Abstract
The development of a prophylactic vaccine that targets human papillomaviruses (HPV) 6, 11, 16, and 18 to prevent cervical cancer has increased interest in the ethnic and geographical distributions of HPV genotypes. We investigated HPV prevalence and type distribution by restriction fragment mass polymorphism (RFMP) testing a total of 60,775 specimens (aged 18-79 yr, median 44) taken from liquid-based cytology. Overall HPV positive rate of total patients was 34.2%. Among the positive patients, 87.7% was single type infections, and 12.3% was multiple HPV types. HPV-16 was the most prevalent genotype observed in 2,307 (26.0%), followed by type 52 in 2,269 (25.5%), type 58 in 1,090 (12.3%), type 18 in 633 (7.1%), type 56 in 436 (4.9%). The pattern of high risk-HPV positive rate according to age showed U-shape with a peak in HPV prevalence among women less than 30 yr of age, and a second peak among the older females aged 70 to 79 yr. The leading four high-risk HPV genotypes were HPV-16, HPV-52, HPV-58, and HPV-18 in descending order. In conclusion, this study provides the most representative prevalence and type-specific distribution of HPV among Korean women, and demonstrates that the epidemiology of HPV infection is different from that of other regions of the world.
Keywords: Human Papillomavirus, Genotype, Prevalence, Distribution, RFMP, Korean
INTRODUCTION
Infection with human papillomavirus (HPV) is considered to be a pre-requisite for the development of cervical cancer and is associated with neoplastic precursor lesions (1). In Korean women, the cervical cancer is the fifth frequent cancer after breast cancer, stomach cancer, colon cancer and thyroid cancer. To date more than 100 HPV genotypes have been identified and at least 50 are known to infect the female anogenital tract (2). Among these thirteen mucosotropic HPVs (types 16, 18, 31, 33, 35, 39, 45, 5, 52, 56, 58, 59, and 68) have been classified as class I high risk carcinogens to human beings (3). Because the distribution and prevalence of HPV vary by geographic region and the immunity conferred by vaccines is type-specific, the need for HPV genotyping in routine screening population is increasingly recognized. For example, although HPV-16 is the most prevalent type worldwide, some genotypes such as type 52 and 58 are rare in Western countries, whereas they are relatively prevalent in Asian populations (4-6). Hence, an accurate assessment of the regional, community-based distribution of HPV genotypes is extremely important for the prevention of cervical cancer and for public hygiene management. The US FDA-approved Hybrid Capture II HPV DNA test (HC II) (Digene Corporation, Gaithersburg, MD, USA), which can detect 13 carcinogenic HPV types, is the HPV DNA detection method most commonly used. Unfortunately, this cocktail detection method does not identify specific HPV genotypes. The restriction fragmentation mass polymorphism (RFMP) assay (GeneMetrix Co., Yongin, Korea), which utilizes PCR to amplify the HPVL1 gene followed by enzyme restriction and mass measurement using MALDI-TOF, offers a method to detect 38 types of HPV. This new method has manifested sensitivity and reliability comparable to HC II (7-9).
The goal of this study was to establish and investigate an age-specific HPV prevalence, genotype distribution and extent of multiple infections in Korean women to provide the most representative epidemiologic data for the clinical prediction of the outcome of HPV infection, future prevention strategies, and the development of the efficient multivalent HPV vaccine.
MATERIALS AND METHODS
Study population
The residual samples after liquid-based cytology (LBC) tests from 60,775 patients aged 18-79 yr (median age 44-yr-olds) from September 2006 to September 2011 were used for HPV genotypes using RFMP assay. After cytology, residual cells in the LBC sample were centrifuged at 3,500 rpm for 10 min and stored as split cellular pellets of 200 µL at -70℃ prior to nucleic acid extraction.
DNA extraction
QIAamp1 DNA Blood Mini Kits (Qiagen, Valencia, CA, USA) were used to extract cervical cell DNA according to the manufacturer's protocol. Nucleic acid was stored at -70℃ prior to HPV amplification and genotyping.
HPV DNA amplification and genotyping
The presence and genotype of HPV in all samples were tested using RFMP method. The details of the RFMP assay protocol were described previously (8). Briefly, 4 µL of DNA were amplified with PGMY09/11 primers, comprising of two non degenerate pools of L1 consensus primers. Second round primer pairs comprised a sense primer RFMP specific to bases 6584 to 6603 (5'-GCMCAGGGHCAYAAGGATGAA TGG-3') and an antisense primer RFMP specific to bases 6657 to 6626 (5'-GTACTDCKDGTRGTATCHACMACGGATGTAACAAA-3'). The 5-nucleotide sequence (GGATG) embedded in the primers introduced a FokI site (a neoschizomer of BtsCI) in the amplicon. Restriction enzyme digestion of PCR products was performed by mixing the PCR reaction mixtures with 10 µL of buffer containing 50 mM potassium acetate, 20 mM Tris-acetate, 10 mM magnesium acetate, 1 mM dithiothreitol and 1 unit of FokI and BtsCI. The reaction mixtures were incubated at 37℃ for 1 hr. The resulting digest was purified by vacuum filtration through 96-well Oasis® µElution Plates (Waters, Tokyo, Japan). The desalted reaction mixtures were resuspended with matrix solution containing 15 mg/mL 3-hydroxypicolinic acid, 0.023 M ammonium citrate and 12% acetonitrile, and spotted in 3-µL volumes on a polished MTP AnchorChip™ plate (BrukerDaltonics, Bremen, Germany). Mass spectra were acquired with the aid of installed software (flexcontrol 3.0) on a Microflex linear MALDI-TOF mass spectrometer (BrukerDaltonics, Bremen, Germany).This method was able to detect 38 HPV genotypes (6, 11, 16, 18, 26, 30, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 66, 68, 70, 72, 73, 74, 81, 82, 83, 84, 89, and 90).
Statistical analysis
The data were analyzed by an SPSS 12.0 statistical package for Windows. Pearson's chi-squared test was used to evaluate the significant differences between the designated groups. All tests were two-sided, and a P value < 0.05 was considered statistically significant.
Ethics statement
This study was approved by the institutional review board of Green Cross Reference Laboratory of Korea (GCRL-IRB-2012001). Informed consent was exempted by the board due to the retrospective design and anonymous data.
RESULTS
HPV prevalence
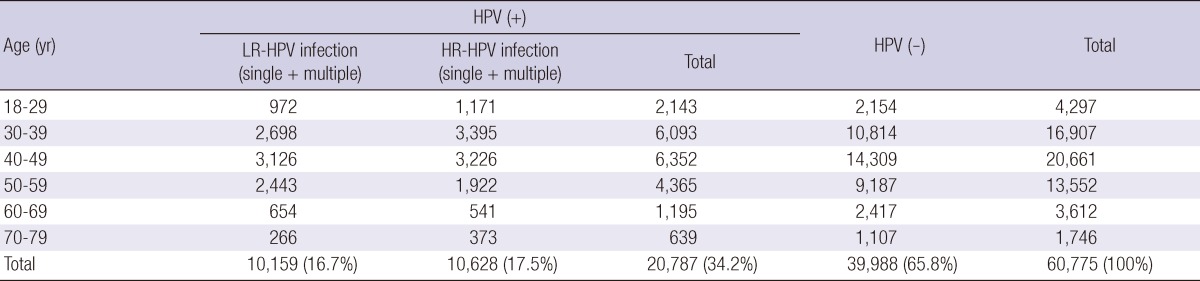
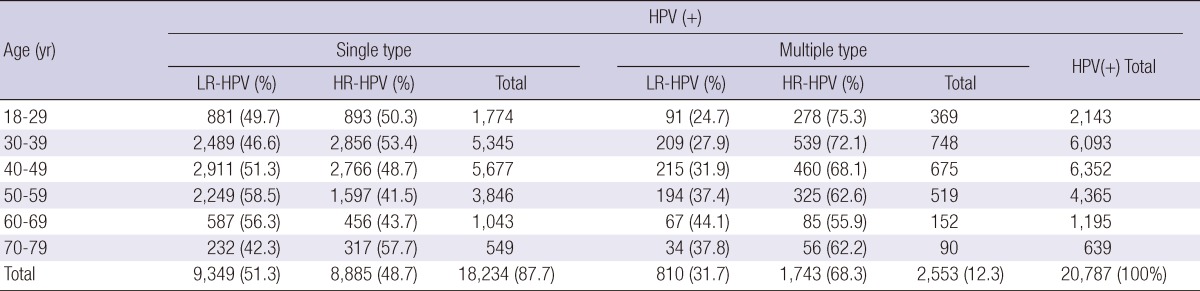
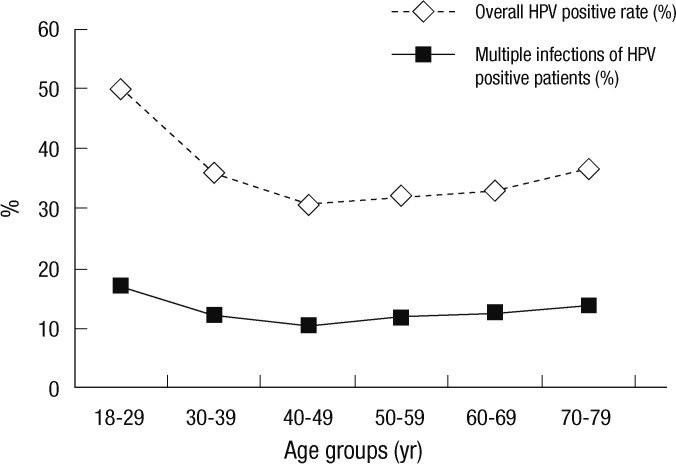
Of the 60,775 Korean women aged between 18 and 79 yr, HPV DNA was detected in 20,787 (34.2%); 10,628 (17.5%) patients were infected with high-risk HPV genotypes and 10,159 (16.7%) patients with low-risk HPV genotypes (Table 1, 2). Among HPV-positive patients of 20,878, 18,234 (87.7%) patients had single infection and 2,553 (12.3%) had multiple infection (Table 3). Age-specific HPV infection prevalence peaked at 49.9% in 18- to 29-yr-olds, and declined to 30.7% in 40- to 49-yr-olds, after which HPV prevalence steadily increased to 36.6% in 70- to 79-yr-olds (Table 1, Fig. 1). In the multiple infections of 2,553, infections with two different genotypes were 2,347 (91.9%) and with three genotypes 206 (8.1%) (Table 4). The number of genotypes was not significantly different among the different age groups (P = 0.26, Table 4). Age-specific prevalence curve of multiple infections in HPVpositive patients revealed a very similar pattern to that of the overall HPV positivity stratified by age (Fig. 1).
Table 1.
Overall HPV prevalence stratified by age
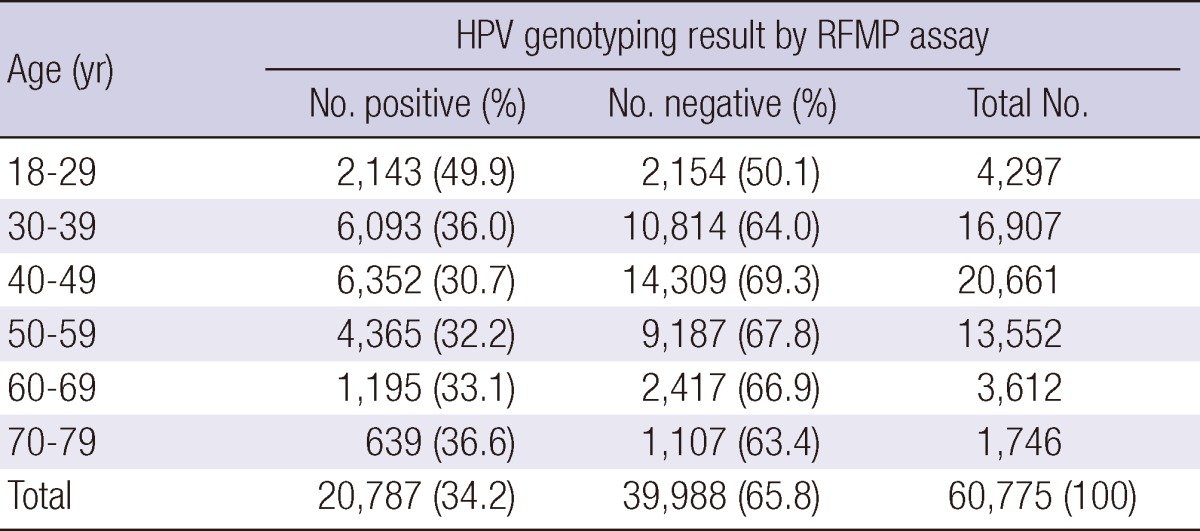
RFMP, restriction fragmentation mass polymorphism; HPV, human papillomavirus.
Table 2.
LR-HPV and HR-HPV positive rate stratified by age
LR-HPV, low risk-HPV; HR-HPV, high risk-HPV.
Table 3.
Single and multiple type infections stratified by age
LR-HPV, low risk-HPV; HR-HPV, high risk-HPV.
Fig. 1.
Overall HPV positive rate and multiple infection rate of HPV positive patients stratified by age.
Table 4.
Number of genotypes in multiple infections stratified by age
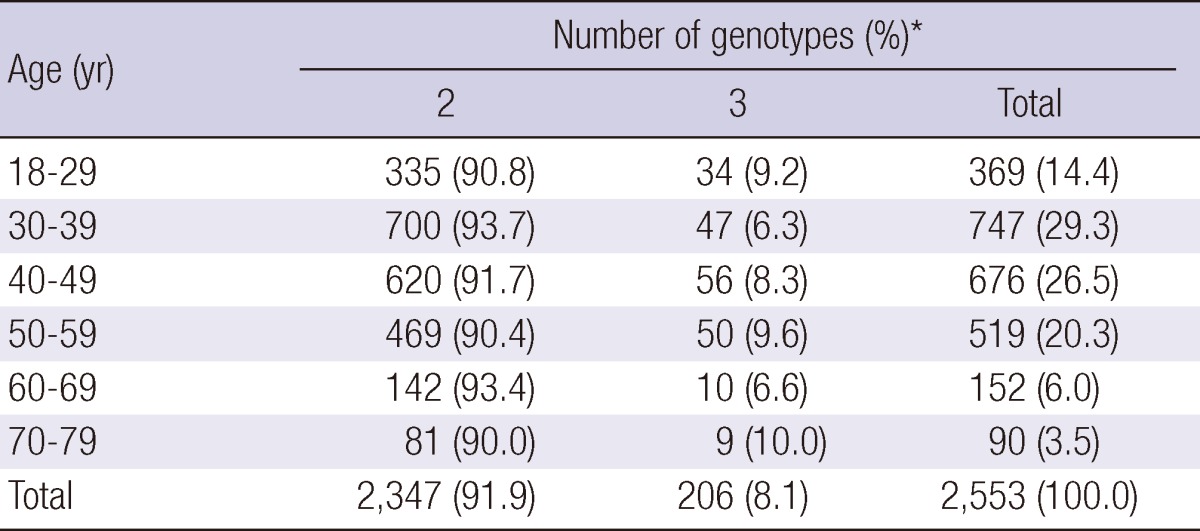
*χ, P = 0.26.
Age-specific HPV genotype distribution
We considered HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 as primary carcinogenic (high-risk) types and all others as non-oncogenic (low-risk) types (3).
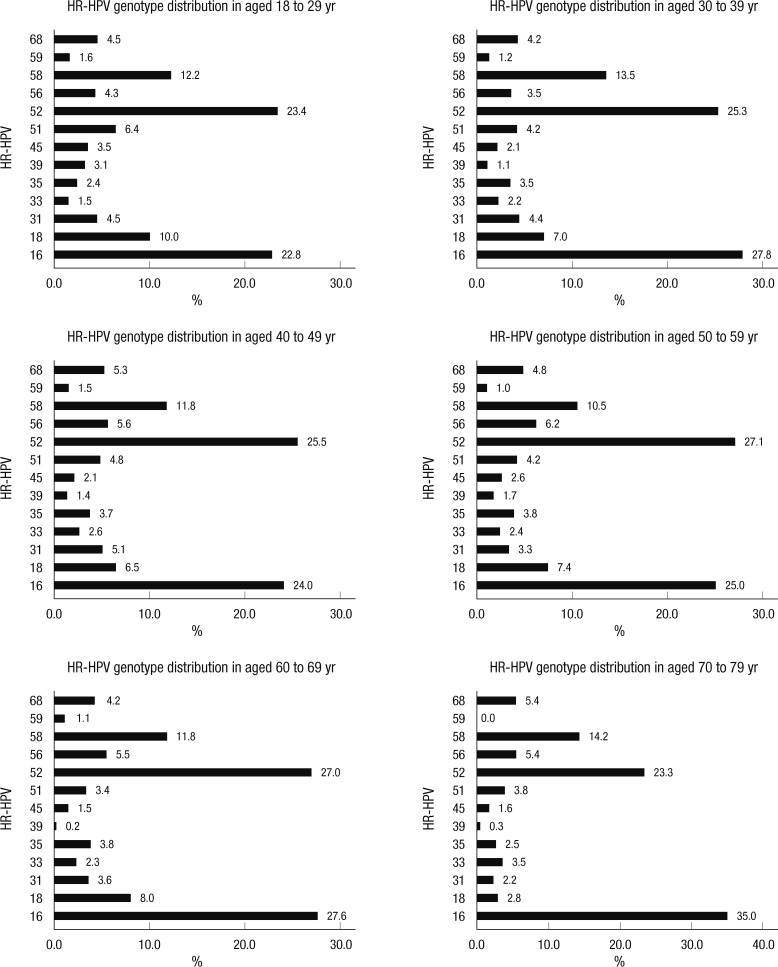
Among the high-risk HPV (HR-HPV) infections, HPV 16 (25.6%) and HPV 52 (25.2%) were the most commonly detected types, followed by HPV 58, 18, 56, 31, 51, 68, 35, 33, 45, 39, and 59 at 11.5%, 7.5%, 5.2%, 5.0%, 4.8%, 4.2%, 3.5%, 2.6%, 2.4%, 1.4%, and 1.3%, respectively (Table 5). The leading four HR-HPV genotypes stratified by age in descending order were HPV type 52, 16, 58, and 18 in 18- to 29-yr-olds, and 40- to 59-yr-olds, HPV 16, 52, 58, and 18 in 30- to 39-yr-olds, and 60- to 69-yr-olds, and HPV 16, 52, 58, 56, and 68 in 70- to 79-yr-olds (Fig. 2).
Table 5.
The distribution of high-risk HPV genotypes of HPV positive patients
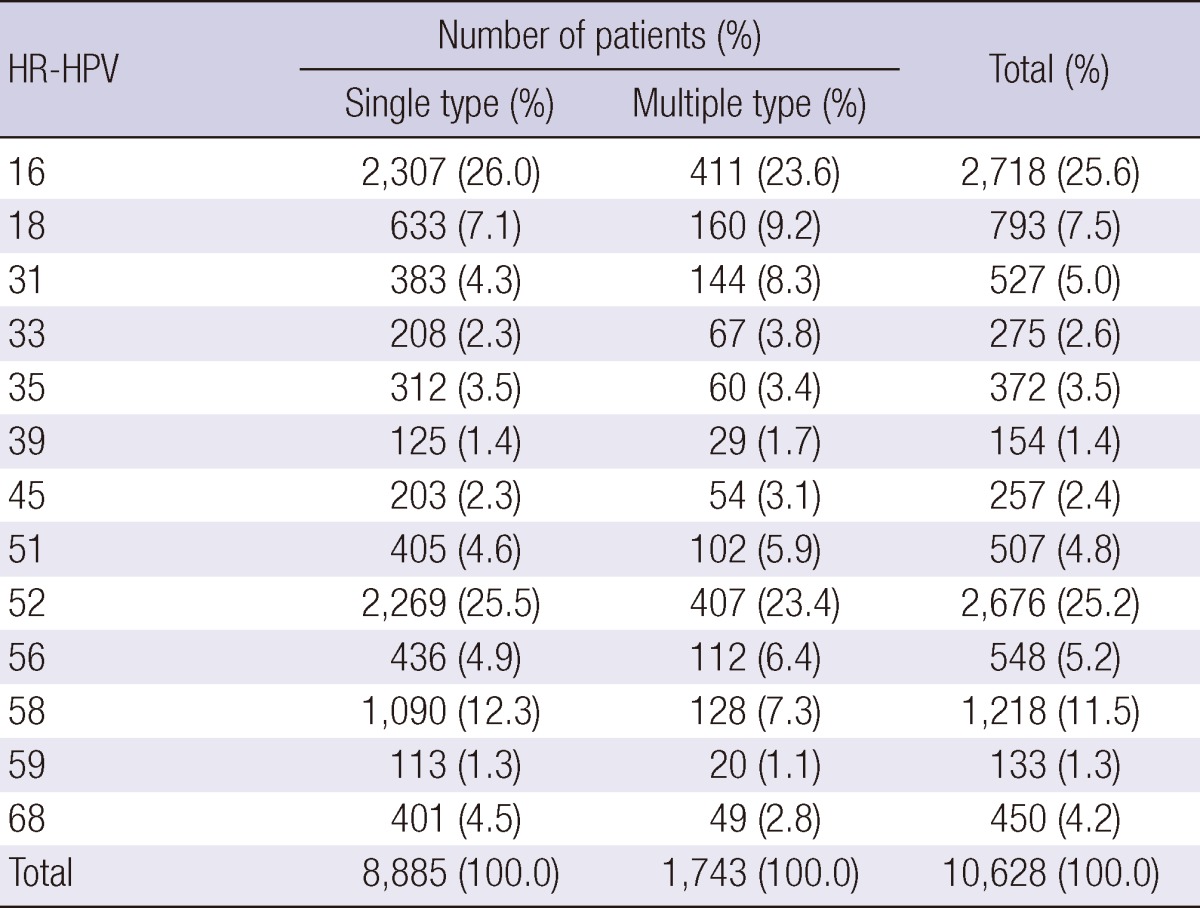
HR-HPV, high-risk human papillomavirus.
Fig. 2.
The distribution of high-risk HPV genotypes according to age groups.
DISCUSSION
HPV is one of the most common sexually transmitted infections worldwide (10). The estimated risk of HPV is notably high over a woman's lifetime (over 80%), however, most women who acquire HPV infection do not develop more serious high-grade cervical neoplasia or invasive cancer because most infections are transient (11).
Our cross-sectional study was performed on patients referred to the gynecology office seeking for an opportunistic cancer screening with liquid based cytology. Population-based data for HPV-type distribution is a prerequisite to the development of new HPV vaccine and cancer screening tests, and to assess the effect and benefits of vaccination. The prevalence of cervical infection with human papillomavirus (HPV) in women varies geographically (12). This study represents, to our knowledge, the largest study of HPV prevalence, including HPV-DNA prevalence and a type specific distribution stratified by age in 60,775 women in Korea. Therefore, the present study provides a more closely represented HPV prevalence of the general population in Korean women for vaccine development and future prophylactic programs in Korea. The cytology results of all samples were composed of 84% of normal cytology, and 16% of abnormal cytology (data not shown).
Aside from geographical differences, variation may also be the result of detection methods employed and varied specimen type and amount. Therefore, precise and accurate HPV DNA test depends on the selection of a proper HPV genotyping method. Although the FDA-approved Hybrid Capture II (HC II) HPV DNA test is the most widely used HPV DNA detection method, it does not have the capability to identify a specific HPV genotype. Furthermore, previous studies have shown that the HC II HPV test cross-reacts with at least 15 HPV genotypes not included in its current high-risk probe cocktail set (13, 14). In this study, HPV genotypes were identified by using RFMP assay which is comparable to HC II with regard to detection of high-risk HPV infection, but is also able to identify 38 HPV genotypes in a single assay, which offers high analytical and clinical sensitivity and the advantage of reliable detection of multiple HPV infections, and it showed a good correlation with direct sequencing data (7, 9). Several studies on the prevalence of overall HPV infection in Korean women have reported rates ranging from 7.2% to 44.8%, and the prevalence of HR-HPV infection ranging from 7.2% to 34.3% (15-20). Because the prevalence of HPV infection varies by country, region within country, detection method, sample size and specimen type, the prevalence of HPV in Korean women shows in wide range according to various articles. As this study was conducted with a very large number of samples of 60,775, using validated precise and accurate detection method, the prevalence of HPV infection observed on this investigation might be the most accurate. Overall, HPV infection rate of our study was 34.2%, and high-risk HPV infection positivity was 17.5%. Those results are lower than the previous study in which overall HPV prevalence accounted for 44.8% and high-risk HPV infection rate was 34.3% (19). Age-specific HPV prevalence varied considerably across geographical regions. Across all geographical regions, observed HPV prevalence was strongly associated with age, and the curves of HPV prevalence according to age were characterized by a U-shape with a relatively higher HPV prevalence in younger and older ages in Africa, Central/South America, Europe, and North America (21). This study also revealed such U-shape curve, which peaked at women between 18- and 29-yr-olds, decline women between the age of 40- and 49-yr-olds, and then steadily increased to women between the age of 70- and 79-yr-olds (Fig. 1). The U-shaped curves of HPV by age may potentially be explained by newly acquired HPV infections and reactivation of latent HPV infections in the older women (22, 23).
Multiple infections among HPV positive cases were accounted for 12.3%, which was lower than previous study which revealed 20.9% (14). Several studies reported that the risk for developing the tissue abnormalities or lesions that typically precede cervical cancer is much higher for women infected with multiple genotypes of the human papilllomavirus. Therefore, incorporating diagnoses of multiple HPV infections into the prediction outcomes of HPV infections is considered important (24, 25). The prevalence of multiple HPV infections in HPV positive cases has shown a worldwide geographical variation ranging from about 9% to 50% in the European countries (26, 27). This variation is also due to different sample size, population, detection methods and the type of the samples.
Distribution of HPV genotypes varies across geographic areas (12, 27, 28). Several studies demonstrated that the most common type is HPV 16 followed by 18 in Europe, Central and South America, by HPV 52 and HPV-58 in Asia, by HPV-53 and HPV-52 in North America (6, 10, 27, 29). These differences in HPV type distribution in countries and regions may be related to different sexual habits and migrations of people (27, 30). Therefore, accurate information on the distribution of HPV genotypes based on the population is very important for both primary cervical cancer screening and prophylactic vaccination policy decision making.
Data on the distribution of HPV genotypes in Korean women remain controversial. Cho et al. showed that the three major HPV types were HPV 16, 18, and 33 (17). Shin et al. showed that the most common HPV types in southern Korean women were 70, 16, and 33 (20). Hwang et al. reported that three major HPV types were HPV-16, HPV-52, and HPV-58 in descending order, which is the same result of our study. The study by Hwang et al. is based on relatively large sample size of 2,470 (19).
Our study showed that the five most prevalent high-risk HPV types were Type 16, 52, 58, 18, and 56 in descending orders. Those five HPV types comprised about 76.7%, and four most prevalent types comprised 72.0% of all the 13 high risk HPV type infections. On the other hand, HPV-16 and 18, which was the most common HPV type in the western countries, comprised 34.0% of all the 13 high risk HPV type infections. The four most prevalent high-risk HPV types were not different across six age groups, except the fourth prevalent high-risk HPV type was HPV-56 and 68 in women between aged 70- and 79-yr-olds. Therefore, high risk HPV types other than HPV-16 and 18 such as genotypes 52, 58, and 56 must be targeted in the design of diagnostic tests and prophylactic vaccines for Korean women.
HPV genotype prevalence and distribution were different compared with the other regions, showing higher frequencies of HPV 52, and HPV 58. The rate of multiple genotype infections was lower than other countries as 12.3%. The most common four HR-HPV genotypes were HPV16, 52, 58, and 18 in descending order. There is a peak in HPV prevalence among women less than 30 yr of age, with a second peak among older females aged 70 to 79 yr in Korea.
In conclusion, this study provides the most representative prevalence and type-specific distribution of HPV among Korean women, and demonstrates that the epidemiology of HPV infection is different from that of other world regions.
Footnotes
This study was supported by a grant from the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (no. A084507).
References
- 1.zur Hausen H. Papillomaviruses and cancer from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.de Villiers EM, Whitley C, Gunst K. Identification of new papillomavirus types. Methods Mol Med. 2005;119:1–13. doi: 10.1385/1-59259-982-6:001. [DOI] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group: epidemiological classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.Huang S, Afonina I, Miller BA, Beckmann AM. Human papillomavirus types 52 and 58 are prevalent in cervical cancers from Chinese women. Int J Cancer. 1997;70:408–411. doi: 10.1002/(sici)1097-0215(19970207)70:4<408::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Lai HC, Sun CA, Yu MH, Chen HJ, Liu HS, Chu TY. Favorable clinical outcome of cervical cancers infected with human papillomavirus type 58 and related types. Int J Cancer. 1999;84:553–557. doi: 10.1002/(sici)1097-0215(19991222)84:6<553::aid-ijc2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papilloma virus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Um TH, Lee EH, Chi HS, Kim JW, Hong YJ, Cha YJ. Comparison of HPV genotyping assays and hybrid capture 2 for detection of high-risk HPV in cervical specimens. Ann Clin Lab Sci. 2011;41:48–55. [PubMed] [Google Scholar]
- 8.Hong SP, Shin SK, Lee EH, Kim EO, Ji SI, Chung HJ, Park SN, Yoo W, William R, Folk WR, et al. High-resolution human papillomavirus genotyping by MALDI-TOF mass spectrometry. Nat Protoc. 2008;3:1476–1484. doi: 10.1038/nprot.2008.136. [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, Hong YJ, Um TH, Lee EH, Chi HS, Koh JS, Yim HW, Cha YJ. Detection and identification of human papillomavirus using a PCR-restriction fragment mass polymorphism assay. Mol Med Report. 2011;4:645–650. doi: 10.3892/mmr.2011.475. [DOI] [PubMed] [Google Scholar]
- 10.de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 11.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–192. [PubMed] [Google Scholar]
- 12.Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the international Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–998. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 13.Poljak M, Marin IJ, Seme K, Vince A. Hybrid capture II HPV test detects at least 15 human papillomavirus genotypes not included in its current high-risk probe cocktail. J Clin Virol. 2002;25:S89–S97. doi: 10.1016/s1386-6532(02)00187-7. [DOI] [PubMed] [Google Scholar]
- 14.Huang SL, Chao A, Hsueh S, Chao FY, Huang CC, Yang JE, Lin CY, Yan CC, Chou HH, Huang KG, et al. Comparison between the Hybrid Capture II Test and an SPF1/GP6þ PCR-based assay for detection of human papillomavirus DNA in cervical swab samples. J Clin Microbiol. 2006;44:1733–1739. doi: 10.1128/JCM.44.5.1733-1739.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SA, Kang D, Seo SS, Jeong JK, Yoo KY, Jeon YT, Kim JW, Park NH, Kang SB, Lee HP, et al. Multiple HPV infection in cervical cancer screened by HPV DNA Chip. Cancer Lett. 2003;198:187–192. doi: 10.1016/s0304-3835(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Choe JY, Park SH, Park YW, Lee SS, Kang YM, Nam EJ, Park W, Kwon SR, Bae SC, et al. Prevalence of human papillomavirus infections and cervical cytological abnormalities among Korean women with systemic lupus erythematosus. J Korean Med Sci. 2010;25:1431–1437. doi: 10.3346/jkms.2010.25.10.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho NH, An HJ, Kim JJ, Kang S, Kim JW. Genotyping of 22 human papillomavirus types by DNA chip in Korean women: comparison with cytologic diagnosis. Am J Obstet Gynecol. 2003;188:56–62. doi: 10.1067/mob.2003.120. [DOI] [PubMed] [Google Scholar]
- 18.Oh YL, Shin KJ, Han J, Kim DS. Significance of high-risk human papillomavirus detection by polymerase chain reaction in primary cervical cancer screening. Cytopathology. 2001;12:75–83. doi: 10.1046/j.1365-2303.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- 19.Hwang HS, Park M, Lee SY. Distribution and prevalence of human papillomavirus genotypes in routine pap smear of 2,470 Korean women determined by DNA chip. Cancer Epidemiol Biomarkers Prev. 2004;13:2153–2156. [PubMed] [Google Scholar]
- 20.Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, Jung KY, Kim HH, Park UD, Cha HS, et al. Prevalence of human papillomavirus infection in women in Busan, South Korea. Int J Cancer. 2003;103:413–421. doi: 10.1002/ijc.10825. [DOI] [PubMed] [Google Scholar]
- 21.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593–4599. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velicer C, Zhu X, Vuocolo S, Liaw KL, Saah A. Prevalence and incidence of HPV genital infection in women. Sex Transm Dis. 2009;36:696–703. doi: 10.1097/OLQ.0b013e3181ad25ff. [DOI] [PubMed] [Google Scholar]
- 23.González P, HildesheimA, Rodríguez AC, Schiffman M, Porras C, Wacholder S, Piñeres AG, Pinto AA, Burk RD, Herrero R. Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiol Biomarkers Prev. 2010;19:3044–3054. doi: 10.1158/1055-9965.EPI-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fife KH, Cramer HM, Schroeder JM, Brown DR. Detection of multiple human papillomavirus types in the lower genital tract correlates with cervical dysplasia. J Med Virol. 2001;64:550–559. doi: 10.1002/jmv.1085. [DOI] [PubMed] [Google Scholar]
- 25.Bello BD, Spinillo A, Alberizzi P, Cesari S, Gardella B, D'Ambrosio G, Roccio M, Silini EM. Cervical infections by multiple human papillomavirus (HPV) genotypes: prevalence and impact on the risk of precancerous epithelial lesions. J Med Virol. 2009;81:703–712. doi: 10.1002/jmv.21429. [DOI] [PubMed] [Google Scholar]
- 26.Forslund O, Antonsson A, Edlund K, van den, Hansson BG, Meijer CJ, Ryd W, Rylander E, Strand A, Wadell G, et al. Population-based type-specific prevalence of high-risk human papillomavirus infection in middle-aged Swedish women. J Med Virol. 2002;66:535–541. doi: 10.1002/jmv.2178. [DOI] [PubMed] [Google Scholar]
- 27.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV International Biological Study on Cervical Cancer (IBSCC) Study Group. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 28.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Cai HB, Ding XH, Chen CC. Prevalence of single and multiple human papillomavirus types in cervical cancer and precursor lesions in Hubei, China. Oncology. 2009;76:157–161. doi: 10.1159/000195885. [DOI] [PubMed] [Google Scholar]
- 30.Barzon L, Giorgi C, Buonaguro FM, Palù G. Guidelines of the Italian Society for Virology on HPV testing and vaccination for cervical cancer prevention. Infect Agent Cancer. 2008;3:14. doi: 10.1186/1750-9378-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]