging tests conducted (neck CT/MRI, chest CT, or FNAC) are listed in Table 2. Fig. 2 shows [18F]-FDG-PET/CT images of a patient who had an elevated Tg level but who was devoid of any definite within-tissue [131I] concentration post-therapeutically. No definite [131I]-positive lesion was found, but [18F]-FDG-PET-positive cervical lymph node metastasis was detected, and precisely located via image fusion with CT scan data.
Table 2.
Clinicopathological characteristics and treatment outcomes of patients with FDG-PET- or [124I]-PET-positive recurrence
FN, false-negative result; TP, true-positive result; RI, radioactive iodine treatment.
Fig. 2.
Patient 8: [18F]-FDG-PET/CT data from a patient with an elevated Tg level but who was tumor-negative on both neck US and a post-therapy [131I] scan. [131I]-negative [18F]-FDG-positive metastases are evident in the cervical lymph node (A), and the CT scan affords exact localization (B).
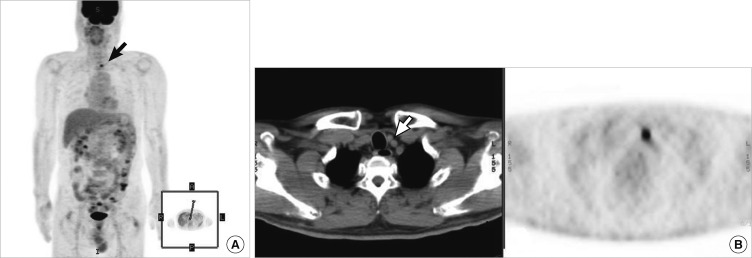
Five patients showing uptake on [124I]-PET/CT scans had lesions that were not visible on post-treatment [131I] scans. Of these patients, four (80.0%) were confirmed to have recurrence, using either histological or other radiological means. One patient underwent further surgery and three received high-dose radiation therapy. Moreover, [124I]-PET correctly restaged all four patients. A false-positive finding was recorded for only one patient; the FNAC test and other imaging data were negative. Fig. 3 shows data on a patient in whom a small cervical lymph node metastasis was not seen upon post-therapeutic [131I] ablation scanning or neck US, but was clearly identified in the [124I]-PET/CT image. Fig. 4 shows that metastasis localization is improved upon use of [124I]-PET compared with conventional imaging, indicating that lung metastasis not found upon post-therapeutic [131I] scanning or chest radiography was visible in the [124I]-PET scan. Also, in three patients (patients 4, 5, and 9) both types of PET/CT, thus using either [18F]-FDG or [124I], were more accurate in terms of disease localization than was PET scanning alone, and the PET/CT results caused the treatment of patients 4 and 9 to be changed (Table 2).
Fig. 3.
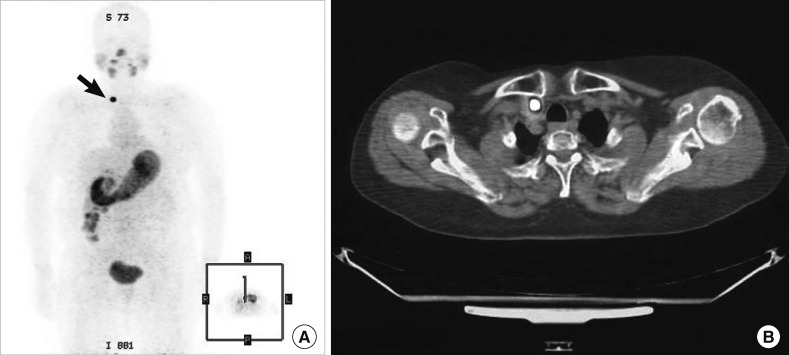
Patient 3: [124I]-PET/CT data from a patient with an elevated Tg level but who was tumor-negative on both neck US and a post-therapy [131I] scan. (A) The post-therapy [131I] scan did not clearly reveal the cervical lymph node metastasis evident on the [124I]-PET scan. (B) Exact localization of the lesions of lateral lymph node metastasis, obtained by fusion of the image with that of the CT scan.
Fig. 4.
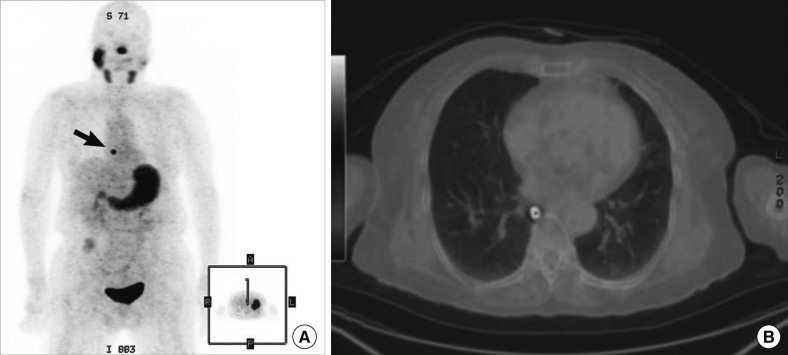
Patient 11: [124I]-PET showed that lung metastases were present; the patient had widely invasive follicular carcinoma. In this patient, no definite positive lesion was evident upon either the diagnostic or post-therapy [131I] scans, despite the presence of an elevated Tg level. The [124I]-PET scan showed definite lung metastases (A), and [124I]-PET/CT precisely localized the lesions (B).
Treatment modalities and follow-up
Based on radiological findings, 15.8% (3/19) of all patients underwent surgery. One patient was negative on [18F]-FDG-PET/CT but showed metastasis on [124I]-PET/CT, whereas the other two patients exhibited local recurrence only on [18F]-FDG-PET/CT. These patients were all disease-free on further follow-up. Tumor response was demonstrated during follow-up (Table 3). The treatment outcomes were: stable disease in six patients, regressive disease in eight, progressive disease in two, and disease-free status in all three patients who underwent surgical treatment.
Table 3.
Treatment methods and status at last follow-up
SLD, selective lymph node dissection; Disease-free, Negative on any kind of imaging study and serum Tg ≤ 1 ng/mL (TSH stimulation); Regressive, Reduced tumor volume on imaging study or decreased serum Tg level; Stable, No change on imaging study and serum Tg level; Progressive, Expanded tumor volume on imaging study or increased serum Tg level.
DISCUSSION
This is the initial report on the results of a prospective study on the effectiveness of PET/CT using both [124I] and [18F]-FDG to locate recurrent DTC lesions in patients with elevated thyroglobulin levels but who do not show pathological lesions when conventional imaging modalities are used. The present study showed that the use of a combination of [124I]-PET/CT and [18F]-FDG-PET/CT revealed the complete extent of the disease and afforded precise anatomical localization of iodine-positive and iodine-negative lesions in 9 of the 19 patients (47.4%). Thus, if [18F]-FDG-PET/CT and [124I]-PET/CT were performed before repeated high-dose RI treatment was considered, such an evaluation would facilitate diagnostic accuracy and might avoid unnecessary exposure of patients to radiation.
Some patients showed increased levels of Tg but conventional imaging tests (the [131I] scan, neck US, and/or chest radiography) do not reveal a pathological lesion. Therefore, other methods were required to locate the disease. In patients with elevated Tg levels, empirical high-dose RI treatment may be indicated, followed by a post-treatment [131I] scan; this is also a useful diagnostic method. However, repeated empirical treatment with RI may unnecessarily expose patients to high levels of radiation; this may be a particular problem in patients who show no [131I] uptake on the post-treatment scan (7-9, 14-18). Therefore, an advanced diagnostic imaging technique, such as [124I]-PET/CT or [18F]-FDG-PET/CT, that provides anatomical localization data, is required. The information provided by such scans is necessary to allow physicians to accurately locate metastatic lesions and to indicate the most efficient therapeutic options. In our patient series, we showed that combination PET/CT using [124I] and [18F]-FDG was more accurate in terms of lesion localization than was conventional imaging, in 9 of our 19 patients (47.4%).
Currently, [18F]-FDG-PET/CT is recognized as useful in examination of DTC patients who present with negative [131I] scan data but pathologically increased Tg levels (14-18). Moreover, any difference between PET findings and conventional [131I] scan information can provide important clinical clues relevant to treatment of recurrent DTC. However, no complete consensus has yet been achieved on whether [18F]-FDG-PET/CT has a diagnostic utility independent of that of Tg level, or even if the scan data are associated with Tg concentration. Some authors have reported that TSH elevation seems to improve scan sensitivity. In such patients, the incremental value afforded by hormonal stimulation of TSH is great because lesions that might be missed owing to their small size or low metabolic activity become apparent (25, 26). This is why TSH significantly increases [18F]-FDG uptake in DTC patients experiencing recurrence; TSH stimulates GLUT-1 expression and thus indirectly increases glucose transport. The stimulation afforded by TSH is real. However, other reports found no increase in the accuracy (sensitivity) of scan data obtained after hormonal TSH stimulation (27, 28). Accordingly, no consensus on the effect of TSH stimulation on FDG-PET accuracy has been attained and it is important to render scanning cost-effective (18). In the present study, the results of PET/CT using [18F]-FDG after TSH stimulation increased diagnostic confidence, resulted in less uncertainty than is associated with interpretation of the data of conventional imaging; yielded additional information on approximately 26.3% of patients (5/19), and triggered changes in therapeutic management of all such patients (for example, patient 8; Fig. 2).
Iodine-124 is a positron-emitting isotope and is therefore suitable for use in PET imaging. The half-life is 4.2 days and the decay scheme is complex, resulting in additional radiation coincidence detection that is either non-destructive in terms of image quality or partially destructive (29, 30). Recently, the isotope has been used for thyroid cancer imaging using high-resolution PET, and a combination of [124I]-PET and CT increased the clinical application of such a modality in thyroid cancer patients; detailed anatomical information and the location of iodine-positive tissue are provided (19-22, 29, 30). The [124I] isotope has also been used in dosimetric studies and for thyroid volume measurement (13, 20-23). Most reports found that [124I]-PET scanning was useful in terms of DTC staging before RI treatment, and did not materially affect the therapeutic radiation dose (18, 20-23). Only a few studies have to date evaluated the efficacy of [124I]-PET in localization of tumor recurrence during follow-up of DTC patients after ablation (19-21). Therefore, we focused on this topic. We found that the combination of [124I]-PET with CT considerably (21.1%; 4/19 patients) improved the ability to detect recurrent lesions compared with conventional imaging.
Why is [124I]-PET/CT an appropriate diagnostic method when empirical high-dose RI treatment is recognized to be valuable in clinical practice? RI treatment of patients negative on the low-dose diagnostic [131I] scan but with elevated Tg levels has been used diagnostically, therapeutically, and prognostically (21). In addition, patients who show no iodine accumulation on post-treatment [131I] scanning (which may indicate tumor dedifferentiation) have a poorer prognosis than do others (31). However, repeat high-dose RI treatment may be associated with a long-term risk of secondary malignancy or infertility. The total radiation exposure during an [124I]-PET scan has been calculated to be 7.0 mSv, at a dose of 74 MBq [124I]. This is similar to the dose used in routine nuclear medicine scans. However, the total exposure to [124I] is very low compared with that from the therapeutic dose of [131I] (340 mSv from a dose of 5,500 MBq) (31). Therefore, if [124I]-PET/CT is performed before empirical repeat RI treatment, the total radiation exposure is markedly decreased. Our results show that such an approach avoids unnecessary delivery of high-dose radiation to patients with recurrence when existing locoregional metastases are not detected by the [131I] scan (for example, in patient 3; Fig. 3). The technique yields useful information when the iodine-avidity of a distant metastatic region has not previously been reliably assessed (for example, in patient 11; Fig. 4).
Our study had several limitations. First, we enrolled only a small number of participants, and a large prospective trial is required to definitively evaluate our data. Second, our follow-up period was relatively short; years or decades of follow-up must pass before statistically reliable data can be obtained; this reflects the epidemiological and prognostic features of DTC. Finally, it is important to establish uniform standards for the conduct of scans, and to develop guidelines permitting comprehensive interpretation of test results, before the possible superiority of our [18F]-FDG-PET-plus-[124I]-PET combination can be precisely compared with traditional follow-up protocols. The technique should be offered to only well-informed patients who thoroughly understand the advantages, limitations, and downside of determining cancer location. Our further studies will address these issues. Also, the histopathological and cytopathological characteristics associated with the ability of a tumor to avidly take up iodine, glucose, or both, require investigation.
Our findings showed that both [124I]-PET/CT and [18F]-FDG-PET/CT are useful diagnostic tools when recurrence occurs in DTC patients who have elevated Tg levels but no definitive abnormalities by conventional imaging. Moreover, a combination of the two modalities provides an even greater diagnostic capacity, by which therapeutic decisions on additional surgery or RI treatment can be informed. However, it should be also noticed that considerable number of patients could not be detected by these imaging techniques, and therefore, continuing and further studies are still required.
Footnotes
This work was supported by Nuclear Research & Development Program of the Korea Science and Engineering Foundation (KOSEF) (M20702010001-07N0201-00110) and Quri Project grant funded by the Korean government (MEST) (grant code: M14943).
References
- 1.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 2.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1058. [PubMed] [Google Scholar]
- 3.Lin JD, Huang MJ, Juang JH, Chao TC, Huang BY, Chen KW, Chen JY, Li KL, Chen JF, Ho YS. Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid. 1999;9:1227–1235. doi: 10.1089/thy.1999.9.1227. [DOI] [PubMed] [Google Scholar]
- 4.Wartofsky L, Sherman SI, Gopal J, Schlumberger M, Hay ID. The use of radioactive iodine in patients with papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 1998;83:4195–4203. doi: 10.1210/jcem.83.12.5293-1. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger MJ. Diagnostic follow-up of well-differentiated thyroid carcinoma: historical perspective and current status. J Endocrinol Invest. 1999;22:3–7. [PubMed] [Google Scholar]
- 6.Galloway RJ, Smallridge RC. Imaging in thyroid cancer. Endocrinol Metab Clin North Am. 1996;25:93–113. doi: 10.1016/s0889-8529(05)70314-5. [DOI] [PubMed] [Google Scholar]
- 7.Lubin E, Mechlis-Frisch S, Zatz S, Shimoni A, Segal K, Avraham A, Levy R, Feinmesser R. Serum thyroglobulin and iodine-131 whole-body scan in diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. J Nucl Med. 1994;35:257–262. [PubMed] [Google Scholar]
- 8.Samaan NA, Schultz PN, Haynie TP, Ordonez NG. Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab. 1985;60:376–380. doi: 10.1210/jcem-60-2-376. [DOI] [PubMed] [Google Scholar]
- 9.Schlüter B, Bohuslavizki KH, Beyer W, Plotkin M, Buchert R, Clausen M. Impact of FDG PET on patients with differentiated thyroid cancer who present with elevated thyroglobulin and negative 131I scan. J Nucl Med. 2001;42:71–76. [PubMed] [Google Scholar]
- 10.Krishnamurthy S, Bedi DG, Caraway NP. Ultrasound-guided fine-needle aspiration biopsy of the thyroid bed. Cancer. 2001;93:199–205. doi: 10.1002/cncr.9029. [DOI] [PubMed] [Google Scholar]
- 11.Frilling A, Görges R, Tecklenborg K. Value of preoperative diagnostic modalities in patients with recurrent thyroid carcinoma. Surgery. 2000;128:1067–1074. doi: 10.1067/msy.2000.110771. [DOI] [PubMed] [Google Scholar]
- 12.Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, Lippi F, Taddei D, Grasso L, Pinchera A. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 13.Lubberink M, Abdul Fatah S, Brans B, Hoekstra OS, Teule GJ. The role of (124)I-PET in diagnosis and treatment of thyroid carcinoma. Q J Nucl Med Mol Imaging. 2008;52:30–36. [PubMed] [Google Scholar]
- 14.Freudenberg LS, Frilling A, Kühl H, Müller SP, Jentzen W, Bockisch A, Antoch G. Dual-modality FDG-PET/CT in follow-up of patients with recurrent iodine-negative differentiated thyroid cancer. Eur Radiol. 2007;17:3139–3147. doi: 10.1007/s00330-007-0682-2. [DOI] [PubMed] [Google Scholar]
- 15.Iagaru A, Kalinyak JE, McDougall IR. F-18 FDG PET/CT in the management of thyroid cancer. Clin Nucl Med. 2007;32:690–695. doi: 10.1097/RLU.0b013e318125037a. [DOI] [PubMed] [Google Scholar]
- 16.Palmedo H, Bucerius J, Joe A, Strunk H, Hortling N, Meyka S, Roedel R, Wolff M, Wardelmann E, Biersack HJ, et al. Integrated PET/CT in differentiated thyroid cancer: diagnostic accuracy and impact on patient management. J Nucl Med. 2006;47:616–624. [PubMed] [Google Scholar]
- 17.Zuijdwijk MD, Vogel WV, Corstens FH, Oyen WJ. Utility of fluorodeoxyglucose-PET in patients with differentiated thyroid carcinoma. Nucl Med Commun. 2008;29:636–641. doi: 10.1097/MNM.0b013e3282f813e1. [DOI] [PubMed] [Google Scholar]
- 18.Bertagna F, Bosio G, Biasiotto G, Rodella C, Puta E, Gabanelli S, Lucchini S, Merli G, Savelli G, Giubbini R, et al. F-18 FDG-PET/CT evaluation of patients with differentiated thyroid cancer with negative I-131 total body scan and high thyroglobulin level. Clin Nucl Med. 2009;34:756–761. doi: 10.1097/RLU.0b013e3181b7d95c. [DOI] [PubMed] [Google Scholar]
- 19.Freudenberg LS, Antoch G, Frilling A, Jentzen W, Rosenbaum SJ, Kühl H, Bockisch A, Görges R. Combined metabolic and morphologic imaging in thyroid carcinoma patients with elevated serum thyroglobulin and negative cervical ultrasonography: role of 124I-PET/CT and FDG-PET. Eur J Nucl Med Mol Imaging. 2008;35:950–957. doi: 10.1007/s00259-007-0634-8. [DOI] [PubMed] [Google Scholar]
- 20.Freudenberg L, Jentzen W, Müller SP, Bockisch A. Disseminated iodineavid lung metastases in differentiated thyroid cancer: a challenge to 124I PET. Eur J Nucl Med Mol Imaging. 2008;35:502–508. doi: 10.1007/s00259-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 21.Phan HT, Jager PL, Paans AM, Plukker JT, Sturkenboom MG, Sluiter WJ, Wolffenbuttel BH, Dierckx RA, Links TP. The diagnostic value of 124I-PET in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:958–965. doi: 10.1007/s00259-007-0660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capoccetti F, Criscuoli B, Rossi G, Ferretti F, Manni C, Brianzoni E. The effectiveness of 124I PET/CT in patients with differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2009;53:536–545. [PubMed] [Google Scholar]
- 23.Jentzen W, Freudenberg L, Eising EG, Sonnenschein W, Knust J, Bockisch A. Optimized 124I PET dosimetry protocol for radioiodine therapy of differentiated thyroid cancer. J Nucl Med. 2008;49:1017–1023. doi: 10.2967/jnumed.107.047159. [DOI] [PubMed] [Google Scholar]
- 24.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 25.Chin BB, Patel P, Cohade C, Ewertz M, Wahl R, Ladenson P. Recombinant human thyrotropin stimulation of fluoro-D-glucose positron emission tomography uptake in well-differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2004;89:91–95. doi: 10.1210/jc.2003-031027. [DOI] [PubMed] [Google Scholar]
- 26.Filetti S, Damante G, Foti D. Thyrotropin stimulates glucose transport in cultured rat thyroid cells. Endocrinology. 1987;120:2576–2581. doi: 10.1210/endo-120-6-2576. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz Franco-Baux JV, Borrego Dorado I, Gómez Camarero P, Rodríguez Rodríguez JR, Vázquez Albertino RJ, Navarro González E, Astorga Jiménez R. F-18-Fluorodeoxyglucose positron emission tomography on patients with differentiated thyroid cancer who present elevated human serum thyroglobulin levels and negative I-131 whole body scan. Rev Esp Med Nucl. 2005;24:5–13. doi: 10.1157/13070351. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Macapinlac H, Larson SM, Yeh SD, Akhurst T, Finn RD, Rosai J, Robbins RJ. [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic (131)I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab. 1999;84:2291–2302. doi: 10.1210/jcem.84.7.5827. [DOI] [PubMed] [Google Scholar]
- 29.Pentlow KS, Graham MC, Lambrecht RM, Daghighian F, Bacharach SL, Bendriem B, Finn RD, Jordan K, Kalaigian H, Karp JS, et al. Quantitative imaging of iodine-124 with PET. J Nucl Med. 1996;37:1557–1562. [PubMed] [Google Scholar]
- 30.Lambrecht RM, Woodhouse N, Phillips R, Wolczak D, Qureshi A, Reyes ED, Graser C, Al-Yanbawi S, Al-Rabiah A, Meyer W. Investigational study of iodine-124 with a positron camera. Am J Physiol Imaging. 1988;3:197–200. [PubMed] [Google Scholar]
- 31.van Tol KM, Jager PL, de Vries EG, Piers DA, Boezen HM, Sluiter WJ, Dullaart RP, Links TP. Outcome in patients with differentiated thyroid cancer with negative diagnostic whole-body scanning and detectable stimulated thyroglobulin. Eur J Endocrinol. 2003;148:589–596. doi: 10.1530/eje.0.1480589. [DOI] [PubMed] [Google Scholar]