Abstract
The study was designed to investigate the effect of serum glucose, insulin and insulin resistance on the risk of prostate cancer (CaP) and on the clinicopathological characteristics in Korean men. Subjects were retrospectively recruited from 166 CaP patients underwent radical prostatectomy and 166 age-matched benign prostatic hyperplasia (BPH) patients. The serum was taken on the morning of the day of operation and insulin resistance was assessed by homeostasis model assessment insulin resistance index (HOMA-IR). Men in highest tertile of insulin was associated with 55% reduced odds of CaP than those with the lowest tertile (OR = 0.45, 95% CI = 0.23-0.89, P = 0.022). The patients in highest tertile of insulin had a more than 5.6 fold risk of locally advanced stage than those in the lowest tertile (OR = 5.62, 95% CI = 1.88-16.83, P = 0.002). Moreover, the patients in the highest tertile HOMA-IR group was associated with an increased risk of locally advanced stage than the lowest tertile group (OR = 3.10, 95% CI = 1.07-8.99, P = 0.037). These results suggest that elevated insulin and insulin resistance are associated with the advanced pathological stage of prostate cancer in Korean patients.
Keywords: Glucose, Insulin, Insulin Resistance, HOMA-IR, Prostatic Neoplasms
INTRODUCTION
Prostate cancer (CaP) is the most common malignancy among men in developed countries, with an estimated 190,000 new cases diagnosed each year in Europe and the USA (1, 2), and it is well known that environmental factors are associated with the risk of developing this malignancy (3). The incidence of CaP is higher in Western countries and a Western lifestyle, characterized by high caloric intake and low physical activity, thus obesity has been suspected to influence the risk of disease (4, 5). Also the incidence of CaP in Korean men has been increasing and obesity seems to play a role of carcinogenesis (6, 7). Although it is still disputed, some studies have shown that the metabolic syndrome, characterized by central obesity, insulin resistance, high serum glucose levels, systemic arterial hypertension and dyslipidemia, may play a role in the development of CaP (8-12).
Elevated serum glucose leads to rapid increment of insulin from the pancreatic beta cells, and high insulin levels can be associated with insulin resistance. In addition, insulin has potent mitogenic and growth-stimulatory effects on the prostate and other tissues, and alterations in these effects could potentially contribute to the development of malignancy (13). Therefore, among the physiopathological entities that comprise metabolic syndrome, glucose, insulin and insulin resistance may link to the risk of CaP.
Moreover, obesity or diabetes could be associated with the clinicopathological outcomes. Some studies reported that obese men had higher-grade and pathologically more advanced CaP (14, 15). On the other hand, although prevalent diabetes is associated with decreased CaP incidence (16), a recent study reported that patients with diabetes showed a higher risk of advanced CaP (17). Given the evidences, the endocrinologic serum parameters such as glucose, insulin and insulin resistance might affect not only the susceptibility but also the clinical outcomes of CaP. To the best of our knowledge, no study of relationship between these serum parameters and clinicopathological outcomes of CaP in Asian population has been conducted.
The present study was designed to investigate the effect of serum glucose, insulin and insulin resistance on the risk of CaP and on the clinicopathological characteristics of CaP in Korean men using a benign prostatic hyperplasia (BPH) group as a control.
MATERIALS AND METHODS
Study population
A retrospective case-control study was conducted including 166 cases with newly diagnosed CaP and 166 controls among age-matched BPH patients. Cases were recruited from the patients who underwent radical prostatectomy and histologically confirmed primary adenocarcinoma of the prostate at our institution between 2004 and 2010. Controls were selected from the database of BPH patients who underwent transurethral resection of prostate (TURP), and one-to-one matched with similar age and closest date of blood sampling according to those of cases. Controls with serum prostate specific antigen (PSA) levels higher than 3 ng/mL underwent a transrectal prostate biopsy before TURP to rule out the presence of cancer, and those with PSA levels higher than 10 ng/mL were excluded from the study to rule out the possibility of CaP. Subjects with a suspicious history of previous management for CaP or incomplete medical records were excluded from the study. Also, the patients were excluded if they were taking the medication that influence on serum glucose or insulin. Gleason grade and TNM 2002 stage were used as prognostic factors. Gleason grade and pathologic stage were measured from specimens of radical prostatectomy.
Specimen and laboratory assays
On the morning of the day of operation, patient serum was taken and stored at -80℃ until use. Serum PSA levels were measured by using a quantified monoclonal IRMA radioimmunoassay (Izotop, Budapest, Hungary). Glucose was measured by a Hitachi 7600 automatic chemical analyzer (Hitachi, Tokyo, Japan) using hexokinase method. Insulin was measured by an Elecsys 2010 autoanalyzer (Roche Diagnostics, Indianapolis, IN, USA), employing an electrochemiluminescence immunoassay principles, and is based on sandwich test principle which employs two monoclonal antibodies directed against insulin. All assays were performed according to the respective manufacturer's instructions. Insulin resistance was assessed by homeostasis model assessment insulin resistance index (HOMA-IR) calculated as fasting glucose (mg/dL) × fasting insulin (mU/mL)/405.
Statistical analysis
Clinical variables such as age, PSA, prostate size and body mass index (BMI) in patient and control groups were compared using the Student's t-test, and categorical variables were compared using chi-square tests. In the evaluation of CaP risk, Gleason scores were classified into 7 or less and 8 or more, and the pathological stage was divided into localized (pT2) and locally advanced disease (pT3, pT4 or any nodal metastasis). Separate analyses were carried out for insulin, glucose, and HOMA-IR. The serum parameters were categorized into approximate tertiles on the basis of the distribution of each variable within the nested subcohort, with the lowest tertile assigned as the reference group. Tertile cut-off points were determined on variable distribution of controls in the full study group. Tests for linear trend used scored categorical trend variables that assigned the tertile median to each person. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using logistic regression models and sequentially adjusted for age and BMI. Statistical analysis was performed using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA), and a P value of < 0.05 was considered statistically significant.
Ethics statement
The collection and analysis of all samples was approved by the institutional review board of Chungbuk National University Hospital (Cheongju, Korea; IRB registration number 2006-01-001). An informed consent was obtained from each patient.
RESULTS
Baseline characteristics
Table 1 shows the baseline characteristics of the 166 CaP patients and 166 BPH controls enrolled in the study. The mean age of CaP patients was 66.4 yr (range 49-78) and that of the BPH controls was 66.4 yr (range 45-77). The serum PSA level was higher in CaP patients than in BPH cases (15.60 ± 26.89 ng/mL vs 2.91 ± 2.21 ng/mL; P < 0.001). The size of the prostate was smaller in CaP patients than in BPH cases (34.85 ± 17.31 gram vs 43.28 ± 23.87 gram; P < 0.001). There were no significant differences between cases and controls regarding age and BMI (P = 0.957 and 0.155, respectively). The prevalence of diabetes mellitus or hypertension was not different in both groups (P = 0.759 and 0.162, respectively). The number of subjects showing a Gleason score ≤ 7 and ≥ 8 was 100 (60.2%) and 66 (39.8%), respectively; and patients with a localized and locally advanced stage were 100 (60.2%) and 66 (39.8%), respectively.
Table 1.
Comparison of clinicopathologic characteristics between prostate cancer cases and controls
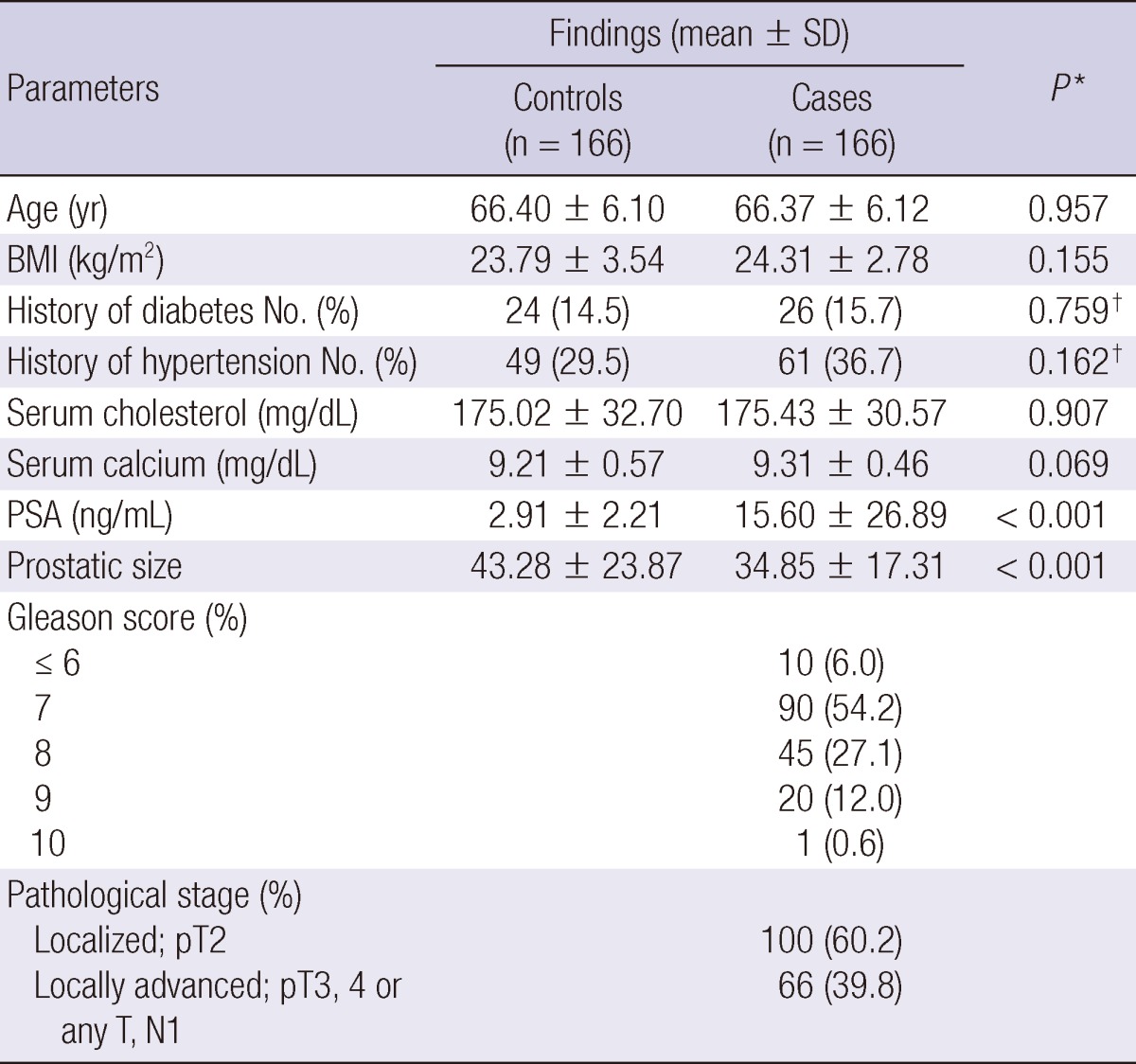
*Based on Student t-test; †Based on chi-square test. BMI, body mass index; PSA, prostate specific antigen.
Glucose, insulin, HOMA-IR and risk of CaP
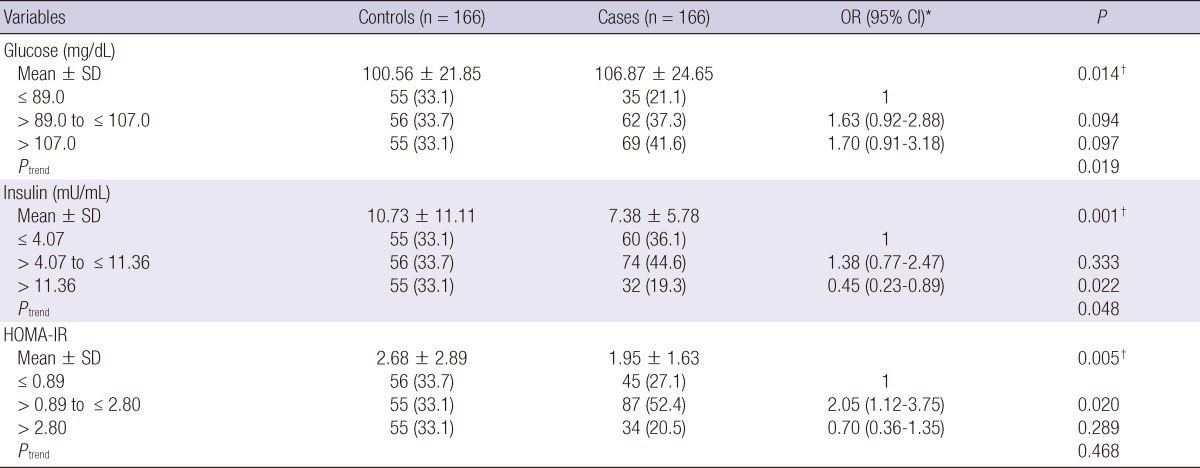
Fasting serum glucose was higher in CaP cases than the controls (106.87 ± 24.65 mg/dL vs 100.56 ± 21.85 mg/dL; P = 0.014), and the risk of CaP was increased with higher glucose group (Ptrend = 0.019) (Table 2). In the logistic regression analysis, however, the risk of CaP was not different across glucose tertiles (each P > 0.05). Serum insulin was lower in the cases than the controls (7.38 ± 5.78 mg/dL vs 10.73 ± 11.11 mg/dL; P = 0.001), and the risk of CaP was decreased with higher insulin group (Ptrend = 0.048). Men in highest tertile of insulin had a 55% reduction in risk of CaP than those with the lowest tertile (OR = 0.45, 95% CI = 0.23-0.89, P = 0.022). HOMA-IR was lower in the cases than controls (1.95 ± 1.63 vs 2.68 ± 2.89; P = 0.005). Interestingly, men with mid tertile HOMA-IR group had a increased risk of CaP than the lowest tertile group (OR = 2.05, 95% CI = 1.12-3.75, P = 0.020).
Table 2.
Age- and body mass index-adjusted associations of tertiles of baseline serum insulin, glucose and HOMA-IR with prostate cancer risk
*ORs were calculated by conditional logistic regression analysis and adjusted for age and BMI; †Based on Student t-test.
Glucose, insulin, HOMA-IR and clinical stage in CaP patients
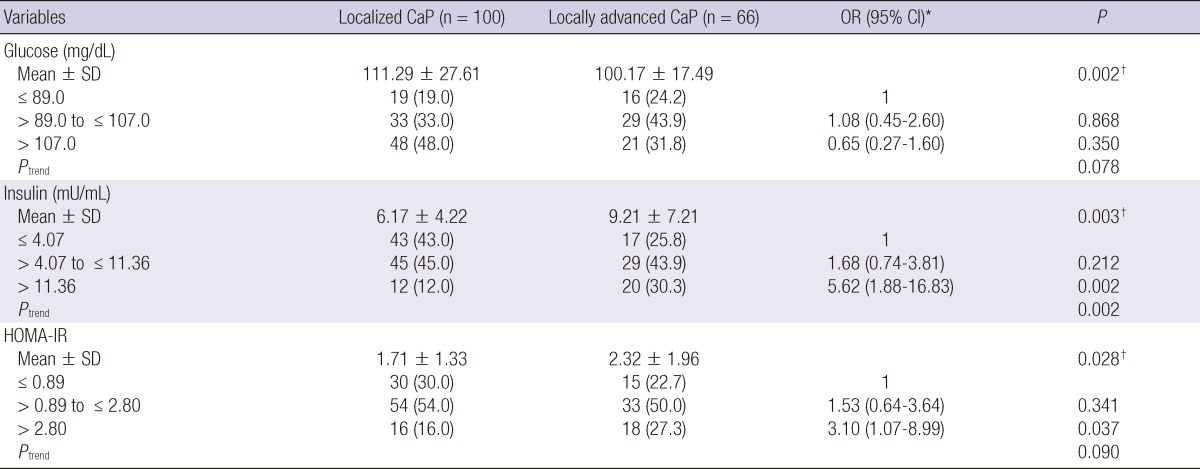
Fasting serum glucose was higher in localized CaP patients than locally advanced cases (111.29 ± 27.61 vs 100.17 ± 17.49; P = 0.002) (Table 3). Serum insulin and HOMA-IR were lower in localized CaP cases than locally advanced cases (6.17 ± 4.22 vs 9.21 ± 7.21; P = 0.003 and 1.71 ± 1.33 vs 2.32 ± 1.96; P = 0.028, respectively). The risk of locally advanced CaP was increased with higher insulin group (Ptrend = 0.002). The patients in highest tertile of insulin had a more than 5.6 fold risk of locally advanced stage than those in the lowest tertile (OR = 5.62, 95% CI = 1.88-16.83, P = 0.002). Moreover, the patients in the highest tertile HOMA-IR group had an increased risk of locally advanced stage than the lowest tertile group (OR = 3.10, 95% CI = 1.07-8.99, P = 0.037).
Table 3.
Age- and body mass index-adjusted associations of baseline serum insulin, glucose and HOMA-IR with pathological stage of prostate cancer
Localized CaP defined as pT2 and locally advanced CaP as pT3, pT4 or any nodal metastasis. *ORs were calculated by unconditional logistic regression analysis and adjusted for age and BMI; †Based on Student t-test.
Glucose, insulin, HOMA-IR and Gleason score in CaP patients
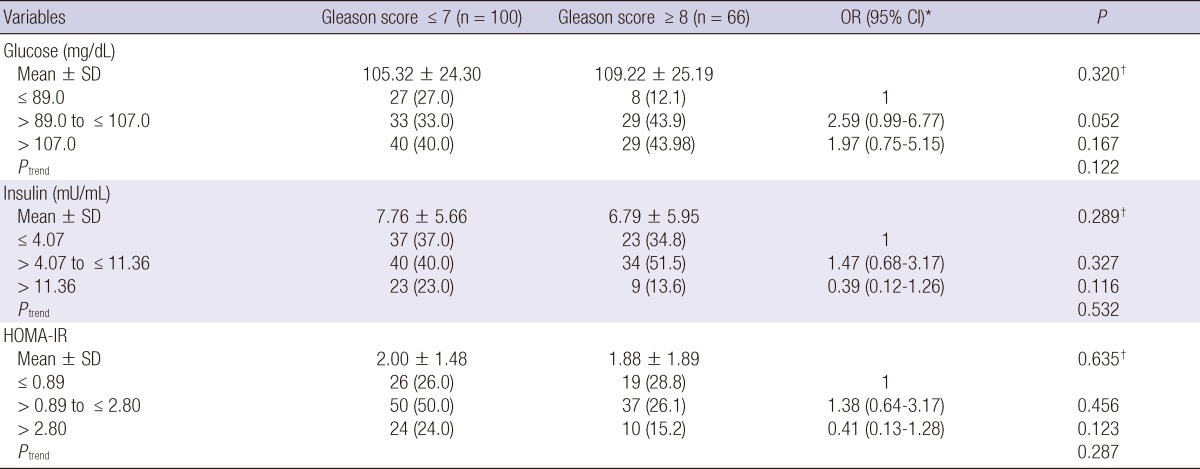
Fasting serum glucose, insulin and HOMA-IR did not show the significant different between high (≥ 8) and low (≤ 7) Gleason score (each P > 0.005) (Table 4). In the logistic regression analysis, these parameters were not different across tertiles (each P > 0.05).
Table 4.
Age- and body mass index-adjusted associations of baseline serum insulin, glucose and HOMA-IR with Gleason score of prostate cancer
*ORs were calculated by unconditional logistic regression analysis and adjusted for age and BMI; †Based on Student t-test.
DISCUSSION
The current study reveals that fasting serum glucose, insulin and insulin resistance are associated with CaP susceptibility and clinicopathological characteristics in Korean men. Men in the highest tertile of insulin was associated with 55% reduced odds of CaP than those with the lowest tertile, and the patients in the highest tertile of insulin had a more than 5.6 fold risk of locally advanced CaP than those in the lowest tertile. Moreover, the patients in the highest tertile HOMA-IR group had an increased risk of locally advanced stage than the lowest tertile group.
Serum glucose is directly controlled by insulin, thus higher glucose level induces insulin secretion from pancreatic beta cells. Such a hyperinsulinemia is associated with insulin resistance, therefore contributes the pathogenesis of type 2 diabetes. The role of insulin in cancer has been studied, and high level of circulating insulin decreases the production of insulin like growth factor I (IGF-I) binding proteins and increase levels of free IGF-I, which promotes carcinogenesis (18). In addition, diet-induced hyperinsulinemia was associated with increased tumor growth in a xenograft model (19). In contrast to biologic evidences, however, studies between the relationship between obesity and CaP incidence are inconsistent (20).
Moreover, several epidemiologic studies suggested that diabetes is associated with low risk of CaP although debates remain (16). In the present study, serum glucose, insulin and HOMA-IR did not show the consistent results in terms of the CaP risk. Higher glucose was positively related to the susceptibility, whereas higher insulin was inversely related to the risk of CaP. We could not explain such a discrepancy, but we believe that the carcinogenesis of prostate is somewhat complex, thus multiple factors (eg, IGF-I, IGF receptors, circulating androgen, etc) could be intermingled in the tumorigenesis.
Reports of the insulin resistance in relation to CaP risk have been conflicting. Hsing et al. conducted a population-based case control study including 128 cases and 306 controls in China, and concluded that insulin resistance was associated with a higher risk of CaP among Chinese men (21). Albanes et al. (22) performed a prospective cohort study with 100 cases and 400 controls, and reported that increased HOMA-IR was associated with the significantly increased risks of CaP. In contrast to previous studies, however, Stocks et al. reported that HOMA-IR was strongly inversely related to overall CaP risk especially among young men and among men with non-aggressive disease through a prospective cohort in Northern Sweden with 392 cases and 392 matched controls (23). In the present study, HOMA-IR did not show the similar result. There are some possibilities that explain the differences. At first, controls in the current study were recruited from the BPH patients who underwent TURP, thus they may be not representative of the normal population. Second, the study populations of previous studies were different from our study. Average BMI of our cases was 24.3 kg/m2, but in two Western studies BMI was relatively higher than ours (> 26.0 kg/m2) and Chinese men was lower (21.9 kg/m2).
The results obtained in this study demonstrate that the insulin level and HOMA-IR are strongly associated with the higher pathological stage in patients who underwent radical prostatectomy, and these findings agree with the results of other studies. Freedland et al. (15) reported that obese men undergoing radical prostatectomy had higher-grade and larger tumor than non-obese patients in Western population. Recently, Li et al. (17) conducted a population-based prospective cohort study in Japanese men and concluded that the patients with diabetes associated with a higher risk of advanced CaP. Given the epidemiologic evidences, insulin and insulin resistance were postulated as major cause of the cancer aggressiveness. Lehrer et al. (24) demonstrated that increased T stage was independently correlated with increased serum insulin levels whereas Gleason score did not show the significance, and this result could bolster our data. Moreover, no study of relationship between insulin resistance and aggressiveness of CaP in Asian population has been conducted. Based on our results, the effort to reduce the insulin level and insulin resistance may influence on the clinical features of prostate cancer patients, although long-term well designed cohort study is needed to validate our results.
Our study had several inherent weaknesses. First, the current study was a cross-sectional study. Therefore, to understand the long-term effect of these parameters, a prospective longitudinal cohort study is needed. Second, as we mentioned previously, controls in the current study may be not representative of the normal population. Finally, all cases underwent radical prostatectomy, thus the cancer stage was consisted with localized or locally advanced stage, and not including metastatic disease. However, we believe our cases had strong advantages not only more accurate stage and Gleason score were measured from the specimen, but also glucose, insulin and insulin resistance have more chance to affect the pathogenesis in early period of carcinogenesis than metastatic stage.
Footnotes
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science and Technology (2012-0000478).
References
- 1.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–1721. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Barnard RJ, Aronson WJ, Tymchuk CN, Ngo TH. Prostate cancer: another aspect of the insulin-resistance syndrome? Obes Rev. 2002;3:303–308. doi: 10.1046/j.1467-789x.2002.00081.x. [DOI] [PubMed] [Google Scholar]
- 6.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 7.Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010;25:1113–1121. doi: 10.3346/jkms.2010.25.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mydlo JH, Tieng NL, Volpe MA, Chaiken R, Kral JG. A pilot study analyzing PSA, serum testosterone, lipid profile, body mass index and race in a small sample of patients with and without carcinoma of the prostate. Prostate Cancer Prostatic Dis. 2001;4:101–105. doi: 10.1038/sj.pcan.4500514. [DOI] [PubMed] [Google Scholar]
- 9.Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–549. doi: 10.1093/oxfordjournals.aje.a010241. [DOI] [PubMed] [Google Scholar]
- 10.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 11.Cho IC, Kwon WA, Kim JE, Joung JY, Seo HK, Chung J, Park WS, Lee KH. Prostate volume has prognostic value only in pathologic T2 radical prostatectomy specimens. J Korean Med Sci. 2011;26:807–813. doi: 10.3346/jkms.2011.26.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim WT, Yun SJ, Choi YD, Kim GY, Moon SK, Choi YH, Kim IY, Kim WJ. Prostate size correlates with fasting blood glucose in non-diabetic benign prostatic hyperplasia patients with normal testosterone levels. J Korean Med Sci. 2011;26:1214–1218. doi: 10.3346/jkms.2011.26.9.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argiles JM, Lopez-Soriano FJ. Insulin and cancer (Review) Int J Oncol. 2001;18:683–687. [PubMed] [Google Scholar]
- 14.Amling CL, Kane CJ, Riffenburgh RH, Ward JF, Roberts JL, Lance RS, Friedrichs PA, Moul JW. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001;58:723–728. doi: 10.1016/s0090-4295(01)01373-5. [DOI] [PubMed] [Google Scholar]
- 15.Freedland SJ, Banez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: an analysis of the duke prostate center database. Prostate Cancer Prostatic Dis. 2009;12:259–263. doi: 10.1038/pcan.2009.11. [DOI] [PubMed] [Google Scholar]
- 16.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Kuriyama S, Kakizaki M, Yan H, Sone T, Nagai M, Sugawara Y, Ohmori-Matsuda K, Hozawa A, Nishino Y, et al. History of diabetes mellitus and the risk of prostate cancer: the Ohsaki Cohort Study. Cancer Causes Control. 2010;21:1025–1032. doi: 10.1007/s10552-010-9530-9. [DOI] [PubMed] [Google Scholar]
- 18.Harish K, Dharmalingam M, Himanshu M. Study Protocol: insulin and its role in cancer. BMC Endocr Disord. 2007;7:10. doi: 10.1186/1472-6823-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkateswaran V, Haddad AQ, Fleshner NE, Fan R, Sugar LM, Nam R, Klotz LH, Pollak M. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99:1793–1800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 20.Buschemeyer WC, 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52:331–343. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 21.Hsing AW, Gao YT, Chua S, Jr, Deng J, Stanczyk FZ. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95:67–71. doi: 10.1093/jnci/95.1.67. [DOI] [PubMed] [Google Scholar]
- 22.Albanes D, Weinstein SJ, Wright ME, Mannisto S, Limburg PJ, Snyder K, Virtamo J. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101:1272–1279. doi: 10.1093/jnci/djp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stocks T, Lukanova A, Rinaldi S, Biessy C, Dossus L, Lindahl B, Hallmans G, Kaaks R, Stattin P. Insulin resistance is inversely related to prostate cancer: a prospective study in Northern Sweden. Int J Cancer. 2007;120:2678–2686. doi: 10.1002/ijc.22587. [DOI] [PubMed] [Google Scholar]
- 24.Lehrer S, Diamond EJ, Stagger S, Stone NN, Stock RG. Serum insulin level, disease stage, prostate specific antigen (PSA) and Gleason score in prostate cancer. Br J Cancer. 2002;87:726–728. doi: 10.1038/sj.bjc.6600526. [DOI] [PMC free article] [PubMed] [Google Scholar]