How did the study come about?
The Tromsø Study was initiated in 1974 in an attempt to help combat the high mortality of cardiovascular diseases in Norway, that was particularly pronounced among middle-aged men. In the mid-1970s, Norwegian men had a 20% risk of dying of myocardial infarction (MI) before the age of 75 years. The situation in Northern Norway was even worse.1 The primary aim of the Tromsø Study was to determine causes of the high cardiovascular mortality, and also to develop ways of preventing heart attacks and strokes. This was reflected through the first name of the study: The Tromsø Heart Study. However, during the 37 years since the first examination of the Tromsø Study took place, increasing emphasis has been put on other chronic diseases and conditions, in particular atrial fibrillation, venous thromboembolism, diabetes mellitus, osteoporosis and fractures. It has been a deliberate policy to invite a wide range of faculty research groups to join in with subprojects in the surveys, and there are currently some 100 different ongoing research projects based on the data from the consecutive six surveys.
The study was initially funded by the University of Tromsø, and has been so for the entire period since 1974, but there have also been substantial contributions, directly and indirectly from, for example, the National Screening Services, the Research Council of Norway, Northern Norway Regional Health Authority, Norwegian Council on Cardiovascular Diseases and Norwegian Foundation for Health and Rehabilitation. Teams of investigators approach public research programmes for funding of the different examinations conducted.
Tromsø is the largest city in Northern Norway. It is situated ∼400 km north of the Arctic Circle, and has approximately 67 000 inhabitants. The physical living conditions are dominated by dramatic changes in the light with 2 months of midnight sun and 2 months of the polar night. However, due to the Gulf Stream, the climate is relatively mild, the latitude (69°N) taken into account.
What does the cohort cover?
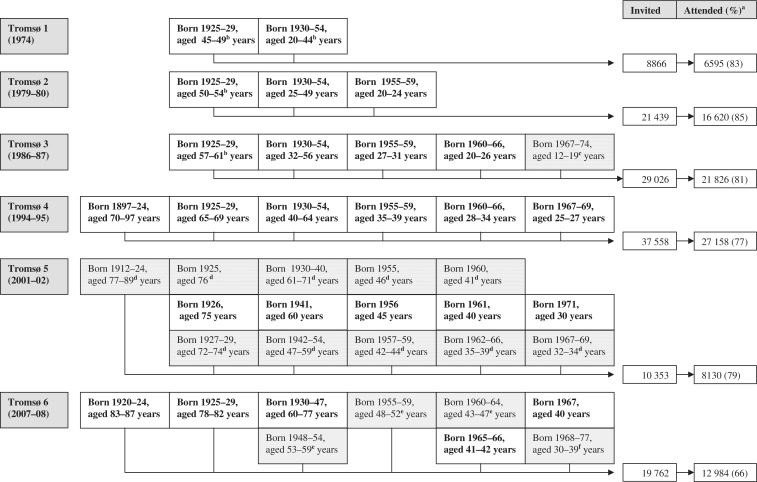
The Tromsø Study consists of six surveys (referred to as Tromsø 1–6) that have been conducted in the municipality of Tromsø from 1974 to 2008 (Table 1 and Figure 1). The Tromsø Study population includes subjects who have attended at least one of the six surveys, 40 051 subjects in total. As detailed below, the attendance rates have been high (>75% in surveys 1–5), but somewhat lower in the last survey (66%) conducted in 2007–08, due to lower attendance rate among the relatively young and those who never had participated in the previous Tromsø Study surveys.
Table 1.
The Tromsø Studya
| Age group (years) | Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Attendees |
Non-attendees |
Attendees |
Non-attendees |
||||||
| n (%) | Age, mean (years) | n | Age, mean (years) | n (%) | Age, mean (years) | n | Age, mean (years) | ||
| Tromsø 1 (1974) | 20–49 | 6595 (74.4) | 33.7 | 2271 | 30.2 | – | |||
| Tromsø 2 (1979–80) | 20–54b | 8477 (73.8) | 35.7 | 3004 | 31.3 | 8144 (81.8) | 32.9 | 1815 | 28.7 |
| Tromsø 3 (1986–87) | 12–67c | 10 963 (71.7) | 37.6 | 4318 | 32.5 | 10 863 (79.0) | 35.4 | 2882 | 29.9 |
| Tromsø 4 (1994–95) | 25–97 | 12 865 (69.6) | 46.6 | 5615 | 40.9 | 14 293 (74.9) | 47.2 | 4785 | 44.1 |
| Tromsø 5 (2001–02) | 30–89 | 3511 (75.7) | 59.9 | 1125 | 46.0 | 4619 (80.8) | 59.4 | 1098 | 50.8 |
| Tromsø 6 (2007–08) | 30–87 | 6054 (62.9) | 57.5 | 3571 | 54.5 | 6930 (68.4) | 57.5 | 3207 | 58.1 |
aExamination year, age groups included and attendance rate. Number of subjects (n), mean age in the six different surveys according to gender and attendance.
b20–49 years in women.
cAll men aged 20–61 years and women aged 20–56 years were invited (see text).
Figure 1.
The Tromsø Study. Invitation by birth cohort and attained age in Tromsø 1–6. Invitation of total birth cohorts is marked as bold, shading indicates that samples of birth cohorts were invited. aAdjusted for deaths, emigration from Tromsø during the survey period etc. bMen only. c10% of total birth cohort and offspring of high-risk men who participated in a family intervention trial after the second survey. dRestricted to those who participated in the second visit in Tromsø 4. e 40% of the total birth cohorts. f10% of the total birth cohorts
Enrolment methods
The six surveys had the same general design. Based on the official population registry, residents of the municipality of Tromsø were invited to take part in the survey. A personal invitation was mailed about 2 weeks before a suggested time of appointment. The subjects were free to attend whenever suitable within the time frame of the study (∼1 year). The invitation leaflet included information about the survey and the examination. Non-attendees were given one reminder.
Tromsø 4–6 also included a second visit with a more extensive examination of the participants. Subjects in the population who were eligible for the second-visit examinations were identified before they were to attend the first visit of the survey. If they attended this, they were invited to the second-visit examination 2–4 weeks later.
Who is in the sample?
Table 1 gives the basic features of the Tromsø Study with regard to year of examination, the number of subjects who attended, age groups included and attendance rate. The table also gives the mean age according to gender and attendance in each of the six surveys.
The aim has been to include large, representative samples of the Tromsø population, with invitation of whole birth cohorts and random samples. We have obtained repeated measurement from the same population.
Figure 1 shows the general outline of the study. Only invitation to the main survey (first visit) is shown. In Tromsø 1–4, new birth cohorts were consecutively added to the invited populations. Subjects living in Tromsø, in the birth cohorts examined in previous surveys, were invited to the next survey even if they had not attended the previous survey. The fifth survey differs from the other surveys in that a larger proportion of those invited were selected on the basis that they had participated in the second visit of the fourth survey (Figure 1).
Tromsø 3 also included a sample of 1134 youngsters (aged 12–19 years). Approximately 66% of these children and adolescents were part of a family intervention trial2 initiated after Tromsø 2, but a 10% random sample was also invited. Included in the survey (data not shown) were also 89 women born before 1930 who were married to high-risk men included in the family intervention trial.
The fourth survey represented a new development in the history of the Tromsø Study. Due to a closer collaboration with researchers engaged in clinical medicine, a large proportion of the participants in this survey, as well as later in Tromsø 5 and 6, were also invited to a second visit with new, extended examinations by non-invasive procedures and measurements using advanced technology (see ‘Physical examinations’ section). Due to lack of capacity and funding, not all subjects in Tromsø aged ≥25 years could be invited, but these second-visit participants from 1994 and 1995 represent a cohort within the cohort, and they have been the basis for the invitations to the two later surveys.
In Tromsø 4, all men in the Tromsø municipality aged 55–74 years (born between 1920 and 1939) and women aged 50–74 years (born between 1920 and 1944) as well as smaller (5–8%) random samples of the other age groups aged < 85 years were invited to the more extensive second-visit examination. Subjects who were part of the family intervention trial2 were also invited. A total of 7965 (or 76% of the 10 542 eligible subjects) attended this second visit.
Subjects who had previously taken part in the second visit in Tromsø 4 were eligible for a second-visit examination in Tromsø 5. A total of 5939 subjects participated (85% of the eligible population).
The population eligible for the second visit in Tromsø 6 were first-visit participants aged 50–62 or 75–84 years, a 20% random sample of men and women aged 63–74 years and subjects, if not already included in the two groups above, who had attended the second visit of Tromsø 4 and were aged <75 years in 1994. Out of the 11 484 subjects who were eligible, 7307 (64%) attended.
Tromsø 1–6 — participation in repeated surveys and characteristics of non-attendees
A total of 53 731 different subjects have been invited to at least one of the six surveys and 40 051 (75%) have attended at least one. Repeated measurements are available for many of the subjects. A total of 1235 men have taken part in all 6 surveys from 1974 to 2008, 3467 men and women have attended 5 surveys, 4125 subjects 4 surveys, 6330 3 surveys, 8185 2 surveys and 16 709 individuals have attended only 1 survey. We further note that 3631 men attended all 4 surveys during 1974–95 and 5286 women attended all the 3 surveys conducted during 1979–95.
Subjects who did not attend tended to be younger and the proportion of men to be higher than in attendees (Table 1). Further detailed information about the age and sex distribution according to attendance is given elsewhere (www.tromsostudy.com). Non-attendees tended also to be single. In the most recent survey, Tromsø 6, 59% of the attendees were married whereas 41% of the non-attendees were so, and the higher attendance rate in married subjects was a consistent pattern over the age groups. Similar results have been found in Tromsø 2.3
Legal restrictions put on us by the Norwegian Data Inspectorate preclude detailed analyses of mortality or morbidity according to attendance. However, the total age- and sex-adjusted mortality for subjects who were invited to Tromsø 4 was 6.9/1000 person-years in subjects who had attended all Tromsø 2–4 surveys and 11.1/1000 person-years in subjects who had been invited to all three, but only attended Tromsø 4, demonstrating lower mortality in subjects who were consistent attendees.
What has been measured?
Table 2 gives a brief overview of the types of data collected in the different parts of the Tromsø Study. For a comprehensive overview of the data collected, we refer to our NESSTAR website (http://tromsoundersokelsen.uit.no/tromso/).
Table 2.
Brief overview of data collected in the different surveys that form the Tromsø Study
| Tromsø Study survey number | ||||||
|---|---|---|---|---|---|---|
| Type of informationa | 1 | 2 | 3 | 4 | 5 | 6 |
| Marital status, age, sex | x | x | x | x | x | x |
| Questionnaire data | x | x | x | x | x | x |
| Interview | x | x | x | x | x | x |
| Measured weight and height | x | x | x | x | x | x |
| Measured waist and hip circumference | x | x | x | |||
| Measured blood pressure | x | x | x | x | x | x |
| Blood sample (blood lipids) | x | x | x | x | x | x |
| Blood sample (hormones) | x | x | x | |||
| Blood samples (haematology) | x | x | x | |||
| Blood samples (other blood analyses) | x | x | x | x | ||
| Electrocardiography (ECG) | x | x | x | x | ||
| Echocardiography | x | x | x | |||
| Ultrasound examination of the carotid artery | x | x | x | |||
| Ultrasound examination of the abdominal aorta | x | x | ||||
| Spirometry | x | x | ||||
| Bone mineral densitometry | x | x | x | |||
| Urinary analyses (microalbuminuria) | x | x | x | |||
| Examination of sight (visual acuity) | x | x | ||||
| Examination of number of falls | x | |||||
| Cognitive testing | x | x | ||||
| Retinal photography, optical coherence tomography | x | |||||
| Pain sensitivity | x | |||||
aNote that some of the examinations have been conducted only in parts of the population. For a close to complete overview of the data collected, please see our website (http://tromsoundersokelsen.uit.no/tromso/).
Questionnaires
In all surveys, a questionnaire has been enclosed in the invitation. In Tromsø 2–6, the participants were given a second questionnaire and they were asked to return it by mail in a pre-addressed stamped envelope. The large majority, typically ∼90%, did so. The questionnaires are posted on our website (www.tromsostudy.com). In Tromsø 1 and 2, the first questionnaire covered prevalent cardiovascular diseases and cardiovascular symptoms, diabetes, physical activity, smoking habits, employment, family history of coronary heart disease (CHD) and ethnicity (i.e. Norwegian, Sami or Finnish grandparents).
Over the years, both the first and the second questionnaires have expanded and include questions about a wide range of diseases and symptoms, dietary habits, other lifestyle aspects, use of medication, sleeping patterns, socio-economic status, use of health-care services and menstruation and childbirths (for women). In order to supplement the information from the questionnaires, a short interview was included in most of the surveys. The topics have differed (e.g. family history of CHD, menopause, current and former use of medications).
Physical examinations
In Tromsø 1, the physical examination consisted of measurements of blood pressure, height and weight. In the later surveys, particularly in Tromsø 4–6, an increasing number of physical examinations were performed and also included, for example, hip and waist circumference, bone mineral densitometry of the forearm (single X-ray absorptiometry, SXA) and registration of balance and falls. In Tromsø 4–6, the second visit included ultrasound of carotid artery, echocardiography, electrocardiography, bone densitometry of the hip (dual-energy X-ray absorptiometry, DEXA) and spirometry. Ultrasound examination of the abdominal aorta was done in Tromsø 4 and 5. Cognitive testing was performed in Tromsø 5 and 6 and the last survey also included pain sensitivity (heat pain threshold and cold pressor test), retinal photography and optical coherence tomography. The reason for including these and other new physical examinations has been to get a more comprehensive picture of the microvascular damage as assessed by examination of the retina. This is also important with regard to diabetes epidemiology. Pain sensitivity is a new research area in The Tromsø Study.
Blood samples
Blood samples were in Tromsø 1 analysed for haemoglobin and non-fasting serum total cholesterol, triglycerides and glucose. The number of blood analyses has expanded in the subsequent surveys, and covers indicators of renal function, inflammation, haematology, markers of hepatic disorders and hormones (including sex hormones). Blood samples for later analyses of novel biomarkers have been stored at each survey. Examples of such biomarkers that have been measured in stored samples include plasma homocysteine analysed from blood samples from Tromsø 3 and high-sensitive C-reactive protein (CRP) and osteoprotegerin from the second visit of Tromsø 4. DNA samples have been stored from Tromsø 3 and onwards, and DNA has been extracted from all Tromsø 4 participants.
Urine samples
In Tromsø 4–6, morning urine samples from three consecutive days were collected from those who attended the second visit. Presence of protein, glucose, blood, nitrite and leucocytes were assessed in each of the three samples, and quantification of microalbuminuria and creatinine levels was performed.
Follow-up of the participants
All participants are being followed up with regard to mortality and disease incidence. The national 11-digit unique personal identification number facilitates complete follow-up regarding cause of death and allows linkage to official registries of some diseases (like cancer) and cause of death. Cancer registration is mandatory by law and the data quality is considered to be high.4 Emigration from the municipality, from Norway and date of death are registered by the Population Register of Norway. Recent examples of linkage between data from the Tromsø Study and cause-specific mortality or cancer incidence include relationships between weight loss5 or albuminuria6 and mortality, and between albuminuria and cancer incidence.7
The University Hospital of North Norway is the only hospital in Tromsø. Admissions to other hospitals are unlikely because of long distances as the nearest hospital is >200 km from Tromsø. We are thus able to follow the subjects who have taken part in the surveys with regard to incident (fatal and non-fatal) cases of cardiovascular endpoints (MI, stroke, atrial fibrillation and venous thromboembolism), diabetes mellitus and non-vertebral fractures. Adjudication of first-ever cases of these conditions is performed by independent endpoint committees. Each case is reviewed separately. Those linkages have allowed numerous publications during the last years, and we refer to our website (www.tromsostudy.com) for details.
There are also ample possibilities for linkage of data from the Tromsø Study with data from other registries like the Norwegian Patient Registry, the Norwegian Prescription Database and the Medical Birth Registry.
Clinical trials
All six surveys provided the starting point for a number of subgroup studies. The surveys have also been the basis for clinical research, often in collaboration with staff at the Department of Clinical Medicine, University of Tromsø and the University Hospital of North Norway. The most frequently cited of the studies concerned the effect of omega-3 fatty acids on blood pressure.8 Several case–control studies have also been conducted based on data from the Tromsø Study.
What has been found?
Although the initial and primary aim of the Tromsø Study was to determine causes of the high cardiovascular mortality, a number of other research areas and hypothesis have been addressed. Some of the projects, like the case–control studies and clinical trials, have been undertaken in order to test a specific hypothesis (e.g. serum homocysteine and risk of MI),9 whereas other findings are based on post hoc analyses (e.g. the association between coffee drinking and serum cholesterol10).
Approximately 50 PhD theses and a number of well-known publications have been based on data from the Tromsø Study. The most well-known publication from Tromsø 1 was the identification of a protective effect of high HDL cholesterol on the risk of MI.11 From Tromsø 2, it was reported that coffee drinking increased serum cholesterol.10 It was later (based on data from Tromsø 3) found that it was boiled (unfiltered) coffee that had this effect on the blood cholesterol level.12 The later studies (Tromsø 4–6) have resulted in a large number of publications, many in high-ranking journals. Including the 2 publications mentioned above,10,11 the 10 most cited publications based entirely on data from the different surveys include information concerning serum homocysteine,9 echolucent (‘soft’) carotid plaques13 and serum Apo A114 as risk factors for clinical cardiovascular diseases, associations between level of education and risk factors for cardiovascular risk factors,15 determinants of γ-glytamyl-transferase,16 the prevalence of and risk factors for abdominal aortic aneurysms17 as well as the prevalence of ankylosing spondylitis.18 By early 2011, six publications based entirely on data from the Tromsø Study have been cited >10 times per year after publication.9,11,13,17,19,20 Data from the Tromsø Study are increasingly being included in large, international collaborative analyses like MOnica Risk, Genetics, Archiving and Monograph (MORGAM) (genetics of cardiovascular diseases), Finland-United States Investigation of NIDDM Genetics (FUSION) (genetics of type 2 diabetes), Wellcome Trust Sanger Institute (genetics of pain), deCODE (genetics of atrial fibrillation), Consortium on Health and Ageing: Network of Cohorts in Europe and the United States (CHANCES) (a PF7 project on ageing and health), Global Burden of Diseases, Injuries and Risk Factors Study and the Emerging Risk Factors Collaboration, for example.21–24 Data from some of the surveys are also included in the Norwegian Cohort of Norway (CONOR) cohort.25
A list of publications can be found on our website (www.tromsostudy.com).
What are the main strengths and weaknesses?
The main strength of the Tromsø Study is the longitudinal design. With its repeated surveys with high attendance rates conducted within the same community, the Tromsø Study is able to demonstrate how the prevalence of different risk factors, such as smoking, change with time and, even more important, provides ample possibilities to study longitudinal trends, quantify tracking and describe the development of risk factors for diseases in the same individual up to six times during a period of up to 34 years. We have in this way for example been able to demonstrate that cross-sectional studies give a misleading picture of how weight changes with advancing age in men.26
It is also a major strength that prospective studies with a number of different endpoints can be performed because there is only one hospital in the community and because all Norwegians have a unique personal identification number.
Furthermore, it is a significant strength that we (since 1994) have collected much information from clinical examinations by non-invasive procedures (e.g. ultrasound of the abdominal aorta and the carotid artery, echocardiography, electrocardiography, bone mineral densitometry, cognitive function) thereby obtaining information also about intermediate and surrogate endpoints and preclinical stages. The surveys have also provided information about prevalence of a number of conditions [e.g. abdominal aortic aneurysms (AAAs), carotid stenosis and osteoporosis]. It is therefore possible to investigate risk factors for the disease, relations to other diseases and the relationships between these different measures.
Overall attendance rate is high. However, as in other population studies in Norway and in other countries, the attendance rates are falling. Tromsø 1–4 and 6 included all adults in Tromsø, all subjects in selected age groups or representative samples of them. Subjects aged <20 years (except for a small sample in 1986–87) have not been invited to any of the surveys that constitute the Tromsø Study. Furthermore, we have no cross-sectional information from those aged <30 years in Tromsø 5 and 6, i.e. after 1995. However, currently a survey (FitFutures) (www.fitfutures.no) with many elements, very similar to the main Tromsø Study, being conducted among high-school students in Tromsø. Data from this survey will be included in the Tromsø Study database.
As the Tromsø Study is based in the seventh largest Norwegian city with relatively few immigrants, it is limited with regard to ethnic diversity; the vast majority of the participants are Caucasian subjects.
Can I get hold of data? Where can I find out more?
Our website (www.tromsostudy.com) gives a brief overview of the different surveys and an overview of the data collected (http://tromsoundersokelsen.uit.no/tromso/). Not all data from the second visit of the last survey (Tromsø 6) are currently (February 2011) included, but we continuously expand the database with data from smaller projects conducted under the Tromsø Study umbrella. We invite other research groups to apply for access to the data (see instructions on our website).
Conflict of Interest: None declared.
References
- 1.Thelle DS, Førde OH, Try K, Lehmann EH. Tromsø Heart Study—Methods and main results of cross-sectional study. Acta Med Scand. 1976;200:107–18. [PubMed] [Google Scholar]
- 2.Knutsen SF, Knutsen R. The Tromsø Survey: the Family Intervention study—the effect of intervention on some coronary risk factors and dietary habits, a 6-year follow-up. Prev Med. 1991;20:197–212. doi: 10.1016/0091-7435(91)90020-5. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen BK, Thelle DS. The Tromsø Heart Study: responders and non-responders to a health questionnaire, do they differ? Scand J Soc Med. 1988;16:101–4. doi: 10.1177/140349488801600207. [DOI] [PubMed] [Google Scholar]
- 4.Larsen IK, Småstuen M, Parkin DM, Bray F. Data Quality at the Cancer Registry of Norway. Cancer in Norway 2006—Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo: Cancer Registry of Norway; 2007. [Google Scholar]
- 5.Wilsgaard T, Jacobsen BK, Mathiesen EB, Njølstad I. Weight loss and mortality: a gender-specific analysis of the Tromsø study. Gend Med. 2009;6:575–86. doi: 10.1016/j.genm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen L, Jenssen T, Heuch I, Jacobsen BK. The combined effect of albuminuria and inflammation on all-cause and cardiovascular mortality in nondiabetic persons. J Intern Med. 2008;264:493–501. doi: 10.1111/j.1365-2796.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- 7.Jørgensen L, Heuch I, Jenssen T, Jacobsen BK. Association of albuminuria and cancer incidence. J Am Soc Nephrol. 2008;19:992–98. doi: 10.1681/ASN.2007060712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bønaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromsø study. N Engl J Med. 1990;322:795–801. doi: 10.1056/NEJM199003223221202. [DOI] [PubMed] [Google Scholar]
- 9.Arnesen E, Refsum H, Bønaa KH, Ueland PM, Førde OH, Nordrehaug JE. Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995;24:704–9. doi: 10.1093/ije/24.4.704. [DOI] [PubMed] [Google Scholar]
- 10.Thelle DS, Arnesen E, Førde OH. The Tromsø heart study. Does coffee raise serum cholesterol? N Engl J Med. 1983;308:1454–57. doi: 10.1056/NEJM198306163082405. [DOI] [PubMed] [Google Scholar]
- 11.Miller NE, Thelle DS, Førde OH, Mjøs OD. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1977;1:965–68. doi: 10.1016/s0140-6736(77)92274-7. [DOI] [PubMed] [Google Scholar]
- 12.Bønaa K, Arnesen E, Thelle DS, Førde OH. Coffee and cholesterol: is it all in the brewing? The Tromsø Study. BMJ. 1988;297:1103–4. doi: 10.1136/bmj.297.6656.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathiesen EB, Bønaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the Tromsø study. Circulation. 2001;103:2171–75. doi: 10.1161/01.cir.103.17.2171. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa T, Fidge N, Thelle DS, Førde OH, Miller NE. The Tromsø Heart Study: serum apolipoprotein AI concentration in relation to future coronary heart disease. Eur J Clin Invest. 1978;8:179–82. doi: 10.1111/j.1365-2362.1978.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen BK, Thelle DS. Risk factors for coronary heart disease and level of education. The Tromsø Heart Study. Am J Epidemiol. 1988;127:923–32. doi: 10.1093/oxfordjournals.aje.a114895. [DOI] [PubMed] [Google Scholar]
- 16.Nilssen O, Førde OH, Brenn T. The Tromsø Study. Distribution and population determinants of gamma-glutamyltransferase. Am J Epidemiol. 1990;132:318–26. doi: 10.1093/oxfordjournals.aje.a115661. [DOI] [PubMed] [Google Scholar]
- 17.Singh K, Bønaa KH, Jacobsen BK, Björk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromsø Study. Am J Epidemiol. 2001;154:236–44. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 18.Gran JT, Husby G, Hordvik M. Prevalence of ankylosing spondylitis in males and females in a young middle-aged population of Tromsø, northern Norway. Ann Rheum Dis. 1985;44:359–67. doi: 10.1136/ard.44.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–82. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 20.Johnsen SH, Mathiesen EB, Joakimsen O, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: A 6-year follow-up study of 6226 persons: The Tromsø study. Stroke. 2007;38:2873–80. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Erqou S, Walker M, et al. The Emerging Risk Factors Collaboration: analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22:839–69. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 22.The Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Weerd M, Greving JP, Hedblad B, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41:1294–97. doi: 10.1161/STROKEAHA.110.581058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gudbjartsson DF, Holm H, Gretarsdottir S, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–78. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Næss Ø, Søgaard AJ, Arnesen E, et al. Cohort profile: cohort of Norway (CONOR) Int J Epidemiol. 2008;37:481–85. doi: 10.1093/ije/dym217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen BK, Njølstad I, Thune I, Wilsgaard T, Løchen ML, Schirmer H. Increase in weight in all birth cohorts in a general population: The Tromsø Study, 1974–1994. Arch Intern Med. 2001;161:466–72. doi: 10.1001/archinte.161.3.466. [DOI] [PubMed] [Google Scholar]