Abstract
Background Current physical activity guidelines imply that, by comparison with moderate physical activity (MPA), the benefits of engaging in vigorous physical activity (VPA) are attributed to the greater energy expenditure dose per unit of time and do not relate to intensity per se. The purpose of this study was to determine whether VPA influences the metabolic syndrome (MetS) independent of its influence on the energy expenditure dose of moderate-to-vigorous physical activity (MVPA).
Methods Participants consisted of 1841 adults from the 2003–06 National Health and Nutrition Examination Survey, a representative cross-sectional study. MPA and VPA were measured objectively over 7 days using Actigraph accelerometers. MetS was determined using an established clinical definition. Associations between physical activity and the MetS were determined using logistic regression and controlled for relevant covariates.
Results Analyses revealed that VPA remained a meaningful predictor of the MetS after controlling for the total energy expenditure dose of MVPA such that the relative odds of the MetS was 0.28 (95% confidence interval 0.17–0.46) in the group with the highest VPA compared with the group with no VPA. VPA had a greater influence on the MetS than an equivalent energy expenditure dose of MPA. For instance, between 0 and 500 MET min/week of MPA the adjusted prevalence of the MetS decreased by 15.5%, whereas between 0 and 500 MET min/week of VPA the prevalence decreased by 37.1%.
Conclusion These cross-sectional findings suggest that VPA per se has an important role in cardiometabolic disease prevention.
Keywords: Physical activity, metabolic syndrome, exercise, accelerometer
Introduction
The health benefits of moderate-to-vigorous physical activity (MVPA) are well recognized by exercise scientists1 and health-care practitioners.2 The USA,3 UK4 and the World Health Organization5 have recently published physical activity guidelines that provide the public with targets for the amount and intensity of physical activity needed for good health. The key recommendation within these guidelines is that adults ‘should do at least 150 min a week of moderate-intensity, or 75 min a week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activity’.
The guidelines recognize that, in comparison with vigorous physical activity (VPA), about half as many calories are expended with an equivalent duration of moderate physical activity (MPA).1,3–5 Because the guidelines indicate that 75 min of VPA are equivalent to 150 min of MPA, they imply that there are little or no added health benefits of VPA other than the shorter time frame needed to expend the appropriate amount of energy. In other words, the benefits of engaging in VPA are attributed to the greater expenditure energy dose per unit of time, and do not relate to the intensity per se. This needs to be considered in light of the scientific committee report from which the guidelines were developed. The report recognized that unanswered issues exist in response to the question of how much of what type of activity is appropriate for health promotion.1 The report’s research recommendations indicate that future investigations need to evaluate the effects of physical activity intensity at fixed energy expenditure doses.1 Therefore, the purpose of this study was to determine whether VPA is associated with health, in this case cardiometabolic health, independent of its influence on the energy expenditure dose.
Methods
Participants
The study is based on the 2003–04 and 2005–06 cycles of the U.S. National Health and Nutrition Examination Survey (NHANES), a nationally representative cross-sectional survey.6 NHANES consisted of a home interview and a physical examination conducted in a mobile examination centre. Consent was obtained from all participants. NHANES was approved by the National Center for Health Statistics.
To be considered for the present study, participants had to be 20–64 years old, not pregnant and to have completed the home and morning mobile exam-centre visits. Out of 3071 eligible participants, 564 were excluded due to incomplete physical activity accelerometry data, 656 were excluded because they had not fasted or were missing metabolic syndrome (MetS) data and 10 were excluded due to missing covariate data. This left a final sample of 1841.
Physical activity
MPA and VPA were measured with Actigraph AM-7164 accelerometers (Actigraph, Ft. Pensacola, FL), a uniaxial accelerometer that records average intensities in 1-min intervals or epochs. Participants were asked to wear the accelerometer on their right hip for 7 consecutive days following their mobile exam-centre visit, except when sleeping or when the accelerometer could get wet. Accelerometers were mailed back to the survey collaborators. Accelerometer data were downloaded by survey collaborators and outliers and unreasonable values were removed.
Additional data reduction was completed by the authors based on existing precedence.7–9 Initially, we removed incomplete days, defined as <10 h of wear time. Non-wear time was determined as periods of > 60 min with zero counts.7–9 The second data reduction step involved removing participants with an insufficient number of complete days (<4 complete days).7–9 Next, we estimated the average daily MPA and VPA energy expenditure. A regression equation was used to estimate metabolic equivalents (METs) from the count-per-minute value for each epoch.10 Based on the intensity thresholds corresponding to the American1 and WHO5 physical activity guidelines, MPA was defined as 3.0–5.99 METs and VPA as ≥6.0 METs. METs were summed over each day to create MET minute/day values for MPA and VPA. For instance, if a participant was above the VPA threshold for 3 min with METs of 6.0, 7.0 and 8.0, their VPA MET min/day value would have been 21. MET min/day values were averaged over the number of complete days and multiplied by 7 to convert to MET min/week.
Participants were divided into four groups for MVPA and MPA (which accounted for 92% of MVPA): inactive (0–249 MET min/week), somewhat active (250–499 MET min/week), active (500–999 MET min/week) and very active (≥1000 MET min/week). These cut-points are based on their equivalence to the physical activity guideline—250 MET min/week represents an energy expenditure dose equivalent to half of the guideline, 500 MET min/week is equivalent to the minimal guideline (i.e. 150 min of MPA or 75 min of VPA or an equivalent combination) and 1000 MET min/week is equivalent to double the minimal guideline.5
To derive comparably sized VPA groups to those derived for MVPA, the percentiles within the sample that were equivalent to the MET min/week cut-points for MVPA were calculated. Since more than half of the participants accumulated zero MET min/week of VPA, only three VPA groups were created: none (<7.1 MET min/week, equivalent to <73rd percentile and the inactive and somewhat active MVPA groups), moderate (7.1–41.2 MET min/week, equivalent to the 73rd to 88th percentiles and the active MVPA group) and high (≥41.2 MET min/week, equivalent to ≥89th percentile and the very active MVPA group).
Metabolic syndrome
The primary outcome was the MetS, a clustering of cardiometabolic risk factors that increases cardiovascular disease, diabetes and all-cause mortality risks.11 The MetS was classified based on the 2009 Joint Interim Societies definition12 and was present if three or more of the following were present: high waist circumference (men ≥94 cm, women ≥80 cm), high triglycerides (≥1.7 mmol/l), low HDL cholesterol (men <1.0 mmol/l, women <1.3 mmol/l), elevated blood pressure (systolic ≥130 mmHg or diastolic ≥85 mmHg or hypertension medication use) and elevated glucose (≥5.6 mmol/l or diagnosed diabetes).
Waist circumference was measured to the nearest 0.1 cm at the iliac crest. Four blood pressure measurements were obtained in a seated position using a manual sphygmomanometer. The average of the last three readings was used. Blood samples were obtained after an overnight fast for the measurement of lipids and glucose as described in detail elsewhere.13 Briefly, cholesterol and triglycerides were measured enzymatically in a series of coupled reactions hydrolysing cholesterol ester and triglyceride to cholesterol and glycerol, respectively.14 HDL cholesterol was measured using the direct HDL immunoassay method.15 Fasting plasma glucose was determined using a hexokinase enzymatic method.16
Covariates
Covariates included sex, ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic and other), smoking (current smoker, previous smoker, non-smoker), alcohol (<15 drinks/week, 15–30 drinks/week, or >30 drinks/week), key dietary variables and the poverty-to-income ratio. Dietary variables were assessed via a single 24-h recall and consisted of total fat (≤35% or >35% total calories), saturated fat (≤10% or >10% total calories) and sodium (≤2300 or >2300 mg/day).17 The poverty-to-income ratio was based on family income, size and composition.18
Statistical analysis
Analyses were completing using SAS version 9.2 (SAS Institute Inc., Cary, NC) and accounted for the survey design and morning/fasted subsample weights. Spearman correlations were used to examine relations between continuous physical activity variables. Differences between physical activity groups were determined using general linear models. Relations between physical activity and the MetS were determined using logistic regression and controlled for the covariates. Bivariate logistic models estimated the effects of MVPA, MPA and VPA on their own. Multivariate logistic models, which included both MVPA and VPA (or MPA and VPA), estimated the independent effects of the intensity and energy expenditure dose. Logistic regression findings are presented as odds ratios (ORs) and their associated 95% confidence intervals (CIs).
Results
Descriptive characteristics
The average age of the 1841 participants was 42 years. Slightly more than half were men and 34.6% had the MetS. The average MVPA, MPA and VPA values were 470, 432 and 38 MET min/week, respectively. Just under one-third of the participants achieved the physical activity guideline of 500 MET min/week based on MVPA (30.3%) or MPA alone (28.8%), whereas only 1.7% did so based on VPA alone. Additional information on the study variables is shown in Table 1.
Table 1.
Descriptive characteristics of participants in the 2003–06 NHANES
| Total (N = 1841) | Men (N = 942) | Women (N = 892) | |
|---|---|---|---|
| MetS variables | |||
| Waist circumference | |||
| Mean (cm) | 97.6 ± 16.1 | 101.5 ± 15.2 | 93.6 ± 16.1 |
| High waist (%) | 72.2 | 65.5 | 78.9 |
| Fasting glucose | |||
| Mean (mmol/l) | 5.6 ± 1.4 | 5.7 ± 1.3 | 5.5 ± 1.5 |
| High glucose (%) | 34.8 | 42.8 | 26.9 |
| Blood pressure | |||
| Mean systolic blood pressure (mmHg) | 120 ± 16 | 122 ± 13 | 117 ± 17 |
| Mean diastolic blood pressure (mmHg) | 72 ± 11 | 73 ± 11 | 71 ± 10 |
| High blood pressure (%) | 35.2 | 39.8 | 30.7 |
| Triglycerides | |||
| Mean (mmol/l) | 1.62 ± 1.45 | 1.82 ± 1.50 | 1.43 ± 1.37 |
| High triglycerides (%) | 30.9 | 38.0 | 23.8 |
| HDL cholesterol | |||
| Mean (mmol/l) | 1.41 ± 0.40 | 1.27 ± 0.33 | 1.54 ± 0.42 |
| Low HDL cholesterol (%) | 24.7 | 18.7 | 30.6 |
| MetS (%) | 34.6 | 38.5 | 30.7 |
| Physical activity variables | |||
| MVPA | |||
| Mean, MET min/week | 470 ± 560 | 583 ± 638 | 356 ± 441 |
| ≥500 MET min/week (%) | 30.3 | 38.8 | 21.8 |
| MPA | |||
| Mean, MET min/week | 432 ± 481 | 540 ± 541 | 332 ± 376 |
| ≥500 MET min/week (%) | 28.8 | 37.2 | 20.3 |
| VPA | |||
| Mean, MET min/week | 38 ± 163 | 44 ± 184 | 33 ± 137 |
| ≥500 MET min/week (%) | 1.7 | 2.1 | 1.3 |
Data presented as mean ± standard deviation (SD) for continuous variables and prevalence (%) for dichotomous variables.
Relationships between physical activity variables
The MVPA, MPA and VPA variables were related to each other. Spearman correlations (r values) between the MET min/week values were 0.96 for MVPA vs MPA, 0.61 for MVPA vs VPA and 0.37 for MPA vs VPA (P < 0.001). The average MVPA, MPA and VPA values of the different physical activity groups are shown in Table 2. The very active MPA group had higher VPA levels than the remaining MPA groups (P < 0.001). The high VPA group had higher MPA levels than the none and low VPA groups (P < 0.001).
Table 2.
Description of physical activity variables within physical activity groups
| MET min/week (mean ± SD) |
|||
|---|---|---|---|
| Physical activity group | MVPA | MPA | VPA |
| MVPA | |||
| Inactive (N = 915) | 117 ± 68 | 115 ± 67 | 3 ± 9 |
| Somewhat active (N = 388) | 355 ± 74 | 340 ± 80 | 15 ± 36 |
| Active (N = 302) | 706 ± 145 | 677 ± 145 | 29 ± 71 |
| Very active (N = 236) | 1620 ± 700 | 1405 ± 548 | 215 ± 401 |
| MPA | |||
| Inactive (N = 968) | 127 ± 76 | 121 ± 71 | 5 ± 21 |
| Somewhat active (N = 347) | 390 ± 166 | 358 ± 70 | 31 ± 141 |
| Active (N = 296) | 729 ± 202 | 677 ± 127 | 51 ± 149 |
| Very active (N = 230) | 1612 ± 715 | 1451 ± 512 | 161 ± 364 |
| VPA | |||
| None (N = 1337) | 288 ± 315 | 287 ± 314 | 1 ± 2 |
| Low (N = 295) | 655 ± 495 | 637 ± 494 | 18 ± 10 |
| High (N = 209) | 1218 ± 966 | 947 ± 757 | 270 ± 406 |
Data presented as mean ± SD.
Relations between physical activity and the MetS
The odds of the MetS decreased in a gradient manner when moving from the inactive (OR 1.00), to the somewhat active (OR 0.78, 95% CI 0.60–1.02), to the active (OR 0.48, 95% CI 0.36–0.66), to the very active MVPA group (OR 0.25, 95% CI 0.17–0.39). Similar inverse associations with the MetS were observed with MPA alone and VPA alone (Model 1 in Table 3).
Table 3.
OR (95% CI) for the MetS according to physical activity level
| Physical activity group | Model 1a | Model 2b |
|---|---|---|
| MVPA | ||
| Inactive (N = 915) | 1.00 (referent) | 1.00 (referent) |
| Somewhat active (N = 388) | 0.78 (0.60–1.02) | 0.88 (0.67–1.15) |
| Active (N = 302) | 0.48 (0.36–0.66)d | 0.57 (0.42–0.79)d |
| Very active (N = 236) | 0.25 (0.17–0.39)d | 0.41 (0.26–0.63)d |
| MPA | ||
| Inactive (N = 968) | 1.00 (referent) | 1.00 (referent) |
| Somewhat active (N = 347) | 0.71 (0.54–0.93)c | 0.77 (0.58–1.01) |
| Active (N = 296) | 0.47 (0.35–0.64)d | 0.56 (0.41–0.77)d |
| Very active (N = 230) | 0.27 (0.18–0.40)d | 0.40 (0.26–0.61)d |
| VPA | ||
| None (N = 1337) | 1.00 (referent) | 1.00 (referent) |
| Low (N = 295) | 0.66 (0.50–0.88)c | 0.83 (0.61–1.12) |
| High (N = 209) | 0.19 (0.11–0.29)d | 0.28 (0.17–0.46)d |
Data presented as OR (95% CI). All ORs were adjusted for age, sex, ethnicity, socio-economic status, smoking, alcohol, dietary fat, saturated fat and sodium.
aModel 1: Models were adjusted for the covariates but not the other physical activity variables in the table.
bModel 2: MVPA and MPA were adjusted for the covariates and VPA. VPA was adjusted for the covariates and MVPA.
cP < 0.05 vs referent.
dP < 0.01 vs referent.
The energy expenditure dose and intensity of MVPA were independently associated with the MetS (Model 2 in Table 3). After controlling for VPA, the total dose of MVPA was associated with the MetS. In comparison with the inactive MVPA group, the active and very active MVPA groups were only 0.57 (95% CI 0.42–0.79) and 0.41 (95% CI 0.26–0.63) times as likely, respectively, to have the MetS. VPA remained a predictor of the MetS after controlling for the total energy expenditure dose of MVPA, such that the odds of the MetS was 0.28 (95% CI 0.17–0.46) in the high VPA group by comparison with the group who accumulated no VPA.
The analyses for the MetS were repeated for individual MetS components (Table 4). The patterns for the individual components were similar to those observed for the MetS. An exception was for high glucose; VPA was not associated with high glucose after controlling for the dose of MVPA.
Table 4.
OR (95% CI) for the MetS components according to physical activity level
| Physical activity group | High waist | High glucose | High blood pressure | High triglycerides | Low HDL |
|---|---|---|---|---|---|
| MVPAa | |||||
| Inactive (N = 915) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Somewhat active (N = 388) | 0.93 (0.68–1.25) | 0.82 (0.62–1.08) | 0.89 (0.67–1.18) | 0.80 (0.61–1.06) | 0.82 (0.61–1.10) |
| Active (N = 302) | 0.76 (0.55–1.05) | 0.72 (0.52–0.98)c | 0.74 (0.53–1.02) | 0.72 (0.53–0.99)c | 0.59 (0.41–0.84)d |
| Very active (N = 236) | 0.80 (0.54–1.18) | 0.32 (0.20–0.49)d | 0.52 (0.34–0.81)d | 0.59 (0.40–0.89)c | 0.62 (0.39–0.99)c |
| MPAa | |||||
| Inactive (N = 868) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Somewhat active (N = 347) | 0.79 (0.59–1.06) | 0.82 (0.62–1.08) | 0.91 (0.68–1.21) | 0.71 (0.53–0.94)c | 0.79 (0.59–1.07) |
| Active (N = 296) | 0.78 (0.57–1.07) | 0.65 (0.47–0.89)c | 0.72 (0.52–1.00) | 0.75 (0.55–1.02) | 0.60 (0.42–0.85)c |
| Very active (N = 230) | 0.75 (0.52–1.08) | 0.36 (0.24–0.55)d | 0.62 (0.41–0.94)c | 0.58 (0.39–0.86)c | 0.56 (0.36–0.88)c |
| VPAb | |||||
| Model 1 | |||||
| None (N = 1337) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Low (N = 295) | 0.73 (0.54–0.98)c | 1.00 (0.73–1.36) | 0.76 (0.55–1.04) | 0.80 (0.59–1.09) | 0.82 (0.54–1.14) |
| High (N = 209) | 0.43 (0.30–0.62)d | 1.06 (0.71–1.58) | 0.42 (0.27–0.66)d | 0.67 (0.45–1.00) | 0.51 (0.31–0.81)d |
| Model 2 | |||||
| None (N = 1337) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |
| Low (N = 296) | N/A | 1.12 (0.81–1.54) | 0.84 (0.61–1.17) | 0.91 (0.66–1.25) | 0.90 (0.63–1.26) |
| High (N = 209) | 1.32 (0.87–2.00) | 0.50 (0.32–0.80)c | 0.83 (0.55–1.27) | 0.60 (0.37–0.99)c |
Data presented as OR (95% CI). All ORs adjusted for age, sex, ethnicity, socio-economic status, smoking, alcohol, dietary fat, saturated fat and sodium.
aMVPA and MPA were adjusted for the covariates and VPA.
bModel 1 for VPA was adjusted for the covariates and MVPA. Model 2 for VPA was adjusted for the covariates, MVPA and waist circumference.
cP < 0.05 vs referent.
dP < 0.01 vs referent.
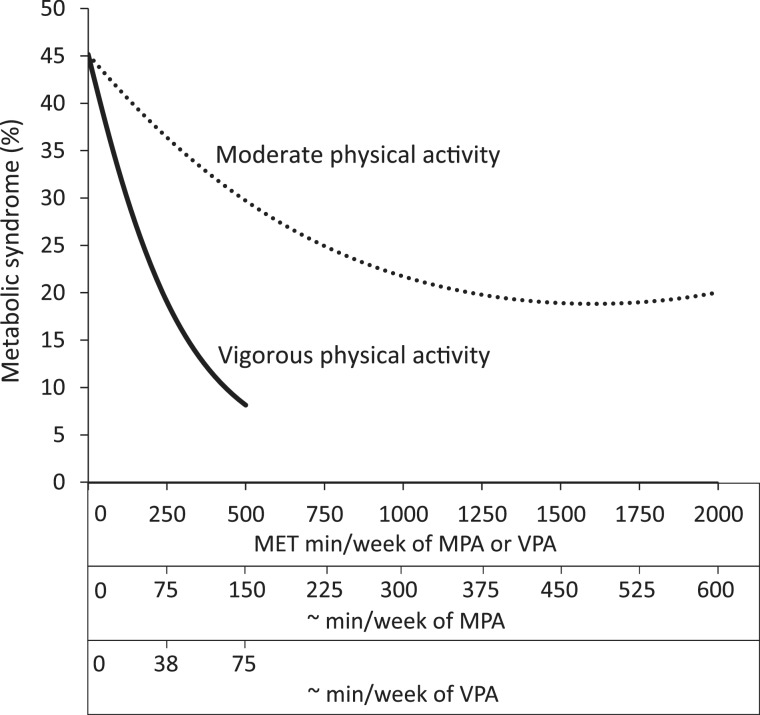
The influence of an equivalent energy expenditure dose of MPA and VPA on the MetS is illustrated in Figure 1. An equivalent energy expenditure dose of VPA had a greater influence on the prevalence of the MetS. For instance, after controlling for covariates and VPA, the prevalence of the MetS decreased by 8.8% between 0 and 250 MET min/week of MPA (45.2–36.4%) and by 15.5% between 0 and 500 MET min/week of MPA (45.2–29.7%). Conversely, after controlling for MPA, the prevalence of the MetS decreased by 26.2% between 0 and 250 MET min/week of VPA (45.2–19.0%) and by 37.1% between 0 and 500 MET min/week of VPA (45.2–8.1%). In other words, ∼75 min/week of VPA were associated with 2.4-fold greater difference in the prevalence of the MetS than ∼150 min/week of MPA.
Figure 1.
Estimated prevalence of the MetS according to MET min/week of MPA and VPA. Prevalence estimates were adjusted for age, sex, ethnicity, socio-economic status, smoking, alcohol, dietary fat, saturated fat and sodium. Minutes per week of MPA and VPA are approximations and were determined by dividing the corresponding MET min/week values by 3.33 and 6.67, respectively. Prevalence estimates were plotted from MPA and VPA MET min/week values of 0 to values that corresponded to the 98th percentile of the sample
Addition analyses explored whether VPA's influence on the MetS was mediated by abdominal obesity. First, waist circumference values were compared across VPA groups. After controlling for covariates and MVPA, adjusted waist circumference values were 98.9 cm in the none VPA group, 96.8 cm in the low VPA group (P = 0.12 vs none), and 94.4 cm in the high VPA group (P = 0.001 vs none). Second, we considered whether the relations that were observed between VPA and the MetS components (Model 1 of Table 4) were attenuated after further controlling for waist circumference (Model 2 of Table 4). Whereas the OR were attenuated somewhat, high VPA remained a predictor of high blood pressure and low HDL cholesterol.
Discussion
The intensity of MVPA was independently associated with the MetS and most of its components. In other words, VPA was a meaningful predictor of the MetS after controlling for the energy expenditure of MVPA, and was more strongly associated with the MetS than MPA. These findings suggest that VPA per se may have a role in health promotion.
As noted in the scientific committee report that formed the basis for the new physical activity guidelines,1 few studies have examined the effects of different physical activity intensities that are independent of their contribution to the energy expenditure dose. In a study of 13 485 men, Lee and colleagues reported that the trend between physical activity energy expenditure and all-cause mortality was more marked for VPA than for MPA.19 In a study of 3043 men, Slattery and colleagues reported that those who performed any VPA had a reduced risk of cardiovascular and all-cause mortality for any level of light-to-moderate intensity physical activity.20 Finally, in a study of 70 102 women, Hu and colleagues reported that equivalent energy expenditures from walking and vigorous activities resulted in comparable magnitudes of risk reduction for diabetes.21 Strengths of these three studies include the large samples and prospective designs. An important limitation of these studies is that questionnaires were used to estimate physical activity dose and intensity. The correlations between questionnaire and direct measures of physical activity, such as those obtained by accelerometry, are low-to-moderate.22 It is intriguing that the two previous studies on all-cause and cardiovascular mortality reported independent effects of VPA,19,20 whereas the previous study on diabetes did not.21 This suggests that the effects of VPA may vary depending on the outcome examined. Indeed, we observed that VPA was independently related to all components of the MetS with the exception of a high glucose.
Our findings suggest that VPA per se should be considered as a component of public health guidelines and considered when developing physical activity programmes. This observation is important as most research interventions prescribe activities that are in the moderate intensity range.1 Furthermore, current physical activity guidelines primarily promote VPA as a time-efficient approach for expending an appropriate amount of energy in comparison with MPA, rather than as an approach for achieving greater health benefits.3–5 The physical activity guidelines do recognize, to a small degree, the benefits of physical activity overload and progression, and our preliminary findings suggest that this aspect of the guidelines may require additional emphasis.3
In addition to the apparent independent health impact of VPA, the ability to expend energy twice as quickly with VPA than with MPA may encourage some inactive people to engage in physical activity as a lack of time is a common physical activity barrier.23 However, we recognize that promoting VPA to the general public is challenging given the apparent distaste most people have for VPA. For instance, in the present study 7 in 10 participants accumulated no VPA whatsoever. Even among those who achieved the MVPA guideline, less than one in five accumulated 10% of their MVPA energy expenditure through VPA.
We are uncertain as to what the biological mechanisms are that explain the observation that VPA had a greater influence on the MetS than an equivalent energy expenditure of MPA. One possibility is the effect of intensity on abdominal obesity. Experimental evidence suggests that, for a given caloric expenditure, high intensity interval training induces greater reductions in abdominal obesity than lower intensity steady-state exercise.24 Accordingly, we observed that, after statistical control for the MVPA energy expenditure dose, waist circumference differed across the VPA groups. We also observed that the odds of the blood pressure and lipid MetS components were lower in the high VPA group following adjustment for waist circumference, implying that non-obesity mechanisms are also relevant. Consistent with this notion, a VPA intervention (85–90% of VO2max) induced greater improvements in endothelial function and ambulatory 24-h blood pressure than an MPA intervention (60% VO2max), independent of weight loss.25
A strength of this study was the use of accelerometers, which measured the energy expenditure dose and intensity of physical activity in an objective way.26 However, the accelerometers did not capture water-based activities (i.e. swimming),were uniaxial and therefore only captured movement in one direction and were limited in their ability to capture some land-based activities (e.g. cycling).27 Assuming that the accelerometer measurement error was non-differential, the observed associations were underestimated. A main limitation of our study was its cross-sectional design, which precludes us from making definitive causal statements. Our degree of confidence that the physical activity variables were causally related to the MetS is increased because the associations we observed were strong, followed dose–response patterns and were biologically plausible, and because previous prospective cohort and randomized trials have reported similar relationships.28 Another limitation is that residual confounding may have been an issue due to measurement issues (e.g. non-differential misclassification) with the covariates, particularly the dietary covariates. Furthermore, 40% of the eligible NHANES sample was excluded because of incomplete or missing data. Although this influenced the descriptive information (i.e. means, prevalences), it is unlikely that this significantly influenced the associations between the physical activity and MetS variables as these relations are consistent in different socio-demographic groups.1 Finally, our calculation of MPA and VPA did not include any restriction on bout length, even though current guidelines are that physical activity be accumulated in bouts of at least 10 min. This limits the comparison of our study with previous epidemiological studies that used self-reported measures of physical activity that occurred in bouts.
In conclusion, the cross-sectional findings from this study suggest that MPA and VPA are independently associated with the MetS. Therefore, as indicated in current physical activity guidelines, accumulating 150 min/week of MPA or 75 min/week of VPA should reduce chronic disease risk. However, contrary to what is implied by the physical activity guidelines, the cardiometabolic health benefits of 75 min/week of VPA appear to be greater than the benefits of 150 min/week of MPA. This suggests that adults should accumulate at least some VPA over the course of the week to attain optimal cardiometabolic health. Additional studies, particularly randomized controlled trials, are needed to confirm the findings presented here.
Funding
Supported with a grant from the Heart and Stroke Foundation of Ontario (NA 7363); to I.J., who also received support from a Canada Research Chair position and an Early Researcher Award from the Ontario Ministry of Research and Innovation (to I.J.).
Conflict of interest: None declared.
KEY MESSAGES.
Current physical activity guidelines imply that, in comparison with MPA, the benefits of engaging in VPA are attributed to the greater energy expenditure dose per unit of time and do not relate to intensity per se.
This cross-sectional study of 1841 adults used minute-by-minute accelerometer measures of physical activity over 7 days to determine whether VPA is associated with the MetS independent of its influence on the energy expenditure dose of MVPA.
The results indicated that VPA remained a meaningful predictor of the MetS after controlling for the total energy expenditure dose of MVPA.
The results also indicated that VPA had a greater influence on the MetS than an equivalent energy expenditure dose of MPA.
References
- 1.Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. Physical Activity Guidelines Advisory Committee. http://www.health.gov/PAguidelines/Report/ (date last accessed 28 February 2012) [Google Scholar]
- 2.Sobal J, Valente CM, Muncie HL, Jr, Levine DM, Deforge BR. Physicians' beliefs about the importance of 25 health promoting behaviors. Am J Public Health. 1985;75:1427–28. doi: 10.2105/ajph.75.12.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2008 Physical Activity Guidelines for Americans. Washington, DC: ODPHP Publication; U.S. Department of Health and Human Services. No. U0036, 2008. http://www.health.gov/paguidelines (date last accessed 28 February 2012) [Google Scholar]
- 4.Bull FC, Groups EW. Physical Activity Guidelines in the U.K.: Review and Recommendations. School of Sport, Exercise, and Health Sciences, Loughborough University, Loughborough, UK; May 2010. [Google Scholar]
- 5.Global Recommendations on Physical Activity for Health. Geneva: 2010. World Health Organization. http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/index.html (date last accessed 28 February 2012) [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Data. National Center for Health Statistics (NCHS) 2003–2006. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm (date last accessed 28 February 2012) [Google Scholar]
- 7.Masse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37:S544–54. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 8.Colley R, Gorber SC, Tremblay MS. Quality control and data reduction procedures for accelerometry-derived measures of physical activity. Health Rep. 2010;21:63–69. [PubMed] [Google Scholar]
- 9.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–88. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 10.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Ardern CI, Janssen I. Metabolic syndrome and its association with morbidity and mortality. Appl Physiol Nutr Metab. 2007;32:33–45. doi: 10.1139/h06-099. [DOI] [PubMed] [Google Scholar]
- 12.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–45. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CL, Rifkind BM, Sempos CT, et al. Declining serum total cholesterol levels among US adults. The National Health and Nutrition Examination Surveys. JAMA. 1993;269:3002–8. [PubMed] [Google Scholar]
- 14.National Health and Nutrition Examination Survey Codebook for Data Production (2005-2006) Total Cholesterol (TCHOL_D) Person Level Data, 2007. [Google Scholar]
- 15.National Health and Nutrition Examination Survey Codebook for Data Production (2005-2006) HDL-Cholesterol (HDL_D) Person Level Data, 2007. [Google Scholar]
- 16.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 17.Dietary Guidelines for Americans 2005. Washington, DC: U.S. Department of Health and Human Services; 2005. U.S. Department of Health and Human Services, U.S. Department of Agriculture. http://www.health.gov/dietaryguidelines (date last accessed 28 February 2012) [Google Scholar]
- 18.Current Population Survey: Definitions and Explanations. Washington DC, USA: U.S. Census Bureau, Population Division, Fertility & Family Statistics Branch. [Google Scholar]
- 19.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000;151:293–99. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 20.Slattery ML, Jacobs DR, Jr, Nichaman MZ. Leisure time physical activity and coronary heart disease death. The US Railroad Study. Circulation. 1989;79:304–11. doi: 10.1161/01.cir.79.2.304. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–39. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 22.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes (Lond) 2008;32:684–91. doi: 10.1038/sj.ijo.0803781. [DOI] [PubMed] [Google Scholar]
- 25.Molmen-Hansen HE, Stolen T, Tjonna AE, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Cardiovasc Prev Rehabil. 2012;19:151–60. doi: 10.1177/1741826711400512. [DOI] [PubMed] [Google Scholar]
- 26.Bjornson KF. Physical activity monitoring in children and youths. Pediatr Phys Ther. 2005;17:37–45. doi: 10.1097/01.pep.0000154107.30252.fe. [DOI] [PubMed] [Google Scholar]
- 27.Treuth MS, Schmitz K, Catellier DJ, et al. Defining accelerometer thresholds for activity intensities in adolescent girls. Med Sci Sports Exerc. 2004;36:1259–66. [PMC free article] [PubMed] [Google Scholar]
- 28.Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]