Abstract
The metabolic syndrome (MetS) is the clustering of cardiovascular risk factors and known as a powerful predictor of diabetes and cardiovascular disease. Glycated hemoglobin (HbA1c) is used as one of the diagnostic criteria for diabetes and category of increased risk for diabetes. We examined the usefulness of HbA1c as a diagnostic tool for MetS and to determine the cut-off value of HbA1c as a criterion for MetS, in non-diabetic Korean subjects. We analyzed 7,307 participants (male: 4,181, 57%) in a medical check-up program, and applied the newly recommended guidelines of the International Diabetes Federation for diagnosis of MetS. The mean HbA1c was 5.54% in all subjects and showed no significant difference between genders. Using receiver-operating characteristic curve, HbA1c value corresponding to the fasting plasma glucose value of 100 mg/dL was 5.65% (sensitivity 52.3%, specificity 76.7%). The prevalence of MetS was 8.5% according to the IDF guideline and 10.9% according to HbA1c value of 5.7%, showing 69.5% agreement rate. The detection rate of MetS increased to 25.7% using the HbA1c criterion of 5.7% instead of fasting hyperglycemia. This study suggests that HbA1c might be used as a diagnostic criterion for MetS and the appropriate cut-off value of HbA1c may be 5.65% in this Korean population.
Keywords: Metabolic Syndrome, Fasting Hyperglycemia, HbA1c
INTRODUCTION
The metabolic syndrome (MetS) is the clustering of closely related cardiovascular risk factors (1), such as obesity, dyslipidemia, hypertension and hyperglycemia and is known as a powerful predictor of diabetes and cardiovascular disease (CVD) (2, 3). It was reported that number of MetS component was related to the severity of coronary atherosclerosis especially in non-diabetic patients (4). The underlying pathophysiology of MetS is insulin resistance, but direct measurement of insulin sensitivity is complex and not readily available. Instead, various anthropometric, hemodynamic, and biochemical parameters have been used to diagnose MetS (5). Recently, a harmonizing definition of MetS was proposed in a joint statement by several organizations in 2009 (6). However, the fasting plasma glucose (FPG) cut-off values for MetS and diabetes may be different between populations. In a study involving middle-aged Koreans, it was suggested that the risk of diabetes starts to increase at the FPG level of below 6.1 mM/L (7).
Glycated hemoglobin (HbA1c) was proposed to be used as one of the diagnostic criteria for diabetes and category of increased risk for diabetes including impaired glucose tolerance (IGT) and impaired fasting glucose (IFG). They proposed HbA1c value of 5.7% to 6.4% to be considered a category of increased risk for diabetes (IRD) (8). Recently, it was reported that although HbA1c criteria alone identified 42% fewer subjects with IRD than does FPG criteria, about 20% more subjects could be detected by including new HbA1c criteria in addition to FPG criteria among Koreans. And they suggested that addition of new HBA1c criteria might be useful in detecting the subjects with IRD (9). HbA1c represents both fasting and postprandial glycemic states and is an index of mean blood glucose. Therefore, HbA1c has traditionally been used in monitoring the long-term glycemic control. But recently, measurement of HbA1c has become standardized and facilitated as a diagnostic tool for diabetes as well as for determining the high risk of diabetes. Use of HbA1C has some advantages over the FPG test and the oral glucose tolerance test (OGTT) because of its convenience of not requiring fasting sample, requiring less time and its superior technical attributes (8). HbA1C has been shown not only to be a predictor of diabetes development (10, 11) but also an important surrogate for cardiovascular disease in both cross-sectional and longitudinal studies (12, 13).
The prevalence of diabetes in some ethnics may not show an agreement between the two different diagnostic criteria based on the level of HbA1c or FPG, and moreover, one method may identify different individuals than the other does because HbA1c level reflects different aspects of glucose metabolism in different individuals. There are several reports proposing that HbA1c is better than FPG in predicting cardiovascular risk and mortality even in non-diabetic subjects (14-16). And there are several proposals suggesting that HbA1c could be an important marker for MetS, though it remains debatable (17-19).
The aims of this study are to examine the usefulness of HbA1c as a diagnostic tool for MetS and to determine the cut-off value of HbA1c for use as a criterion for MetS, particularly in non-diabetic Korean subjects.
MATERIALS AND METHODS
Subjects
We conducted a cross-sectional study of 8,346 participants in the annual medical check-up program of the Health Promotion Center of Yeungnam University Hospital from January 2009 to August 2010.
Patients with diabetes mellitus or taking glucose-lowering agents were excluded and those with diabetes newly identified during screening (HbA1c ≥ 6.5% or FPG > 126 mg/dL), chronic kidney disease (GFR < 60 mL/min/1.73 m2) and anemia (Hgb < 12 g/dL) were also excluded from the study. Finally, 7,307 subjects were included in the study.
Diagnostic criteria of MetS
We applied the newly recommended guidelines of the International Diabetes Federation (IDF) for diagnosis of MetS (20). They include central obesity defined with obligatory Asian-specific cut points of waist circumference (≥ 90 cm in male, ≥ 80 cm in female) plus any two of the following four factors: 1) Hypertriglyceridemia ≥ 150 mg/dL, or specific treatment for this lipid abnormality; 2) Low HDL-C < 40 mg/dL in male and 50 mg/dl in female, or specific treatment for this lipid abnormality; 3) Elevated blood pressure ≥ 130/85 mmHg, or treatment of previously diagnosed hypertension; 4) Raised FPG ≥ 100 mg/dL.
Analytical methods
The height, body weight (BW) and waist circumference (WC) were measured and body mass index (BMI) was calculated from dividing the weight (kg) with height square (m2). WC was measured on standing participants with a soft tape midway between the lowest rib and the iliac crest. Systolic and diastolic blood pressures (BP) were measured with a standard sphygmomanometer after at least 10 min of rest. The subjects' blood was drawn for the evaluations of metabolic, biochemical, and hematological parameters after a 10-12 hr overnight fasting. FPG, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), blood urea nitrogen (BUN), creatinine were measured using the hexokinase method (AU 5400 Autoanalyser; Olympus, Tokyo, Japan) and the serum hs-CRP was measured with the Latex agglutination method and analyzed with Olympus AU5400 automatic analyzer (Olympus Corp., Tokyo, Japan). HbA1c was measured with high-performance liquid chromatography (HLC-723 G7; Tosoh Corporation, Tokyo, Japan) according to the standardized Diabetes Control and Complications Trial assay.
Statistical methods
All statistical analyses were performed using the statistical program SPSS for Windows ver. 18.0 (SPSS Inc., Chicago, IL, USA). Results are expressed as mean ± standard deviation (SD). Student's t-test (unpaired) and chi-square test were performed for comparisons of parameters between groups. The predictive values for the prediction of IFG and MetS were obtained using the receiver-operating characteristic (ROC) curve and the area under the curve (AUC). The optimal cut-off point was defined as the closest point on the ROC curve to the point where 1-specificity was 0 and sensitivity was 100%. Confidence intervals were set to 95% and the result with P < 0.05 was considered significant.
Ethics statement
The protocol was approved by the institutional review board of Yeungnam University Medical Center (YUH-12-03-04). Informed consent was exempted by the board by reason of retrospective study.
RESULTS
Baseline characteristics of clinical and metabolic parameters
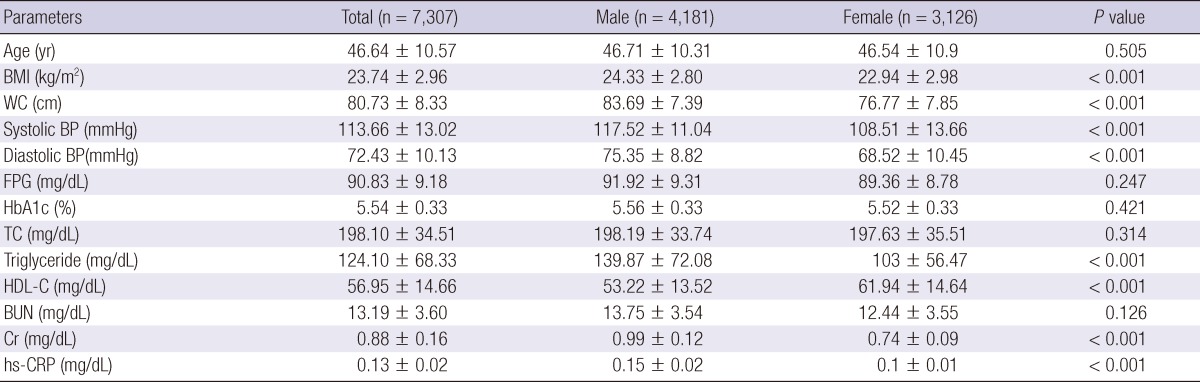
Initial 8,346 subjects were screened to exclude the individuals with history of diabetes (n = 75), with newly diagnosed diabetes (n = 867) and with renal disease or anemia (n = 97); and 7,307 subjects were finally included in the analyses. Males were 4,181 (57%) and the mean age of all subjects was 46.6 yr. Age distribution was the highest in the age of forties.
Waist circumference, BMI, systolic/diastolic BP, triglyceride and hs-CRP were significantly higher, whereas the HDL-C was significantly lower in males. Mean HbA1c value was 5.54% in all subjects; 5.56% in males and 5.52% in females showing no significant difference between genders (Table 1). BP, total cholesterol, triglyceride and FPG were found positively related with HbA1c (P < 0.001), whereas HDL-C was negatively related with HbA1c (P < 0.001) in both male and female subjects (Table 2).
Table 1.
Clinical characteristics of the subjects
mean ± SD, P value with male. BMI, body mass index; BP, blood pressure; WC, waist circumference; FPG, fasting plasma glucose; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; BUN, blood urea nitrogen; Cr, creatinine; hs-CRP, high sensitive C-reactive protein.
Table 2.
Correlation between HbA1c and cardiovascular risk factors in total subjects
mean ± SD. BP, blood pressure; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; FPG, fasting plasma glucose.
Cut-off value for the prediction of IFG and MetS
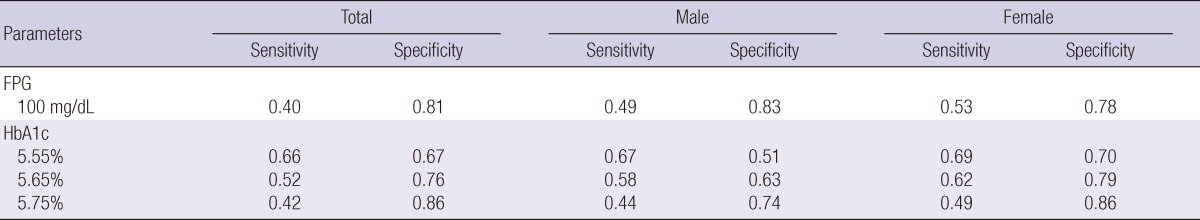
ROC curve were used to obtain the HbA1c value corresponding to the FPG value of 100 mg/dL. Fasting hyperglycemia, i.e. the cut-off value of FPG of 100 mg/dL, predicted the presence of MetS with 40.4% sensitivity and 81.2% specificity, and the corresponding HbA1c was 5.65% with 52.3% sensitivity and 76.7% specificity. When analyzed for each gender, the result showed the same corresponding HbA1c value of 5.65% with 58.6% sensitivity and 63.8% specificity in males and 62.3% and 79.1% respectively in females (Table 3).
Table 3.
Cut-off values of HbA1c for MetS from ROC analysis
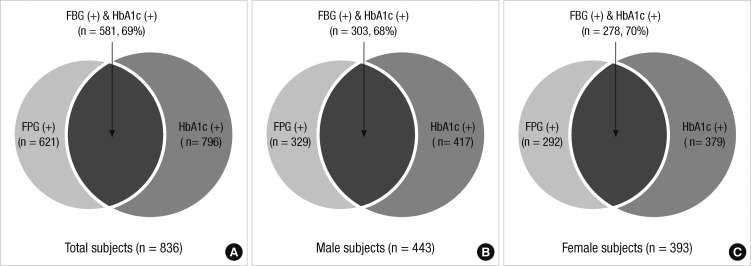
The prevalence of MetS was compared between the categories of the FPG value of 100 mg/dL and the HbA1c value of 5.65%. According to the IDF Guideline which specifies the FPG value of 100 mg/dL, the prevalence of MetS was 8.5% (621/7,307) in all subjects; 7.9% (329/4,181) in males and 9.3% (292/3,126) in females. Using the HbA1c value of 5.7% as the diagnostic criteria, the prevalence of MetS were 10.9% (796/7,307) in all subjects; 10.0% (417/4,181) in males and 12.1% (379/3,126) in females. Among all subjects, 175 subjects (2.4%) were additionally diagnosed with MetS using the criterion of HbA1c ≥ 5.7%. Of 836 subjects diagnosed with MetS based on the criterion of FPG or HbA1c, 581 (69.5%) met both FPG criterion of 100 mg/dL and HbA1c criterion of 5.65% simultaneously. On the other hand, 215 subjects among those diagnosed with MetS met only the HbA1c criterion of 5.65% but not the FPG criterion (Fig. 1). Therefore, the detection rate of MetS increased to 25.7% using the HbA1c criterion of 5.65% instead of fasting hyperglycemia.
Fig. 1.
Prevalence of metabolic syndrome in total subjects, male and female according to modified IDF criteria.
DISCUSSION
HbA1c, a product of nonenzymatic glycosylation of the β-chain of hemoglobin, is formed in proportion to the increases in plasma glucose levels. It has become a preferred tool because HbA1c assay has better technical benefits than the measurement of plasma glucose, and it has less biologic variability and is clinically more convenient (21). However, there are some limitations in interpretation of HbA1c. HbA1c is frequently misleading in patients with some types of anemia and hemoglobinopathies and there are some problems in standardization of HbA1c measurement (8). However, the worldwide standardization of HbA1c measurement has significantly reduced the bias between them (22).
A considerable number of epidemiologic studies have indicated that postprandial hyperglycemia commonly precedes fasting hyperglycemia in the transition from normal glucose tolerance to overt diabetes (23). And postprandial hyperglycemia contributes to the level of HbA1c more than fasting hyperglycemia does as HbA1c level increases through the normal range (24).
In our study, the prevalence of MetS was 8.5% based on fasting hyperglycemia by the IDF guideline, while it was 10.9% based on the HbA1c level of 5.65% as a diagnostic criterion. In other words, using the criterion of HbA1c ≥ 5.65%, more than 2.4% of all subjects could be diagnosed as having MetS. Therefore, diagnosis of MetS based only on fasting hyperglycemia may involve some risk to miss the high risk subjects for CVD.
Many epidemiological data showed that glycated hemoglobin is an independent risk factor for CVD with no apparent threshold, while FPG is not. In a community-based study of non-diabetic elderly who were followed for an average of 8 yr, HbA1c, but not FPG, was significantly related to CVD and IHD mortality in women without diabetes but not in men (15). The Hoorn study was performed to determine the predictive value of FPG, 2PPPG and HbA1c in a population-based cohort of older subjects without known diabetes that followed up for 8 yr (16). After the exclusion of subjects with newly diagnosed diabetes or with pre-existent cardiovascular disease, a 1.4% increase of HbA1c was associated with a higher age-adjusted and sex-adjusted risk of all-causes (RR 1.61) and cardiovascular mortality (RR 2.12). Therefore, they suggested that HbA1c is a better predictor of CVD and IHD mortality than FPG even in subjects without diabetes. Our study also showed that HbA1c was significantly related to several CVD risk factors including BMI, WC, BP, TC, FPG and HDL-C and could support the significance of HbA1c as a surrogate for CVD.
However, few studies have investigated the usefulness of HbA1c as a predictor of MetS. There was a study including 219 non-diabetic, obese, first-degree relatives of African-American patients with type 2 diabetes to examine the significance of HbA1c in MetS (17). HbA1c was divided into tertiles as follows: 4.7% (3.3%-4.8%) for tertile 1, 5.4% (4.9%-5.6%) for tertile 2, and 5.8% (5.7%-6.4%) for tertile 3. The metabolic abnormalities in the upper tertile 3 subjects included a reduced insulin action and reduced glucose effectiveness, as well as elevated systolic and diastolic BPs. These results demonstrated that HbA1c could be considered as a surrogate marker of MetS. But it remains to be elucidated whether these findings could be extrapolated to other racial and ethnic populations who are not genetically predisposed to diabetes. In our study, HbA1c value corresponding to FPG value of 100 mg/dL was 5.65% in Korean subjects, which was the most appropriate cut-off value for the identification of MetS in this population. The agreement rate between these two criteria was 69.5%. This value of HbA1c was similar to the result by Ong et al. (18) in which there was 91.3% agreement between HbA1c level of 5.7% and FPG level of 100 mg/dL when either was used in diagnosing MetS. Sung et al. reported that HbA1c level of 5.45% (sensitivity 53.7%, specificity 70%) was able to predict the presence of MetS defined according to both ATPIII and IDF guidelines in Korean subjects (25). Another study reported the cut-off value of HbA1c for MetS in Korean subjects was 5.35% (26). They also showed gender difference in the cut-off value of HbA1c for MetS, i.e. higher HbA1c in females compared with males. Therefore, they recommended that cut-off values should be determined separately for men and women. However, we were unable to determine gender difference in our study. All these discrepancies in cut-off values and gender difference between the studies are probably due to the differences of age distribution, the use of different methods of HbA1c assay even though they were standardized, and the different mean value of HbA1c, namely, higher one in our study.
Our study has several practical limitations. First, we excluded the subjects with diabetes as well as those with newly diagnosed diabetes by applying the criteria of fasting hyperglycemia and HbA1c at the baseline. However, there is a possibility that a considerable number of subjects with diabetes along with postprandial hyperglycemia were included, because we didn't perform OGTT. Second, we did not check any insulin resistance marker which might be needed to consider along with the underlying mechanism of MetS. Third, this study was a cross-sectional study and there is a limitation in elucidating a clear relationship between the values of HbA1c and MetS as well as the development of diabetes and CVD. Further large prospective studies are needed to assess the relationship between variable glycemic measures and MetS and to identify the cut-off value of HbA1c for determining the MetS.
In conclusion, our study suggests that HbA1c might be used as a diagnostic criterion for MetS and the appropriate cut-off value of HbA1c may be 5.65% in this population. Compared with the use of FPG, the use of HbA1c significantly increased the identification rate of MetS. Therefore, at the point of primary prevention, we can increase the chance of preventing the development of diabetes and CVD by adopting the therapeutic lifestyle changes early.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Stern MP. Diabetes and cardiovascular disease: the common soil hypothesis. Diabetes. 1995;44:369–381. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SE, Ahn SG, Kim JY, Park JS, Shin JH, Tahk SJ, Lee SK, Kim TJ, Han N. Differential relationship between metabolic syndrome score and severity of coronary atherosclerosis as assessed by angiography in a non-diabetic and diabetic Korean population. J Korean Med Sci. 2011;26:900–905. doi: 10.3346/jkms.2011.26.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magliano DJ, Shaw JE, Zimmet PZ. How to best define the metabolic syndrome. Ann Med. 2006;38:34–41. doi: 10.1080/07853890500300311. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Kim DJ, Cho NH, Noh JH, Kim HJ, Choi YH, Jung JH, Min YK, Lee MS, Lee MK, Kim KW. Fasting plasma glucose cutoff value for the prediction of future diabetes development: a study of middle-aged Koreans in a health promotion center. J Korean Med Sci. 2005;20:562–565. doi: 10.3346/jkms.2005.20.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes:2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HK, Bae SJ, Choe JO. Impact of HbA1c criterion on the detection of subjects with increased risk for diabetes among health check-up recipients in Korea. Diabetes Metab J. 2012;36:151–156. doi: 10.4093/dmj.2012.36.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flagel KM, Ebberhardt MS, Goldstein DE. Use of GHb (HbA1c) in screening of undiagnosed diabetes in the US population. Diabetes Care. 2000;23:187–191. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K, Matsumoto M, Akimoto K. Fasting plasma glucose and HbA1c as risk factors for type 2 diabetes. Diabet Med. 2008;25:1157–1163. doi: 10.1111/j.1464-5491.2008.02572.x. [DOI] [PubMed] [Google Scholar]
- 12.Stratton IM, Adler AI, Neil AW, Turner R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications on insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 14.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Barrett-Connor E, Wingard DL, Shan J, Edelstein S. GHb is a better predictor of cardiovascular disease than fasting or postchallenge plasma glucose in women without diabetes: the Rancho Bernardo Study. Diabetes Care. 1996;19:450–456. doi: 10.2337/diacare.19.5.450. [DOI] [PubMed] [Google Scholar]
- 16.de Vegt F, Dekker JM, Ruhé HG, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42:926–931. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- 17.Osei K, Rhinesmith S, Gaillard T, Schuster D. Is glycosylated hemoglobin A1c a surrogate for metabolic syndrome in nondiabetic, first-degree relatives of African-American patients with type 2 diabetes? J Clin Endocrinol Metab. 2003;88:4596–4601. doi: 10.1210/jc.2003-030686. [DOI] [PubMed] [Google Scholar]
- 18.Ong KL, Tso AW, Lam KS, Cherny SS, Sham PC, Cheung BM. Using glycosylated hemoglobin to define the metabolic syndrome in United States adults. Diabetes Care. 2010;33:1856–1858. doi: 10.2337/dc10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenzo C, Wagenknecht LE, Hanley AJ, Rewers MJ, Karter AJ, Haffner SM. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2010;33:2104–2109. doi: 10.2337/dc10-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 21.Mitka M. Hemoglobin A1c poised to become preferred test for diagnosing diabetes. JAMA. 2009;301:1528. doi: 10.1001/jama.2009.479. [DOI] [PubMed] [Google Scholar]
- 22.Little RR, Sacks DB. HbA1c: how do we measure it and what does it mean? Curr Opin Endocrinol Diabetes Obes. 2009;16:113–118. doi: 10.1097/MED.0b013e328327728d. [DOI] [PubMed] [Google Scholar]
- 23.Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, Anfossi G, Costa G, Trovati M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813–819. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 24.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c) Diabetes Care. 2003;26:881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 25.Sung KC, Rhee EJ. Glycated haemoglobin as a predictor for metabolic syndrome in non diabetic Korean adults. Diabet Med. 2007;24:848–854. doi: 10.1111/j.1464-5491.2007.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Choi SR, Lee JR, Shin JH, Lee SJ, Han MA, Park J, Bae HY, Kim SY. Association of hemoglobin A1c with cardiovascular disease risk factors and metabolic syndrome in nondiabetic adults. Korean Diabetes J. 2008;32:435–444. [Google Scholar]